To determine the thrombotic and hemorrhagic risk in bariatric surgery with multimodal rehabilitation programs, comparing two guidelines of pharmacological prophylaxis recommended in the Guide to the Spanish Society for Obesity Surgery and the Obesity Section of the AEC.
MethodsCohorts retrospective study from January-2010 to December-2019. Cases of vertical gastrectomy or gastric bypass were recorded, systematically applying multimodal rehabilitation protocols. Two reduced chemoprophylaxis regimens were analyzed, starting after surgery and maintained for 10 days; one with fondaparinux (Arixtra®) at a fixed dose of 2.5mg/day and the other with enoxaparin (Clexane®) with a single daily dose adjusted to BMI: 40mg/day for BMI of 35–40 and 60mg/day for BMI 40–60.
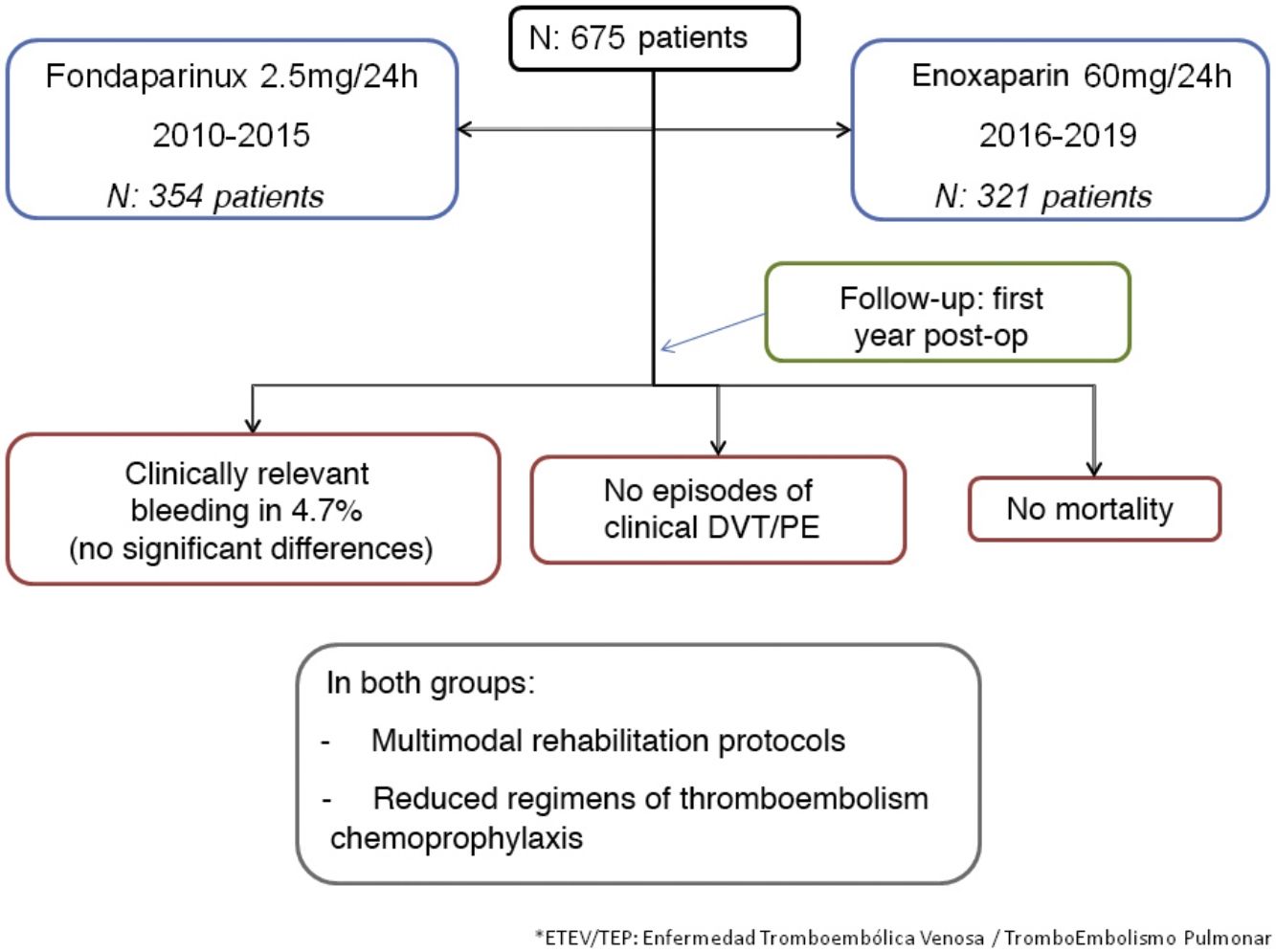
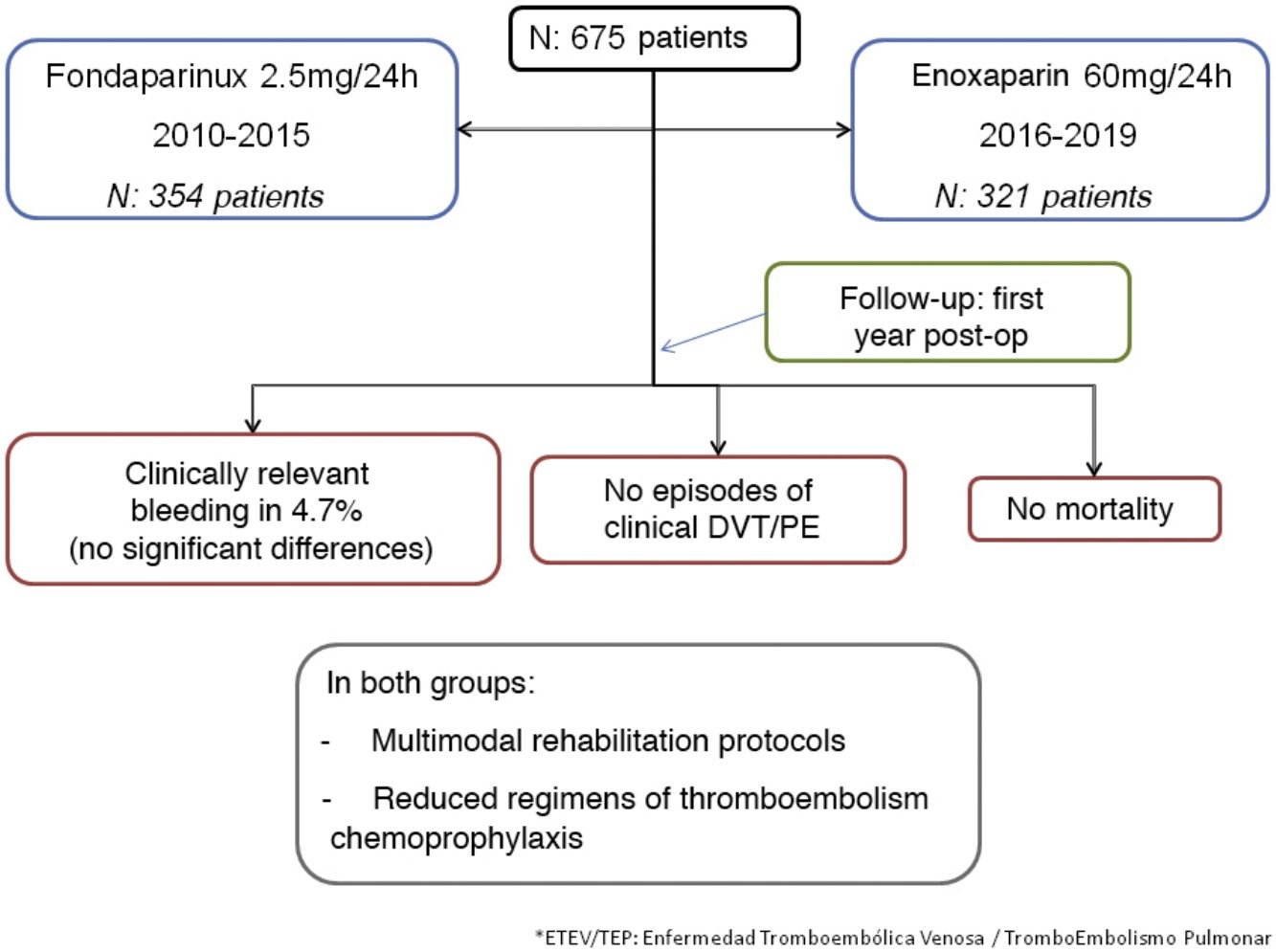
Results675 patients were included; 354 with Fondaparinux-Arixtra® during the period 2010−2015 and 321 with Enoxaparin-Clexane® during the period 2016−2019. There were no cases of DVT or clinical PE. However, the incidence of hemorrhage requiring reoperation, transfusion, or a decrease of more than 3g/dL hemoglobin was 4.7%, with no difference between groups. Mortality was nil. The average stay was 2.8 days and the outpatient follow-up was 100% during the first 6 months and 95% at 12 months.
ConclusionsThe combination of multimodal rehabilitation programs and mechanical and pharmacological thromboprophylaxis by experienced teams, reduces the risk of thromboembolic events and could justify reduced chemoprophylaxis regimens to decrease the risk of postoperative bleeding.
Determinar el riesgo trombótico y hemorrágico en la cirugía bariatrica con programas de rehabilitación multimodal, comparando dos pautas de profilaxis farmacológica recomendadas en la Guía de la Sociedad Española de Cirugía de Obesidad y la Sección de Obesidad de la AEC.
MétodosEstudio retrospectivo de cohortes desde enero-2010 a diciembre-2019. Se registraron los casos de gastrectomía vertical o Bypass gástrico, aplicando sistemáticamente protocolos de rehabilitación multimodal. Se analizaron dos pautas reducidas de quimioprofilaxis, de inicio tras la cirugía y mantenida durante 10 días; uno con fondaparinux (Arixtra®) a dosis fija de 2,5mg/día y otro con enoxaparina (Clexane®) con dosis única diaria ajustada al IMC: 40mg/día para IMC de 35-40 y 60mg/día para IMC de 40-60.
ResultadosSe incluyeron 675 pacientes; 354 con Fondaparinux-Arixtra® durante el periodo 2010-2015 y 321 con Enoxaparina-Clexane® durante el periodo 2016-2019. No hubo ningún caso de TVP o TEP clínico. No obstante, la incidencia de hemorragia con necesidad de una reoperación, trasfusión o con un descenso de más de 3g/dL de hemoglobina fue del 4,7%, sin diferencias entre los grupos. La mortalidad fue nula. La estancia media fue de 2,8 días y el seguimiento ambulatorio fue del 100% durante los primeros 6 meses y del 95% a los 12 meses.
ConclusionesLa combinación de programas de rehabilitación multimodal y tromboprofilaxis mecánica y farmacológica por equipos experimentados, reduce el riesgo de eventos tromboembólicos y podría justificar las pautas reducidas de quimioprofilaxis para disminuir el riesgo de una hemorragia postoperatoria.
Bariatric surgery is on the rise in Spain, with increasingly safer procedures but exposure to postoperative complications. The risk of deep vein thrombosis (DVT) ranges between 0.2% and 2%1 depending on the type of surgery, associated comorbidities, degree of obesity, clinical or radiological definition of thrombosis, and follow-up.2 The diagnosis of pulmonary embolism (PE) is less common but can be fatal.
A recent publication has described the advantages of multimodal rehabilitation or enhanced recovery protocols in reducing complications after bariatric surgery.3,4 One of the most relevant results is the lower incidence of thrombotic events,5,6 which, however, is accompanied by an increased risk of hemorrhage.3,7
The best prevention strategy is to apply preventive measures, including low-molecular-weight heparin, (LMWH), mechanical compression, early ambulation.8–12 Currently, there is no consensus on the dosage, duration of pharmacological prophylaxis, some authors propose a duration of 3–4 weeks,13 but others propose shorter regimens, reserving extended prophylaxis for high-risk patients.5,14
In 2016, the Spanish Society of Obesity Surgery (SECO) and the Obesity Division of the Spanish Association of Surgeons (AEC) published guidelines for thromboembolism prophylaxis in bariatric surgery,15 applying enhanced recovery programs to recommend shorter and reduced chemoprophylaxis guidelines.
The objective of this study was to determine the risk of thrombosis and hemorrhage in bariatric surgery using multimodal rehabilitation programs, comparing the results of 2 short (10-day) regimens of pharmacological thromboembolic prophylaxis: one based on fondaparinux (Arixtra® GlaxoSmithKline) and another on enoxaparin (Clexane® Sanofi Aventis).
MethodsStudy design and data collectionWe conducted a retrospective cohort study from January 2010 to December 2019. Data were collected prospectively, including patients with sleeve gastrectomy (SG) or with a gastric bypass (GBP) or another diversion technique. This study was approved by the hospital Ethics and Research Committee.
All patients were treated following the hospital’s multimodal rehabilitation protocol.16,17 Likewise, all followed a preoperative weight loss program with a hypocaloric/high-protein diet for 3–6 weeks prior to the intervention. Systematically, intermittent pneumatic compression systems were used in the operating room with a circumferential, sequential, progressive and simultaneous design (model DVT-2600®, Sorevan). High-risk cases (history of venous thrombosis, super-obesity, etc.) were more strictly monitored before surgery, with more prolonged and intense preoperative habilitation and weight loss, while pneumatic compression was maintained uninterruptedly after surgery until ambulation of the patient.
After surgery, patients remained in the resuscitation room 4–6h for hemodynamic and analgesia monitoring, mobilization, and respiratory physiotherapy. In the hospital ward, patients transferred to an armchair to initiate early oral tolerance (infusions and water). Oral intake progressed the following day, and patients walked freely until they were discharged.
Types of surgeryThe surgical technique did not change in any of the periods, and all procedures were laparoscopic. SG and derivative techniques were performed with laparoscopic mechanical suture (Echelon Flex® and Powered Echelon Flex® 60, Ethicon), and the staple line was reinforced with bovine pericardium (PeriStrip Dry®, Baxter).
The gastrojejunal anastomosis was created with continuous double flat manual suture using resorbable monofilament material (Monocryl®, Ethicon). The biliopancreatic loop anastomosis was created with mechanical or electrical endostapler and a vascular staple height of 2.5 mm (ETS Flex® 45 manual or Powered Echelon Flex®, Ethicon). A nasogastric tube was never inserted, and closed suction drains were only used in revision surgery.
All patients had follow-up visits after 15 days, one month, and quarterly during the first year, and every six months the year after.
The surgeries were performed by 2 specialists with experience of more than 500 cases of laparoscopic diversion surgeries before starting the study.
CohortsThe two consecutive cohorts were analyzed following the pharmacological regimens recommended in the Guidelines by SECO and the Obesity Division of the AEC:15
- 1
Period 2010−2015: fondaparinux at a dose of 2.5mg/24 h, subcutaneous
- 2
Period 2016−2019: enoxaparin at doses adjusted to BMI according to the most conservative recommendations of the guidelines (40mg/day at a BMI of 35–40; 60mg/day between 40 and 60; and 40mg/12 h in patients with a BMI >60.
The change in protocol was motivated by both the cost of fondaparinux and the difficulty of acquiring it in non-hospital pharmacies.
Pharmacological prophylaxis began 6–8h after the end of the intervention and was maintained for 10 days in all patients, including during hospitalization. No preoperative dose was administered, and anti-Xa activity was not quantified.
Study variablesDVT or PE were defined clinically by the presence of pain or edema in the lower limbs, chest pain, sudden dyspnea, etc. If the patient was asymptomatic, no imaging test was performed to detect a thrombus.
A hemorrhagic episode was defined by the need to transfuse, reoperate, or when a clinical sign of bleeding was observed (hematochezia, melena, hemoperitoneum or tachycardia), associated with a drop in hemoglobin of more than 3g/dL compared to levels prior to hospital admission, in accordance with studies that quantify bleeding in bariatric surgery without incidents.18
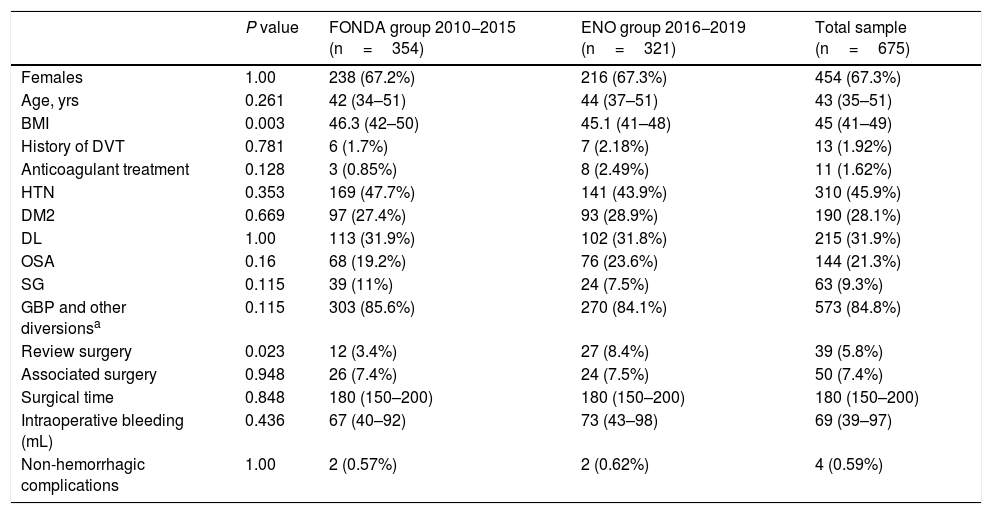
The study variables analyzed included the incidence of bleeding and the type of treatment (reoperation, transfusion, or medical). Bleeding that was considered ‘severe’ required reoperation or transfusion, and the number of units transfused was quantified. Table 1 shows the control variables that were registered. Outpatient follow-up was carried out during the first postoperative year.
Clinical characteristics of patients in both groups and the total sample.
| P value | FONDA group 2010−2015 (n=354) | ENO group 2016−2019 (n=321) | Total sample (n=675) | |
|---|---|---|---|---|
| Females | 1.00 | 238 (67.2%) | 216 (67.3%) | 454 (67.3%) |
| Age, yrs | 0.261 | 42 (34–51) | 44 (37–51) | 43 (35–51) |
| BMI | 0.003 | 46.3 (42–50) | 45.1 (41–48) | 45 (41–49) |
| History of DVT | 0.781 | 6 (1.7%) | 7 (2.18%) | 13 (1.92%) |
| Anticoagulant treatment | 0.128 | 3 (0.85%) | 8 (2.49%) | 11 (1.62%) |
| HTN | 0.353 | 169 (47.7%) | 141 (43.9%) | 310 (45.9%) |
| DM2 | 0.669 | 97 (27.4%) | 93 (28.9%) | 190 (28.1%) |
| DL | 1.00 | 113 (31.9%) | 102 (31.8%) | 215 (31.9%) |
| OSA | 0.16 | 68 (19.2%) | 76 (23.6%) | 144 (21.3%) |
| SG | 0.115 | 39 (11%) | 24 (7.5%) | 63 (9.3%) |
| GBP and other diversionsa | 0.115 | 303 (85.6%) | 270 (84.1%) | 573 (84.8%) |
| Review surgery | 0.023 | 12 (3.4%) | 27 (8.4%) | 39 (5.8%) |
| Associated surgery | 0.948 | 26 (7.4%) | 24 (7.5%) | 50 (7.4%) |
| Surgical time | 0.848 | 180 (150–200) | 180 (150–200) | 180 (150–200) |
| Intraoperative bleeding (mL) | 0.436 | 67 (40–92) | 73 (43–98) | 69 (39–97) |
| Non-hemorrhagic complications | 1.00 | 2 (0.57%) | 2 (0.62%) | 4 (0.59%) |
The qualitative variables present as absolute value (percentage) and the quantitative as median (interquartile range).
Qualitative variables are expressed as a percentage and were compared with a contingency test and Fisher’s exact test. The quantitative variables are expressed as means±standard deviation. Variables whose distribution did not conform to the normal curve are expressed as median and interquartile range, as confirmed with the Kolmogorov–Smirnov non-parametric test. The SPSS 15.0 statistical package from IBM was used.
ResultsBetween 2010 and 2019, 675 patients were operated on. During the 2010−2015 period, 354 were treated with fondaparinux (FONDA group), while in the 2016−2019 period, 321 were treated with enoxaparin (ENO group).
Table 1 shows the baseline characteristics of both groups. There were no significant differences, except in BMI (one point lower in the ENO group) and the higher revision surgery rate in the second period due to the cumulative effect of complications with the time elapsed after the primary surgery. In the correlation analysis between the patients with revision surgery and bleeding episodes, there were no significant differences.
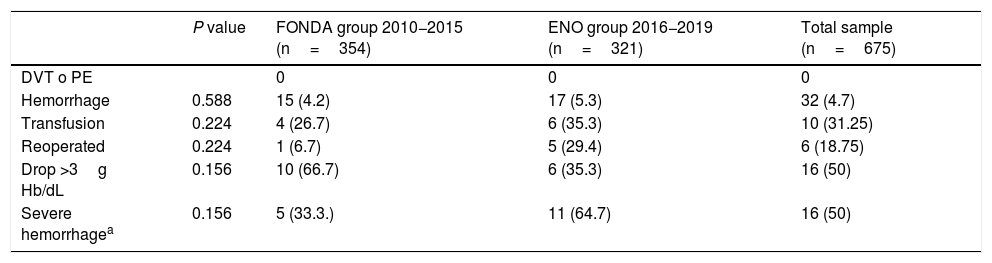
Table 2 shows the results, without registering any cases of clinical DVT or PE. There were 32 cases of bleeding (4.7%), 15 in the FONDA group and 17 in the ENO group, with no significant differences in the most relevant clinical and technical variables described in Table 3. When we analyzed serious bleeding (reoperation or transfusion), there were 11 cases in the ENO group (3.4%) and 5 in the FONDA group (1.4%), with no significant differences. In the ENO group, 6 patients were transfused with a mean of 4.5 units (3–7) and in the FONDA group there were 4 patients with a mean of 5 units (2–10). Most of the hemorrhages were digestive, in the form of melena or rectal bleeding (84%), with no differences between the groups.
Risk of thrombosis and hemorrhage in each group and in the total sample.
| P value | FONDA group 2010−2015 (n=354) | ENO group 2016−2019 (n=321) | Total sample (n=675) | |
|---|---|---|---|---|
| DVT o PE | 0 | 0 | 0 | |
| Hemorrhage | 0.588 | 15 (4.2) | 17 (5.3) | 32 (4.7) |
| Transfusion | 0.224 | 4 (26.7) | 6 (35.3) | 10 (31.25) |
| Reoperated | 0.224 | 1 (6.7) | 5 (29.4) | 6 (18.75) |
| Drop >3g Hb/dL | 0.156 | 10 (66.7) | 6 (35.3) | 16 (50) |
| Severe hemorrhagea | 0.156 | 5 (33.3.) | 11 (64.7) | 16 (50) |
The data are presented as absolute value (percentage).
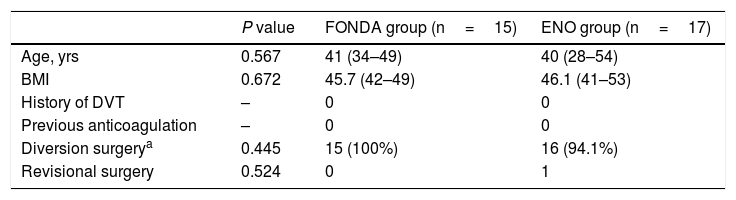
Clinical characteristics and techniques of cases with bleeding.
| P value | FONDA group (n=15) | ENO group (n=17) | |
|---|---|---|---|
| Age, yrs | 0.567 | 41 (34–49) | 40 (28–54) |
| BMI | 0.672 | 45.7 (42–49) | 46.1 (41–53) |
| History of DVT | – | 0 | 0 |
| Previous anticoagulation | – | 0 | 0 |
| Diversion surgerya | 0.445 | 15 (100%) | 16 (94.1%) |
| Revisional surgery | 0.524 | 0 | 1 |
The data are presented as absolute value (percentage).
There were 4 patients with non-bleeding complications: 2 in the FONDA group (2 leaks), and 2 in the ENO group (1 postoperative appendicitis and 1 intestinal obstruction). The reoperation rate was 1%. Overall mortality was zero.
All operations were performed laparoscopically, with no conversions to open surgery. The mean preoperative weight loss was 7 kg. The overall mean stay was 2.8 ± 2 days. Outpatient follow-up participation was 100% during the first 6 months and 95% after 12 months.
DiscussionThe objective of this study was to determine the efficacy and safety of the thromboprophylaxis protocol for bariatric surgery published by SECO and the Obesity Division of the AEC in 2016.15
We have analyzed 2 reduced regimens for pharmacological thromboprophylaxis recommended in the guidelines: one with enoxaparin, and the other with fondaparinux. The latter is a factor Xa inhibitor that has a set dosage, regardless of BMI, which is initiated 6–8h after surgery. Due to these properties, fondaparinux was the treatment of choice in the first study period, from 2010 to 2015. Subsequently, the adjusted enoxaparin regimen proposed in the guidelines was adopted due to the cost and difficulty of purchasing fondaparinux in non-hospital pharmacies.
In the cohort treated with enoxaparin, we used the most conservative of the recommended regimens: prophylaxis initiated 6–8h after surgery, one single daily dose following the hospital care guidelines for postoperative patients, and a duration of 10 days (including hospitalization). The dose was the lowest adjusted to the BMI: 40mg/day at BMI between 35 and 40, and 60 mg/day when the BMI was between 40 and 60.
Both thromboprophylaxis guidelines were very safe, as no case of clinical DVT or PE was detected in the sample analyzed (675 patients) despite the high percentage of diversion techniques (85%), revision surgeries (5.8%), and risk factors, such as type 2 diabetes mellitus (28.1%) or obstructive sleep apnea syndrome (21.3%).
For the early detection of a thromboembolic or hemorrhagic event, patients were closely monitored while hospitalized. The outpatient follow-up included a strict outpatient control, with a follow-up rate of 100% during the first semester and 95% one year after surgery. The lack of clinical thrombosis after 12 months of follow-up was due to a combination of several factors: first, the prioritization of ambulation and mobility immediately after surgery; second, the low incidence (0.6%) of major non-hemorrhagic postoperative complications; and, finally, the short mean hospital stay (2.8 days). Undoubtedly, complications that entail an increase in hospital stay or longer immobilization increase the risk of thrombosis and require a more aggressive approach in the dosage and duration of prophylaxis.5,14
In this study, the application of multimodal rehabilitation measures was very strict and followed the RICA Guidelines of the Spanish Ministry of Health,16 the Spanish Multimodal Rehabilitation Group10 and the SECO Clinical Pathway,11 with special attention to preoperative optimization, multidisciplinary assessment, protocolization of techniques, early ambulation, and oral tolerance. In the study, the use of mechanical pneumatic compression systems was very strict to favor propulsion and venous circulation while the patient was immobilized in the operating room.17 It is surprising that its use is not more extended to all abdominal surgery interventions19 when it is an effective method with few side effects.
In 2018,20 Altieri et al. analyzed an American registry of 11 860 bariatric patients treated with SG or GBP. They reported a striking incidence of thrombosis in a group of 3987 patients without chemoprophylaxis, which was only 0.58% and decreased to 0.18% when a chemoprophylaxis regimen was administered (pre-, postoperative or both). The results of our study, combining minimally invasive surgery, multimodal rehabilitation, and mechanical and pharmacological prophylaxis, show that the current thrombotic risk can be very low.
The incidence of bleeding events in the study was high (4.7%), but these results are within the same range of the American registry by Altieri et al,20 where the incidence of postoperative bleeding requiring transfusion ranged between 1.9% and 5%.
The bleeding risk was similar in both therapeutic groups (4.2% in the FONDA group vs 5.3% in the ENO group), although the incidence of serious events (reoperated or transfused) was higher in the group with enoxaparin, without reaching statistical significance. When we analyzed the subgroup of patients with bleeding, there were no significant differences between the two groups in terms of age, BMI, number of bypass and revision surgeries, or history of thrombosis or previous anticoagulation.
We have analyzed different aspects of the study to explain these results, such as the chemoprophylaxis regimen or the experience of the team. Fondaparinux is prescribed with a set dose, regardless of BMI, while enoxaparin is prescribed based on BMI, without quantifying ideal weight or the actual volume of distribution, which could lead to a supratherapeutic level that would explain the postoperative bleeding. In our opinion, the increased dosage with LMWH recommended for the obese population compared to the non-obese population may be excessive when multimodal rehabilitation programs are applied that reduce the risk factors for thrombosis and equate them with other surgical patients, leading to high doses that may favor the hemorrhagic events observed in this study.
With regards to the experience of the team, bleeding was more frequent in the second period of the study, with enoxaparin prophylaxis, when the main surgeons had accumulated more than 1000 laparoscopic bariatric interventions.
According to the national venous thromboembolism survey published by Arcelus Martínez et al.,19 the multidisciplinary thrombosis committees of each hospital must adapt their postoperative prophylaxis guidelines, as the risk of postoperative hemorrhage can be as serious as the risk of a thrombosis.
The limitations of this retrospective study include the fact that subclinical thromboembolic events have been underestimated, since systematic imaging tests were not performed and only clinical events have been quantified, while assuming that these complications are highly unlikely to go unnoticed under strict outpatient follow-up. However, prospective studies with imaging tests are needed to correctly analyze this problem.
ConclusionsThe combination of multimodal rehabilitation programs and mechanical and pharmacological thromboprophylaxis in bariatric surgery reduces the risk of thrombosis and, therefore, may justify reduced pharmacological prevention regimens to lower the risk of postoperative bleeding. In our study, no differences were found between the use of short courses of fondaparinux and enoxaparin. However, new prospective and multicenter studies are needed to establish the safest and most efficient regimens in the bariatric population.
FundingThis study has received no specific funding from public, commercial, or non-profit organizations.
Conflict of interestsNone.
Please cite this article as: Gorosabel Calzada M, Hernández Matías A, Andonaegui de la Madriz A, León Ledesma R, Alonso-Lamberti Rizo L, Salazar Carrasco A, et al. Riesgo trombótico y hemorrágico en cirugía bariátrica con programas de rehabilitación multimodal comparando 2 pautas reducidas de profilaxis farmacológica. Cir Esp. 2022;100:33–38.