The biological changes that commonly cause degenerative articular cartilage injuries in the knee are primarily associated with misalignment of the joint and metabolic changes related to age, as occurs in osteoarthritis. Furthermore, the capacity for cartilage self-regeneration is quite limited due to the lack of vascularity of the tissue. To date, there is no ideal treatment capable to stimulate cartilage regeneration; thus there is a need to seek alternative therapies for the treatment of such conditions.
The number of publications demonstrating the therapeutic and regenerative benefits of using platelet-rich plasma as a treatment for knee osteoarthritis has been increasing in recent years. In spite of encouraging results, there are still only a few randomised control studies with strong clinical evidence, lacking clarity on points such as the optimum formulation or the mechanism of action of platelet-rich plasma. Up to this point and based on the results of clinical studies, not all patients can benefit from this therapy. It is important to consider aspects such as the age and grade of cartilage degeneration.
The aim of the present paper is to review the recent scientific literature on the treatment of knee osteoarthritis with platelet-rich plasma, and the biological bases of this therapy, as well as presenting the current opinion on this subject.
Los cambios biológicos degenerativos que frecuentemente generan lesiones en el cartílago articular de rodilla se asocian principalmente con defectos en la alineación de la articulación, y con cambios de tipo metabólico relacionados con la edad como ocurre en la osteoartrosis. Además, la capacidad de autorregeneración del cartílago se ve bastante limitada debido a la falta de vascularización del tejido. Hasta la fecha no se cuenta con un tratamiento ideal que logre estimular la regeneración del cartílago, por lo que es necesario buscar alternativas terapéuticas para el tratamiento de este tipo de padecimientos.
En años recientes se ha incrementado el número de publicaciones que intentan mostrar los beneficios terapéuticos y regenerativos al utilizar plasma rico en plaquetas como tratamiento en osteoartrosis de rodilla. A pesar de los resultados alentadores que se han observado, son todavía pocos los estudios controlados aleatorizados, con evidencia clínica contundente, quedando por esclarecer puntos como la formulación óptima o el mecanismo de acción del plasma rico en plaquetas. Hasta este punto, no todos los pacientes pueden tener un beneficio de esta terapia, basándonos en los resultados de los estudios clínicos, en donde es importante tener en cuenta aspectos como la edad y el grado de desgaste del cartílago.
El objetivo del presente trabajo es hacer una revisión de la literatura científica reciente, del tratamiento de la osteoartrosis de rodilla con plasma rico en plaquetas y de las bases biológicas de esta terapia, así como mostrar una opinión actual acerca de este tema.
The articular cartilage is a highly specialised connective tissue in mobile joints, whose main function is to provide an articular surface with low friction and efficient support for mechanical loads. It is a tissue that is devoid of blood vessels, lymphatic vessels and nerve endings, so its intrinsic regenerative ability is very low.1 The lesions caused in this type of tissue are difficult to treat and they still pose a challenge today in the orthopaedic field.
Biological changes, including biomechanical and metabolic ones, as well as traumas, can lead to a loss in cartilage homeostasis, which significantly increases with age. This results in an accelerated reduction of the articular surface, which causes osteoarthrosis.2
This is the most frequent chronic and degenerative articular disease, the most common source of pain and one of the main causes of disability and dependence among the adult population, and generates high costs in healthcare.3 Osteoarthrosis is more common in females (2:1), although after menopause the male:female ratio is 1:1. Its frequency increases with age, so according to radiographic criteria, 30% of the population between the age of 45 and 60 years and over 80% of people over the age of 80 years have osteoarthrosis in at least one joint.4 Worldwide, it is the fourth cause of morbidity in women over the age of 60 and the eighth cause in men.5 In Mexico, a 10.5% predominance of osteoarthrosis has been estimated.6 According to the statistical reports from the Instituto Mexicano de Seguro Social, osteoarthrosis is one of the ten main reasons for consultations in primary healthcare.4
Clinically, this disease is characterised by joint pain, stiffness, motion restriction and variable degrees of swelling.7 It is also characterised by an imbalance between anabolic and catabolic processes,8 which leads to progressive damage of the cartilage, and ultimately to the patient's disability.
Treatments for osteoarthrosis range of techniques that only relieve pain, such as arthroscopy and an initial pharmacological treatment with analgesics and anti-inflammatory drugs, to procedures that involve the creation of grafts with three-dimensional structures in the tissue engineering field. However, up to this moment, there is no effective method of regenerating cartilage.
In recent years, the use of autologous growth factors as a regenerative treatment for chondral tissue has been taken into consideration as a therapeutic alternative. These growth factors can be obtained from circulating platelets in peripheral blood. Thus, platelet-rich plasma therapy has gained great importance in the last few years, and has been the object of several studies and efforts. Its use in tissue regeneration has spread from its implementation in dentistry, maxillofacial surgery and dental implants, the regeneration of tendons and ligaments in orthopaedics, to its implementation in plastic surgery and cosmetology.9
The clinical use of platelet-rich plasma has been proposed as a therapeutic alternative in patients with osteoarthrosis, administering it by means of intra-articular injections directly in the knees of the affected patients. Most of the studies performed so far show promising results, with pain reduction and an improvement in joint function. A couple of years ago, studies on controlled randomised trials began to be published. These provided improved clinical evidence about the first non-controlled trials or the ones in which series of cases were analysed.
Proliferative properties have also been reported10 of chondrogenic11 and anabolic differentiation in platelet-rich plasma12 in in vitro studies, at cellular culture level and in in vivo studies, mainly in mice and rabbits, emphasising the role of platelet-rich plasma in the regeneration of the osteoathrotic cartilage.
What is platelet-rich plasma?This is a preparation that has a higher amount of platelets than normal blood levels. In most cases, platelet-rich plasma is solely defined according to the absolute platelet count, and not according to other blood components. A normal platelet blood count ranges from 150,000 to 350,000platelets/μL, whereas platelet-rich plasma is often defined as a concentration that is two or four times higher than the normal concentration.13 Platelet-rich plasma can be obtained and elaborated from an individual's peripheral venous blood, after one or two centrifugation steps and using basic laboratory material or equipment. This type of extract represents a natural source of growth factors of autologous origin, which is one of its main properties.
The logic behind the use of platelet-rich plasma is that platelets are the first component that acts in the location where the tissue is damaged, so they have the ability to release, together with other active molecules, growth factors that play an essential role in the healing process.14
Elaboration of platelet-rich plasmaAmong the controversial subjects surrounding platelet-rich plasma, the lack of consensus regarding its precise composition and the different techniques available to elaborate it stand out. These can be classified as closed techniques, in which disposable commercial cases are used, where the sample is treated inside just one container (tube or syringe), and open techniques, where the elaboration of platelet-rich plasma is performed manually in more than one tube. In both cases, the elaboration involves one or more centrifugation steps.
There are at least 16 different commercial cases to elaborate platelet-rich plasma, which can significantly vary in platelet, leucocyte and erythrocyte quantities of the final product.15 Platelet concentration can increase up to 9.3 times in association to baseline levels.16 On the other hand, in a manual process with double centrifugation, platelet concentration can increase up to 330%, which would be equal to 3.3 times.17
All available techniques have several features in common. The patient's blood is collected in tubes with anticoagulant and immediately centrifuged. This initial centrifugation separates red blood cells from plasma. The separated plasma can contain several concentrations of platelets with or without white blood cells. Platelets can be activated afterwards using thrombin, calcium chloride, calcium gluconate, by freezing and unfreezing it, and then this is applied in the lesion area when it is still liquid as an injection. The procedure can be carried out in a minimally invasive and ambulatory way, providing an activated preparation directly in the damaged place with an immediate release of the growth factors.
Formulation of platelet-rich plasmaThere can be approximately 1500 proteins in platelets, among which there may be a few growth factors.18 Platelets are non-nucleated cells that contain different intracellular structures, such as glycogen, lysosomes and granules (dense and alpha), to mention just a few.19 In particular, alpha granules store and secrete several types of proteins, such as adhesion proteins, coagulation factors, fibrinolytic factors, proteases and antiproteases, growth factors, cytokines, chemokines, membrane glycoproteins and antimicrobial proteins, among others.20
It has already been observed that some of the bioactive proteins that platelets release are responsible for attracting macrophages, mesenchymal stem cells and osteoblasts, which not only facilitate the removal of necrotic tissue inside the lesion, but also participate in advanced biological functions that range from healing wounds to tumour growth.21
The fact that platelets secrete growth factors and active metabolites can have a positive influence in clinical scenarios that require quick healing and tissue regeneration. In addition, the existence of growth factors connected to platelets and/or within fibrin structures can result in an improved activity regarding soluble recombinant proteins.22
Growth factors in platelet-rich plasmaGrowth factors are soluble and diffusible macromolecules and are generated by a great variety of cellular types. They have specific actions on the growth, differentiation and phenotype of numerous types of cells, among which are chondrocytes.
These molecules interact with membrane cell receptors, which transmit signals to the inside of the cell and generate a cascade reaction that concludes in the regulation of gene expression. Their most common mechanism of action is paracrine or autocrine, and occasionally endocrine. Thus, the cell or cells that receive the signal may be near to or far from the cell that has synthesised and released this factor.23 An ideal growth factor in the regeneration of cartilage should be effective regardless of the patient's age or the existence of osteoarthrosis and will not damage the cartilage or the synovial membrane.
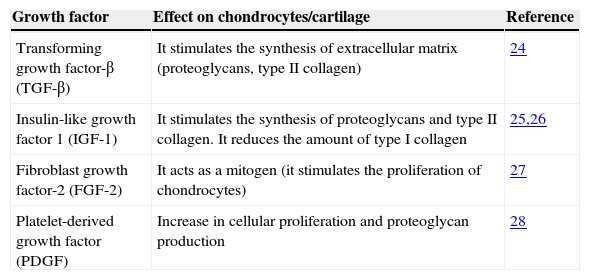
Table 124–27 shows some growth factors that are present in platelet-rich plasma, which have proven to be important in the restoration of the cartilage. In general, these molecules have anabolic features that stimulate the synthesis of proteoglycans, aggrecans and Type II collagen in chondrocytes; they induce the proliferation of synoviocytes and mesenchymal stem cells, and reduce the catabolic effects of cytokines, such as the interleukine-1 (IL-1), and of the extracellular matrix metalloproteases (MMP).28
Main effects on chondrocytes or cartilage of some growth factors found in platelet-rich plasma.
| Growth factor | Effect on chondrocytes/cartilage | Reference |
|---|---|---|
| Transforming growth factor-β (TGF-β) | It stimulates the synthesis of extracellular matrix (proteoglycans, type II collagen) | 24 |
| Insulin-like growth factor 1 (IGF-1) | It stimulates the synthesis of proteoglycans and type II collagen. It reduces the amount of type I collagen | 25,26 |
| Fibroblast growth factor-2 (FGF-2) | It acts as a mitogen (it stimulates the proliferation of chondrocytes) | 27 |
| Platelet-derived growth factor (PDGF) | Increase in cellular proliferation and proteoglycan production | 28 |
The ideal treatment is multidisciplinary and must fulfil the objectives of attaining analgesia, reducing disability and improving articular functionality, as well as improving the patient's quality of life with a lower level of toxicity of drugs.
Pharmacological treatment usually begins with analgesics, such as paracetamol (first-line drug to handle pain in osteoarthrosis), steroidal anti-inflammatory agents, and other non-steroidal anti-inflammatory drugs.29 Drugs like glucosamine, chondroitin sulphate, hyaluronic acid (viscosupplementation) and glucocorticoids have been proposed as non-invasive solutions to treat pain and improve the mobility of joints, all with variable success rates. These drugs can exhibit limitations for their correct administration, such as a potential cardiovascular and gastrointestinal toxicity, an apparently great variation in individual responses to each drug, and the absence of clear clinical data regarding the therapeutic potential of these substances.30 None of the available current treatments can be considered as an ideal procedure to treat chronic severe chondropathy or osteoarthrosis.
Platelet-rich plasma as tissue regenerative therapyAutologous platelet-rich plasma has been proposed as one of the biological therapies that can have potential clinical applications, for its simplicity in isolation and availability, and due to the absence of immunological reactions or disease transmission.
The use of platelet-rich plasma became popular within the field of plastic and maxillofacial surgeries in the 1990s.31 Dental implant surgery with guided bone regeneration is one of the scenarios in which platelet-rich plasma clearly accelerates ossification after the extraction of a dental piece and/or around the titanium implants used.32 As for the use of this therapy in orthopaedics, it started in the beginning of 2000s with the use of platelet-rich plasma in bone grafts, to increase spinal fusion and fracture consolidation. Although the discussion about the potential benefits of platelet-rich plasma in restoring bone33 continues, an increasing amount of evidence at trial level in several animal models supports its use to regenerate soft tissue, such as tendons.34 On the other hand, in tendon explant cultures, and of ligaments in the presence of platelet-rich plasma, a reduction in the expression of the gene that codifies for an extracellular matrix protease (MMP-13) could be observed, as well as an increase in the expression of the cartilage oligomeric matrix protein (COMP) gene, which is part of the extracellular matrix of the cartilage.35
Clinical trials on the use of platelet-rich plasma as a treatment for osteoarthrosisCurrently available clinical trials in medical literature support the use of platelet-rich plasma to treat lesions in the cartilage of the knees, administering it by means of intra-articular injections. In all them, they use clinical scales or rates (WOMAC, IKDC, KOOS, NRS, among others) to assess and measure the effect of the treatment.
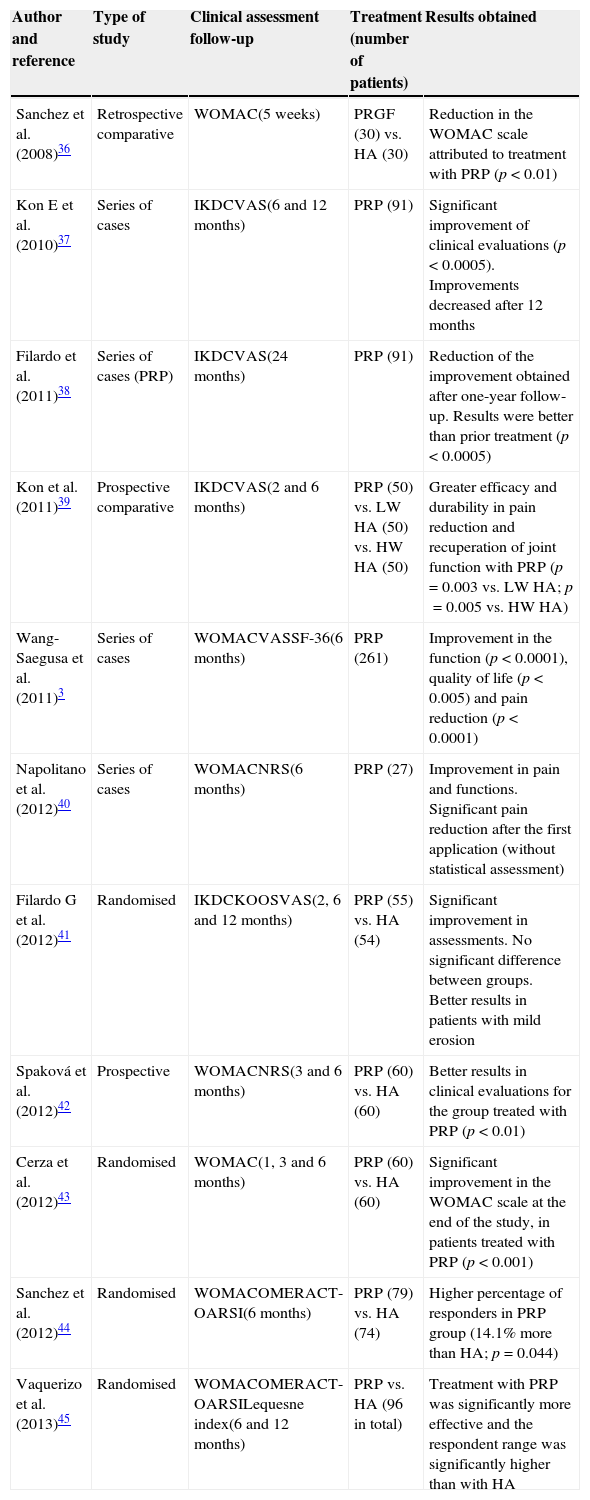
A summary of the main trials is shown in Table 2.3,36–45 These are very heterogeneous trials on the condition of the patients who were treated, the administration interval for injections, as well as the number of applications and the predominance of short to mid-term assessment periods. Despite this, it can be observed that, in most cases, platelet-rich plasma offers better results compared to other approaches implemented (mainly hyaluronic acid).
Clinical trials on the administration of platelet-rich plasma therapy in knee osteoarthrosis.
| Author and reference | Type of study | Clinical assessment follow-up | Treatment (number of patients) | Results obtained |
|---|---|---|---|---|
| Sanchez et al. (2008)36 | Retrospective comparative | WOMAC(5 weeks) | PRGF (30) vs. HA (30) | Reduction in the WOMAC scale attributed to treatment with PRP (p<0.01) |
| Kon E et al. (2010)37 | Series of cases | IKDCVAS(6 and 12 months) | PRP (91) | Significant improvement of clinical evaluations (p<0.0005). Improvements decreased after 12 months |
| Filardo et al. (2011)38 | Series of cases (PRP) | IKDCVAS(24 months) | PRP (91) | Reduction of the improvement obtained after one-year follow-up. Results were better than prior treatment (p<0.0005) |
| Kon et al. (2011)39 | Prospective comparative | IKDCVAS(2 and 6 months) | PRP (50) vs. LW HA (50) vs. HW HA (50) | Greater efficacy and durability in pain reduction and recuperation of joint function with PRP (p=0.003 vs. LW HA; p=0.005 vs. HW HA) |
| Wang-Saegusa et al. (2011)3 | Series of cases | WOMACVASSF-36(6 months) | PRP (261) | Improvement in the function (p<0.0001), quality of life (p<0.005) and pain reduction (p<0.0001) |
| Napolitano et al. (2012)40 | Series of cases | WOMACNRS(6 months) | PRP (27) | Improvement in pain and functions. Significant pain reduction after the first application (without statistical assessment) |
| Filardo G et al. (2012)41 | Randomised | IKDCKOOSVAS(2, 6 and 12 months) | PRP (55) vs. HA (54) | Significant improvement in assessments. No significant difference between groups. Better results in patients with mild erosion |
| Spaková et al. (2012)42 | Prospective | WOMACNRS(3 and 6 months) | PRP (60) vs. HA (60) | Better results in clinical evaluations for the group treated with PRP (p<0.01) |
| Cerza et al. (2012)43 | Randomised | WOMAC(1, 3 and 6 months) | PRP (60) vs. HA (60) | Significant improvement in the WOMAC scale at the end of the study, in patients treated with PRP (p<0.001) |
| Sanchez et al. (2012)44 | Randomised | WOMACOMERACT-OARSI(6 months) | PRP (79) vs. HA (74) | Higher percentage of responders in PRP group (14.1% more than HA; p=0.044) |
| Vaquerizo et al. (2013)45 | Randomised | WOMACOMERACT-OARSILequesne index(6 and 12 months) | PRP vs. HA (96 in total) | Treatment with PRP was significantly more effective and the respondent range was significantly higher than with HA |
VAS, visual analogue scale; HA, hyaluronic acid; HW, high weight; IKDC, International Knee Documentation Committee; KOOS, knee injury and osteoarthritis outcome score; LW, low weight; NRS, numeric rating scale; OMERACT-OARSI, Rheumatology Committee and Osteoarthritis Research Society International Standing Committee for Clinical Trials Response Criteria Initiative; PRP, platelet-rich plasma; WOMAC, Western Ontario and McMaster Universities Index of Osteoarthritis.
In six of these studies, results are based on a series of cases or uncontrolled comparative trials, which pose one of the main disadvantages when assessing platelet-rich plasma as a therapy. However, this therapeutic approach was safe in all of them and improved the patients’ symptoms.
The rest of the publications concern more recent trials, where treatment with platelet-rich plasma is compared with hyaluronic acid treatment in patients treated randomly. The first has better results than the second regarding pain reduction, improvement of joint function and quality of life. There were no significant differences among groups in just one of the trials. Clinical evidence of this type of trial is more relevant and the safety of the proposed therapy is confirmed. The number of patients treated per group ranged from 50 to 80.
ConclusionThe use of platelet-rich plasma directed to the restoration of tissues in several medical fields has increased in the last few years due to the numerous positive results derived from clinically and scientifically based studies concerning its direct administration. Nevertheless, its effectiveness as a therapy to treat osteoarthrosis is not completely proven. This is mostly because initially, clinical evidence came only from trials of series of cases, and they were not compared with any other treatment and/or control group; a small number of individuals were treated, or a heterogeneous population of patients classified according to the type of lesion and/or osteoarthrosis degree. Randomised trials that provide relevant information have started to be published recently, which will help to elucidate this matter. However, in general, clinical trials show an improvement in the patients treated, at least regarding their symptomatology. What is completely clear is that the use of platelet-rich plasma as an alternative treatment is safe, due to the lack of reports of severe adverse events in the patients treated. We must also point out that in studies where diverse joint alteration degrees are treated, younger patients with lower degree lesions exhibit the best results. Thus, we could point out that treatment with platelet-rich plasma could have better results if it is applied as a preventive treatment, before the erosion is irreversible, such as would happen in patients where the only treatment option is to replace the knee with a prosthesis. Another relevant observation is that when patients treated with platelet-rich plasma undergo a long-term follow-up, initial improvement progressively decreases, so the subsequent application could also be an option.
On the other hand, it is important to take into consideration the scientific evidence from in vitro studies or from animal model studies, which show that the use of platelet-rich plasma can reverse the damage caused in the injured cartilage. The benefits of the use of platelet-rich plasma in joints can be observed in them, such as the reversion of inflammatory processes or the formation of an extracellular matrix.
For now, treatment with platelet-rich plasma must be reserved for those patients who, based on recent clinical evidence, can show the best results, in case conservative conventional treatment has failed. Future studies will have to clarify factors such as what is the best formulation or which are the best administration procedures (number of applications, interval between applications).
Conflict of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Simental-Mendía MA, Vílchez-Cavazos JF, Martínez-Rodríguez HG. El plasma rico en plaquetas en osteoartrosis de rodilla: una alternativa de tratamiento. Artículo de revisión. Cirugía y Cirujanos. 2015;83:352–358.