Cervicouterine cancer screening with cytology decreases incidence by more than 50%. The cause of this cancer is the human papilloma virus high risk, and requires a sensitive test to provide sufficient sensitivity and specificity for early detection and greater interval period when the results are negative. The test of the human papilloma virus high risk is effective and safe because of its excellent sensitivity, negative predictive value and optimal reproducibility, especially when combined with liquid-based cytology or biomarkers with viral load, with higher sensitivity and specificity, by reducing false positives for the detection of cervical intraepithelial neoplasia grade 2 or greater injury, with excellent clinical benefits to cervical cancer screening and related infection of human papilloma virus diseases, and is currently the best test for early detection infection of human papillomavirus and the risk of carcinogenesis.
La detección del cáncer cervicouterino con citología disminuyó su incidencia en más del 50%. La causa de este cáncer son virus del papiloma humano de alto riesgo. Se requiere de una prueba sensible que proporcione la sensibilidad y especificidad suficientes para su detección oportuna, y mayor periodo de intervalo cuando los resultados son negativos. La prueba del virus del papiloma humano de alto riesgo es eficaz y segura debido a su excelente sensibilidad, valour predictivo negativo y reproducibilidad óptima, principalmente cuando se combina con citología en base líquida o biomarcadores con carga viral, con mayor sensibilidad y especificidad, reduciendo los falsos positivos para la detección de la neoplasia intraepitelial cervical grado 2 o para lesiones mayores, con excelentes beneficios clínicos para la detección del cáncer cervicouterino y otras enfermedades relacionadas con la infección del virus del papiloma humano. Actualmente es la mejor prueba para la detección temprana de la infección por virus del papiloma humano y el riesgo de carcinogénesis.
Over the last 30 years, the mortality rate from cervical cancer in the United States has decreased by more than 50% as a result of screening with the Papanicolaou or cytology test, which is being offered to most women who were not tested in the past. Cytology has been developed as liquid based since 1928, when Dr. Papanicolaou1–3 first reported cancerous cells in vaginal smears, and published his results in 1941. Testing for human papillomavirus (HPV) high risk (HPV-H) was approved in 2000 and the first vaccine against HPV came onto the market in 2006. New vaccines are currently under development to provide broader coverage of genotypes and greater protection.4,5
EpidemiologyCytology has lowered the incidence and rate of mortality from cervical cancer in developed countries through organised screening programmes. Nevertheless, more than 68,000 and 12,000 new cases are still being reported each year in Europe and the United States respectively, with more than 4000 deaths from cervical cancer in the United States in 2013. However, there is evidence that the cervical cancer mortality rate had decreased even before the HPV vaccine was introduced: the overall rate1,6 fell from 10.2 to 8.5 cases per 100,000 women between 1998 and 2002. In Mexico, 9000 women are treated annually for cervical cancer and 4000 die; the incidence in 2008 was 19.2 and mortality was 9.7 per 100,000 women. The high level of mortality is due to social inequality. The National Programme for the Detection of Cervical Cancer was started in Mexico in 19747,8 and a slight decrease in mortality has been observed since 1992, which has fallen from 13.3 in 2000 to 6–8 per 100,000 in 2008.
Screening recommendations change as technologies develop. The sensitivity of a single Pap test in detecting a cervical intraepithelial neoplasia (CIN) of stage 2 or higher (CIN-2+) or high grade intraepithelial squamous lesions is low,2,7–11 and for it to be implemented demands frequent repetition intervals, a high level of organisation and high costs. Effective biomarkers are needed to predict the risk of CIN. The most important of these are those which genotype HPV-H, which is found in more than 90% of CIN and cervical cancers.12 The clinical course of the new HPV-H screening strategies is reducing the cervical cancer mortality rate, as there are more than 40 different genotypes of HPV-H which cause persistent cervical infections, and the risk of progression of CIN differs considerably according to the HPV-H genotype. But the majority of HPV-H infections are rare and most HPV tests do not include all of them.1 The HPV-H test offers high sensitivity in detecting13–16 CIN-2+, but its specificity is limited because most HPV infections are transient and only a low proportion of HPV infections persist and progress to become intraepithelial squamous lesions. Due to the high prevalence of HPV infections in women under 30, HPV-H testing is not currently recommended in screening women under 30.17
HPV-H screening in women with abnormal cytology plays a role in identifying women at risk of residual or recurrent disease after treatment of CIN. Although the HPV-H test is less specific than cytology, cytology does not always distinguish between transient and chronic infection.4,14,18–21 The expression of oncoproteins E6 and E7 of the HPV-H genotypes, in squamous epithelial cells of the cervix causes the development of neoplastic growth1 and the over-expression of the biomarker p16INK4a (p16)22–27 which is one of the cyclin-dependent kinase inhibitors which prevents the phosphorylation of the retinoblastoma protein (pRb) and, therefore, plays a major role in cell-cycle regulation. Its over-expression is frequently observed in CIN associated with HPV-H infection and is associated with dysfunction of the retinoblastoma protein (pRb) through mutations which occur naturally. It can also be associated with oncoprotein E7 of HPV-16 which causes cell-cycle alterations, over-expressing p16. This biomarker predicts the risk of progression and increases the higher the stage of CIN: CIN-1 (20.7%), CIN-2 (80%) and CIN-3 (89.2%). p16 over-expression is significantly greater in CIN 2 and 3 than in CIN-1 (p<0.001). This biomarker is effective compared to HPV-H testing in patients with CIN-1 and 2. In patients tested for HPV-H, 80% were positive and the HPV-H infection rate also increased in lesions of higher stages, in CIN-1 (65.1%) and CIN-2 and 3 (87.7%) (p<0.001).28,29
However, the patients who were infected with HPV-H showed a greater prevalence of progression of the lesions, with no differences between the groups with positive and negative HPV-H, in either the rate of progression or regression of the lesions between patients infected or otherwise with HPV-16 or HPV-18 (p=0.60).7 Detecting over-expression of p16 is effective in managing cytology with a report of atypical squamous cells of undetermined significance (ASC-US) or low-grade intraepithelial squamous lesions20–25 and for women testing positive for HPV-H. Sensitivity for CIN-2+ is 18%, greater than cytology (p<0.001) in women of all ages, with 95.2% specificity (Table 1).1,30 Specificity of cytology with p16 is greater with the HPV-H test, with 50% fewer false positives. Dual-stain cytology and the combination of biomarkers p16/Ki-67, which are indicative of the transformation of HPV infection, are more sensitive and specific for detecting CIN-2+, principally in women under 30. No other complementary test is currently available, and alternatives to cytology are limited.6,17,19,28,29,31
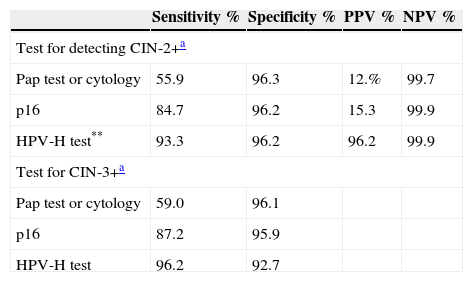
Sensitivity, specificity, positive and negative predictive values in detecting CIN-2+ and CIN-3 in women aged 30–65.
CIN, which is effectively treated, prevents progression to cervical cancer. CIN-1 is a lesion which only requires monitoring and not treatment: 10% of CIN-1 progress to CIN-3 or cervical cancer; 20% of CIN-2 progress to CIN-3 or cervical cancer and 40% of CIN-2 remit spontaneously. Management of CIN-1 and 2 is controversial: some remit spontaneously. They are treated with destructive or excisional procedures in patients at greater risk of progression, and it is observed that those that are low risk remit spontaneously, but it is difficult to predict the outcome for each individual patient.31
Primary detection of cervical cancerHPV-H tests, for detecting and preventing lesions that are precursors of cervical cancer, compared with cytology, offer 60–70% better protection against this cancer. They are effective principally in women aged from 30 to 34, and when they are performed every 5 years offer greater protection than the Pap test undertaken every 3 years.32 The incorporation of the HPV-H test in developed countries in screening strategies for vaccinated women remains to be determined, although it should be similar to that of women who have not been vaccinated. In the short term, detection with the HPV-H test will be cheaper and offer greater security than the conventional Pap test. Despite these benefits, public health programmes will have logistical problems for screening. These problems include determining the type of HPV-H test to be used, how to determine the age and appropriate intervals for testing, how to manage HPV-H positive women and ensure the quality, adherence and application of HPV-H testing in cervical cancer prevention programmes. The HPV-H test is more effective in detecting high-grade intraepithelial squamous lesions and in the prevention of cervical cancer than cytology in women over the age of 35, and than the Pap test or visual examination of the cervix with acetic acid, and this has lowered the incidence and mortality rate of cervical cancer in developing countries.2,14,33
New directives for the detection of cervical cancerNew directives recommended by the different international associations for the early detection of cervical cancer2,15 recommend the HPV-H test combined with the Pap test in women from the ages of 30 to 65. During monitoring after a negative Pap test, it has been demonstrated that screening intervals of 5 years with HPV-H testing are safer than intervals of 3 years using the Pap test alone.
With these recommendations, the proportion of cervical adenocarcinomas has reduced by 40% in women under 30; by 35% between the ages of 30 and 34; by 30% between 35 and 49, and by 23% in women aged over 50. The prevention of cervical cancer in women of reproductive age is a priority, and therefore detection with the HPV-H test should be started at the age of 30. These directives have substantially changed women's health practices and cover HPV infection and the natural history of cervical cancer.2,15
Most HPV infections are transient, the body is capable of eliminating them and only persistent HPV infections result in cervical cancer. Most women, especially under the age of 21, eliminate the infection in 1–2 years. Infections in women over 30 are more likely to persist, and the rates of high-grade lesions increase, but most of these HPV-H related lesions only progress slowly to cervical cancer. On average, it takes 3.7 years for an intraepithelial squamous lesion to progress to cervical cancer. The new directives are1,2,15:
Screening should start at around 21 years of age, regardless of behaviour, risk factors and age of first sexual relationship.
Women aged from 21 to 29 should be Pap tested every 3 years, without HPV-H testing. Combined testing (Pap and HPV-H test) should take place from the age of 30–65 every 5 years. HPV-H is the preferred recommendation, but cytology alone every 3 years is also acceptable. However HPV-H testing alone is not indicated.
After the age of 65, future detection recommendations depend on previous screening. If the results have been negative, these women do not require routine screening and it is not necessary. One previous negative screening means 3 consecutive negative Pap results or 2 consecutive negative combined test results over the past 5 years. Screening cannot stop after the age of 65 for women with a history of CIN-2, CIN-3 and should continue. Women who have had a hysterectomy, with no history of NIC-2 or higher stage, are no longer screened, patients with CIN-2 or CIN-3 prior to hysterectomy continue to be screened with the Pap test every 3 years for 20 years, because recurrent cancer can develop in the vaginal vault years later. There is no clarity on HPV-H testing in this scenario. These routine guidelines do not apply to women who are under immunosuppression, are HIV positive, who have been exposed to diethylstilboestrol in utero, or who have a history of cervical cancer.2,7,8,15
Health promotionThe education of women and healthcare professionals is an essential aspect in HPV-H testing, during primary screening, and the clinical and psychological management of women with normal Pap tests, and positive HPV-H tests are necessary, especially if women under 30 are included. HPV infection is a matter of public interest, but it affects people emotionally and fear of cancer increases their anxiety and this has an impact on their quality of life.34,35
The best time to give information on HPV infection is before the HPV-H test is performed. The patient is more attentive, able to understand and their anxiety can be lessened. Furthermore, it helps with further follow-up procedures (for example, a repeat HPV-H test, cytology, colposcopy and other biological markers) in relation to the stratification of risk.31 A positive HPV-H test does not represent disease, and it is a risk factor. If the HPV-H test is still positive a year after the first test, a cytological triage or non-invasive tests are performed, which prevent the development of cervical cancer. Current algorithms are designed for women who are aware and clinical monitoring is easy with follow-up. But this is not the case in marginalised communities in Latin America, Asia and Africa, with low socio-economical and cultural levels. These communities are those which are most affected by cervical cancer, where prevalence rates of this cancer are higher than those for white women in developed countries.36
DiscussionReports over the past 2 decades on HPV-H testing have definitively demonstrated the association between the HPV-H genotypes and cervical cancer1 in monitoring treated patients, compared with the conventional Pap test or colposcopy, in symptomatic or asymptomatic women, for detecting lesions that are precursors of cervical cancer,32 both primary cervical screening and the management of borderline cytology such as ASC-US. HPV-H tests are not always used in clinical practice, and in national screening programmes, although they are more sensitive than the Pap test1,11–13 in detecting CIN-2+, combined HPV and Pap testing show greater negative predictive values (NPV)14 for CIN-2+. Some CIN-2 remit spontaneously and the greater sensitivity for these precancerous lesions and cervical cancer, grouped as CIN-3+, is merely over diagnosis, as there is a lower future incidence of CIN-3+.1,15
Increased sensitivity has 2 major clinical outcomes: a reduced mortality rate and extended screening interval, with greater detection, lower cost and higher reproducibility. The HPV-H test is more sensitive than liquid-based cytology in detecting CIN-3 or larger lesions, but it is less specific (92.0% versus 53.3%; a difference of 38.7%). Although adding liquid-based cytology to the HPV test increases sensitivity for CIN-3 or greater lesions (96.7%); the number of positive tests has also increased (35.2%). However, the use of the HPV-16 or HPV-18 test offers better information with similar results.13,26,27
Screening for p16 in positive women in the triage study for ASC-US/LSIL (ALTS) designed to compare 3 management options,23 the HPV-H test showed greater sensitivity and identified 96.3% (95% CI: 91.6–98.8) of women with CIN-3 or greater lesions,24 the same positive predictive value as detection with conventional cytology (without increasing the number being sent for colposcopy), and the HPV-H test maintained higher NPV.26 The particular value of the HPV-H test is greater in women vaccinated against HPV. In the near future another value of HPV-H testing is expected due to vaccination and the low prevalence of HPV-related disease.1,4,6,17–19,37–39. Residual or recurrent disease in women with persistent HPV-16 or HPV-18 is higher (82%) than in women with persistence of other types of HPV-H such as HPV 31, 33, 35, 45, 52, and 58 (66.7%) or VPH 39, 51, 56, 59, 68, 26, 53, 66, 73, y 82 (14.3%). This suggests different levels of risk for the progression of CIN. Detecting the persistence of certain HPV-H genotypes has the potential to improve the management of these patients. Obviously, follow-up after treatment must include conventional cytology and HPV-H testing to identify patients at greater risk of recurrence of the disease.1,38,39
In the past 50 years, the relative proportion, and absolute incidence, of pre-invasive and invasive glandular lesions of the cervix have been increasing in western countries. In 1950–1960 cervical adenocarcinomas represented 5% of cervical cancers; in 1970 they represented from 20% to 25% of all cervical cancers, the majority in women of reproductive age who required fertility-conserving surgery. Although the management of in situ adenocarcinoma is controversial, during follow-up of patients with in situ adenocarcinoma who wanted to preserve their fertility,1 the combination of the HPV-H test and the Pap test demonstrated greater sensitivity in detecting persistent lesions, with 100% NPV which is useful and prevents unnecessary hysterectomies. HPV-H tests have been introduced in clinical practice as curative tests, in which the persistence of a specific genotype predicts recurrence short term and the absence of the HPV genotype, associated with preoperative diagnosis, imply successful treatment with low risk of recurrence.1,31,38
ConclusionHPV is the cause for the development of cervical cancer and it is screened using the HPV-H test versus conventional liquid-based cytology; sensitivity, NPV and reproducibility; and management of cytology with ASC-US reporting and follow-up after treatment of the CIN. HPV-H tests are almost 100% sensitive and NPV to identify pre-neoplastic lesions or cervical cancer is the main test in primary screening for these squamous and glandular lesions or in situ adenomacarcinoma; moreover, these lesions are difficult to detect. Genotyping of HPV-H 14 and viral load1,40 reduce the amount of false positive results, respectively. Sensitivity to CIN-3 or greater lesions was maintained at 100%, it being effective and safe in detecting cervical cancer, principally in combination with liquid-based cytology with biomarkers p16/Ki-67 which will be more sensitive and specific, in detecting CIN-2 or greater lesions, and better clinical performance in detecting cervical cancer and HPV related diseases.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Vargas-Hernández VM, Vargas-Aguilar VM, Tovar-Rodríguez JM. Detección primaria del cáncer cervicouterino. Cir Cir. 2015;83:448–453.