Laparoscopic sleeve gastrectomy is a surgical procedure for the treatment of morbid obesity. However, there are still controversies regarding its efficiency in terms of weight reduction and incidence of complications. In this prospective study, the experience is presented of a referral centre for the treatment of morbid obesity with laparoscopic sleeve gastrectomy.
Material and methodsA prospective study on 73 patients subjected to laparoscopic sleeve gastrectomy from February 2009 to September 2013. Patients were followed-up for a period of 12 months, evaluating the development of complications, reduction of gastric volume, and the weight loss associated with the surgery, as well as their impact on the improvement of comorbidities present at beginning of the study.
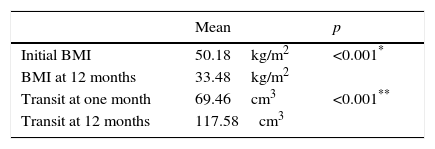
ResultsThere was a statistically a significantly reduction between the preoperative body mass index (BMI) and the BMI at 12 months after laparoscopic sleeve gastrectomy (p<0.001), despite there being an increase in the gastric volume during follow-up, measured at one month and 12 months after surgery (p<0.001). Five patients (6.85%) had complications, with none of them serious and with no deaths in the whole series.
ConclusionsLaparoscopic sleeve gastrectomy is a safe and effective technique for the treatment of morbid obesity. Its use is associated with a significant reduction in the presence of comorbidities associated with obesity. Multicentre studies with a longer period of monitoring are required to confirm the efficacy and safety of this surgical technique.
La gastrectomía vertical laparoscópica es una intervención quirúrgica para el tratamiento de la obesidad mórbida. Existen controversias respecto a su eficiencia en términos de reducción de peso e incidencia de complicaciones. En este estudio prospectivo, presentamos la experiencia en un centro de referencia en el tratamiento de la obesidad mórbida con gastrectomía vertical laparoscópica.
Material y métodosEstudio prospectivo con 73 pacientes tratados mediante gastrectomía vertical laparoscópica, desde febrero de 2009 hasta septiembre de 2013. Los pacientes fueron seguidos durante un periodo de 12 meses. Se evaluaron: el desarrollo de complicaciones, la reducción del volumen gástrico, y la pérdida ponderal asociada a la intervención; así como su impacto en la mejora de las comorbilidades presentes al inicio del estudio.
ResultadosSe observó una significativa reducción entre el índice de masa corporal (IMC) preoperatorio y el IMC a los 12 meses tras la gastrectomía vertical laparoscópica (±DE p<0.001); ello pese a que los pacientes experimentaron un incremento en el volumen de la cavidad gástrica, medido al mes y a los 12 meses tras la intervención (±DE p<0.001). Cinco pacientes (6.85%) presentaron complicaciones, ninguna de ellas graves. No hubo muertes en la totalidad de la serie.
ConclusionesLa gastrectomía vertical laparoscópica es una técnica segura y eficaz para el tratamiento de la obesidad mórbida, su uso se asocia a una importante reducción en la presencia de comorbilidades asociadas a la obesidad. Son necesarios estudios multicéntricos, con un mayor periodo de seguimiento, que confirmen la eficacia y seguridad de esta técnica quirúrgica.
Laparoscopic sleeve gastrectomy is a surgical procedure that is being increasingly used worldwide to treat morbid obesity. It is a restrictive technique which is effective, it seems, not only in reducing gastric volume thus inducing accelerated satiation but neurohumoral mechanisms might also be involved, since a reduction in levels of ghrelin, the appetite stimulating hormone, is also observed.1,2
This technique is the restrictive part which complements other procedures, known as mixed techniques; we refer to the duodenal switch, where a biliopancreatic diversion is also performed. Hess and Hess performed the first duodenal switch in 1988.3 Almogy et al.4 had been performing tubular gastrectomy via the open approach since 1993, in order to reduce the risk of superobese (BMI above 55), male patients aged over 55. In 1999, Gagner and Patterson5 completed the first laparoscopic sleeve gastrectomy in New York's Mount Sinai Hospital, as part of a duodenal switch. A few years later, Regan et al.6 proposed laparascopic sleeve gastrectomy as the first step of gastric bypass, as a treatment alternative for high-risk obese patients, with a view to reducing morbidity and mortality.
Since then many surgical teams have adopted this technique, with good outcomes, although the indications for it remain uncertain. Some institutions maintain that laparoscopic sleeve gastrectomy should be the first step in high-risk patients and patients with a high body mass index (BMI); the idea being to perform a second definitive operation after acceptable weight loss has been achieved in order to reduce surgical risk. By contrast, other teams consider it an alternative to the adjustable gastric band, to gastric bypass or to biopancreatic diversion with duodenal switch.7,8
The fact that this technique has erroneously been considered simple and easily reproducible has encouraged a great many surgeons to perform it. It could seem that, compared to gastric bypass and biopancreatic derivation, it is more a manageable operation from a laparoscopic perspective. However, we should remain aware that its complications, such as the onset of gastric fistulae, can be very serious and most authors agree that a long learning curve is necessary.9–12 Although a low percentage appear, these complications increase morbidity, hospital stay and place the life of the patient at risk.
In light of the above, we prospectively evaluated a series of patients who underwent laparascopic sleeve gastrectomy in our centre for the treatment of morbid obesity.
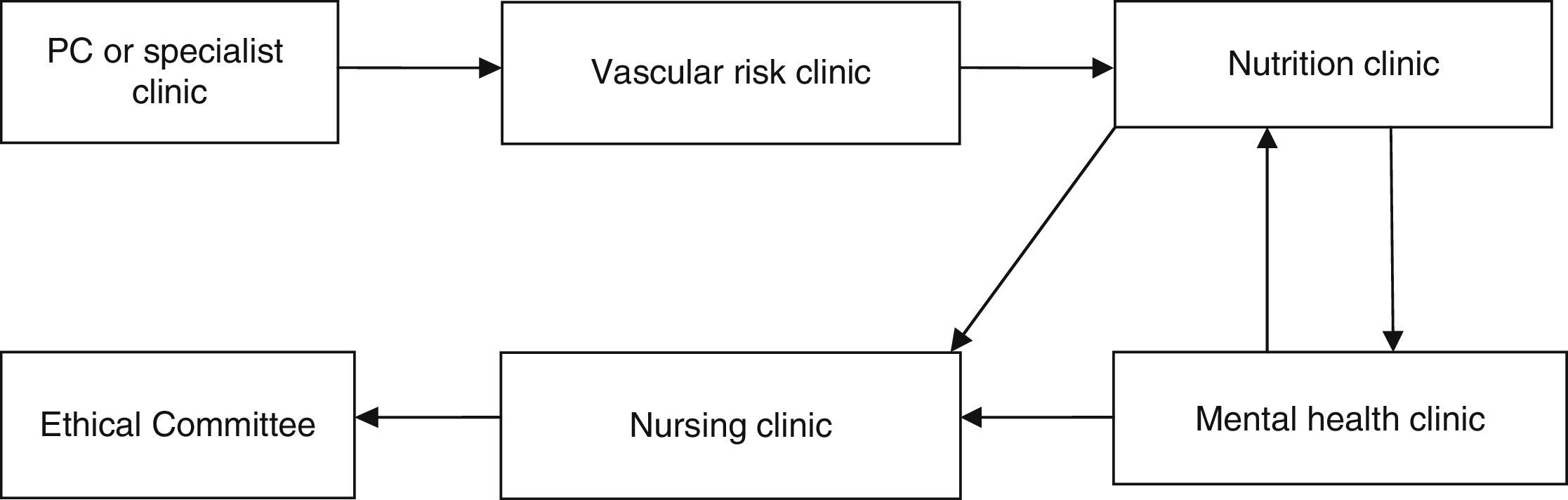
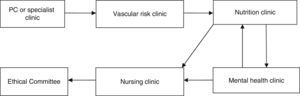
Material and methodsThis was an observational prospective study which included patients operated consecutively between 2 February 2009 and 26 September 2013. All the patients were operated by the bariatric surgery team of the Complejo Hospitalario Torrecárdenas of Almeria (Spain), which comprises 3 surgeons and a multidisciplinary team of endocrinologists, nutritionists and psychologists. The patients who were candidates for surgery had a BMI ≥40kg/m2, or BMI ≥35kg/m2 and also at least one of the following comorbidities: diabetes mellitus, high blood pressure, dyslipidaemia, severe osteoarthritis, obstructive sleep apnoea syndrome and hypoventilation-obesity syndrome. In addition to their BMI, the patients who were candidates for the operation had to meet the following criteria: (a) be aged between 18 and 60 years; (b) have been obese for more than 5 years; (c) other treatments had failed for them; (d) have an acceptable surgical risk; (e) be certain to cooperate during follow-up, and (f) have signed their informed consent. The patients who fulfilled these criteria were assessed by a multidisciplinary team as shown in Fig. 1. Once the operation had been indicated, the patients were evaluated by the bariatric committee and assigned a scale to be put on the waiting list for surgery. The patients were admitted the day before surgery to undergo a complete assessment of their physical and mental parameters and preoperative laboratory tests.
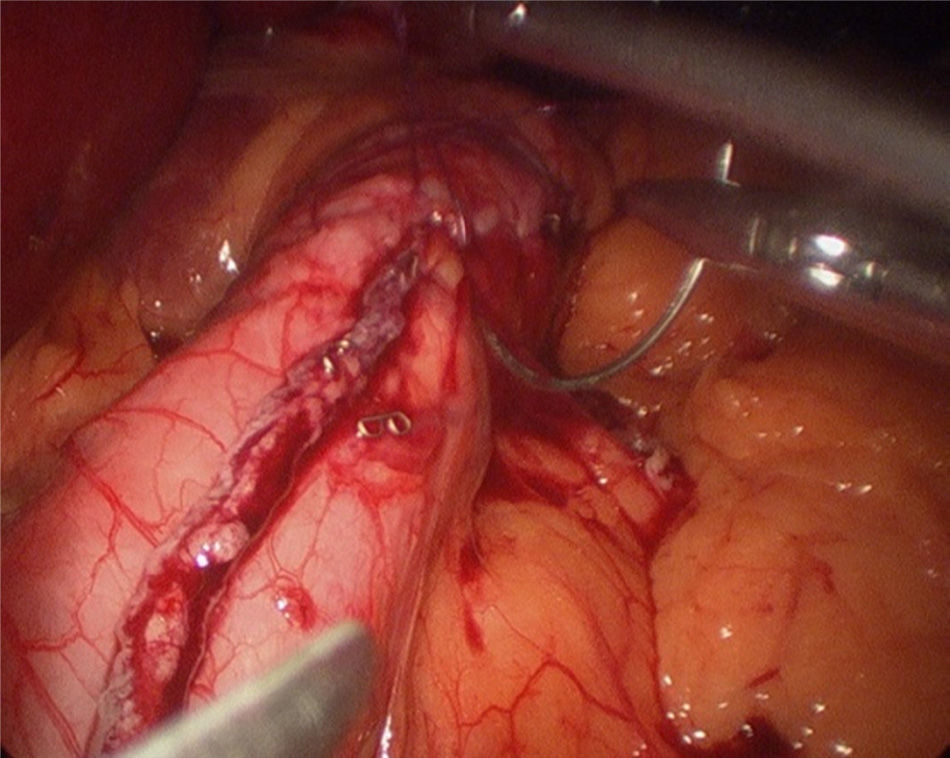
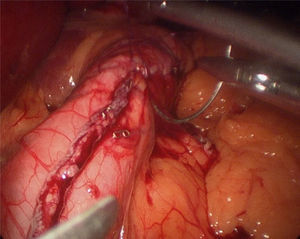
The operation started with the supraumbilical insertion of an optical trocar and placement of 4 accessory trocars (12mm). The greater gastric curvature was dissected using Ligasure, starting at about 4cm from the pylorus, up to the angle of Hiss. Adherences on the posterior gastric face were released. A 34-F Faucher's tube was inserted before performing the gastric section. The section was performed using an endocutter and then reinforced with invaginating suture with 2-0 absorbable monofilament suture (Fig. 2). Methylene blue solution was used to test for leaks, a surgical specimen was taken and a suction drain left. Nasogastric tubes or bladder catheters were not used in the operation. The patients were mobilised the evening after surgery and the next day. A methylene blue test for leaks was performed 48h after surgery and if negative, fluids were given. The patients were discharged 72h after surgery if there had been no incidents.
Follow-upAll the patients were kept on a semi-liquid diet for 4 weeks after the operation and then assessed as outpatients at 1, 3, 6, and 12 months after surgery. All the patients were also closely monitored by the Nutrition Department.
The comorbidities mentioned in the preoperative inclusion criteria were assessed at 12 months after surgery and were defined as present or in remission as follows: remission of diabetes mellitus was defined as antidiabetic drugs discontinued by the endocrinologist, HBA1c ≤6.5% and glycaemia below 126mg/dl; remission of high blood pressure as blood pressure ≤140/90mmHg with withdrawal of antihypertensive drugs; remission of hyperlipidaemia was considered when there was an LDL cholesterol level of below 160mg/dl, and triglycerides <200mg/dl; remission of severe osteoarthritis was considered as a response to the withdrawal of pain relief medication and the absence of associated symptoms; remission of obstructive sleep apnoea syndrome as discontinued use of the continuous positive airway pressure mask, and remission of hypoventilation-obesity syndrome as the absence of daytime hypersomnia and cardiorespiratory symptoms. Remission of the latter 2 disorders was confirmed by polysomnography.
A barium study of upper gastrointestinal transit was performed on all the patients at one month and at 12 months after the operation, except those presenting fistulae, who underwent gastrografin study to assess the measurements and characteristics of the gastric sleeve. In this case, the general volume of the gastric sleeve (V) was equivalent to V=πr2h (in cm3), where h=height and r=the radius in cm.
Statistical analysisThe continuous variables were expressed as means±standard deviations or range. Comparisons between 2 continuous variables were made using the Student's t-test or Wilcoxon's test for normalised or unnormalised variables, respectively. Similarly, comparison between more than 2 continuous variables was made using the Anova or Kruskal–Wallis test, according to whether the distribution was normal or not. Comparison between preoperative and postoperative ordinal variables was made using McNemar's test or the χ2 test and Fisher's exact test where necessary. The statistical package SPSS version 22.0 for Windows was used for the analysis.
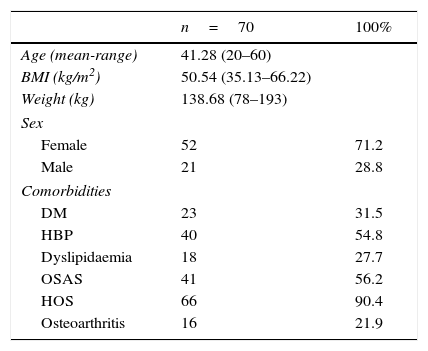
ResultsA total of 73 patients were operated between February 2009 and August 2013. These patients’ preoperative characteristics are shown in Table 1.
Patient characteristics (preoperative data).
| n=70 | 100% | |
|---|---|---|
| Age (mean-range) | 41.28 (20–60) | |
| BMI (kg/m2) | 50.54 (35.13–66.22) | |
| Weight (kg) | 138.68 (78–193) | |
| Sex | ||
| Female | 52 | 71.2 |
| Male | 21 | 28.8 |
| Comorbidities | ||
| DM | 23 | 31.5 |
| HBP | 40 | 54.8 |
| Dyslipidaemia | 18 | 27.7 |
| OSAS | 41 | 56.2 |
| HOS | 66 | 90.4 |
| Osteoarthritis | 16 | 21.9 |
DM: diabetes mellitus; HBP: high blood pressure; OSAS: obstructive sleep apnoea syndrome; HOS: hypoventilation-obesity syndrome.
The majority were female with a mean age of 41.28 (20–60) years (mean [range]), a mean weight of 138.68 (78–193)kg and a mean BMI of 50.54 (35.13–66.22)kg/m2. In terms of comorbidities, the majority presented sleep disorders (90.4% hypoventilation-obesity syndrome and 56.2% obstructive sleep apnoea), 54.8% were hypertensive, 31.5% had type 2 diabetes, 24.7% had dyslipidaemia and 21.9% osteoarthritis. None of the patients had previously undergone bariatric surgery.
All the patients had a significant reduction in mean BMI after laparoscopic sleeve gastrectomy at 12 months, compared with their preoperative measurements (33.5±6.2kg/m2 and 50.2±6.9kg/m2 respectively; p<0.001) (Table 2).
Furthermore, the reduction in BMI following surgery was not affected by the learning curve, since when the series was divided into 3 groups according to the time of the operation, the first 25 patients operated in the first year since starting the technique achieved a reduction in their BMI which was similar to the following 25 operated in the second year and also similar to the last 23 patients of the series operated in the third and fourth years (15.9±5.5; 16.8±6.5 and 17.5±5.8, respectively; p=0.7). The percentage of weight loss was 61.19%, and the percentage of excess BMI lost was 67.92%; and neither were these affected by the learning curve. The effect on weight loss associated with laparoscopic sleeve gastrectomy was maintained during follow-up, despite that fact that the patients experienced a significant increase in gastric cavity volume, established by gastroduodenal study, which showed an increase from 69.46 (18.8–196.3)cm3 at one month after surgery, to 117.58 (25.13–274.88)cm3 at 12 months (p<0.001) (Table 2).
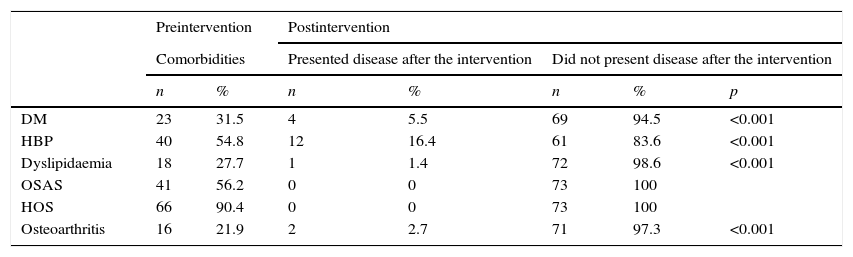
Remission of diabetes mellitus occurred in 83% of the pre-existing cases, hypertension in 70%, osteoarthritis and dyslipidaemia in 86 and 94%, respectively, while obstructive sleep apnoea and hypoventilation-obesity syndrome remitted in all of the patients. These differences between the preoperative data and at 12 months were significant (Table 3).
Comparison of obesity-associated diseases 12 months after the intervention.
| Preintervention | Postintervention | ||||||
|---|---|---|---|---|---|---|---|
| Comorbidities | Presented disease after the intervention | Did not present disease after the intervention | |||||
| n | % | n | % | n | % | p | |
| DM | 23 | 31.5 | 4 | 5.5 | 69 | 94.5 | <0.001 |
| HBP | 40 | 54.8 | 12 | 16.4 | 61 | 83.6 | <0.001 |
| Dyslipidaemia | 18 | 27.7 | 1 | 1.4 | 72 | 98.6 | <0.001 |
| OSAS | 41 | 56.2 | 0 | 0 | 73 | 100 | |
| HOS | 66 | 90.4 | 0 | 0 | 73 | 100 | |
| Osteoarthritis | 16 | 21.9 | 2 | 2.7 | 71 | 97.3 | <0.001 |
DM: diabetes mellitus; HBP: high blood pressure; OSAS: obstructive sleep apnoea syndrome; HOS: hypoventilation-obesity syndrome.
p value obtained by McNemar's test and Fisher's exact test.
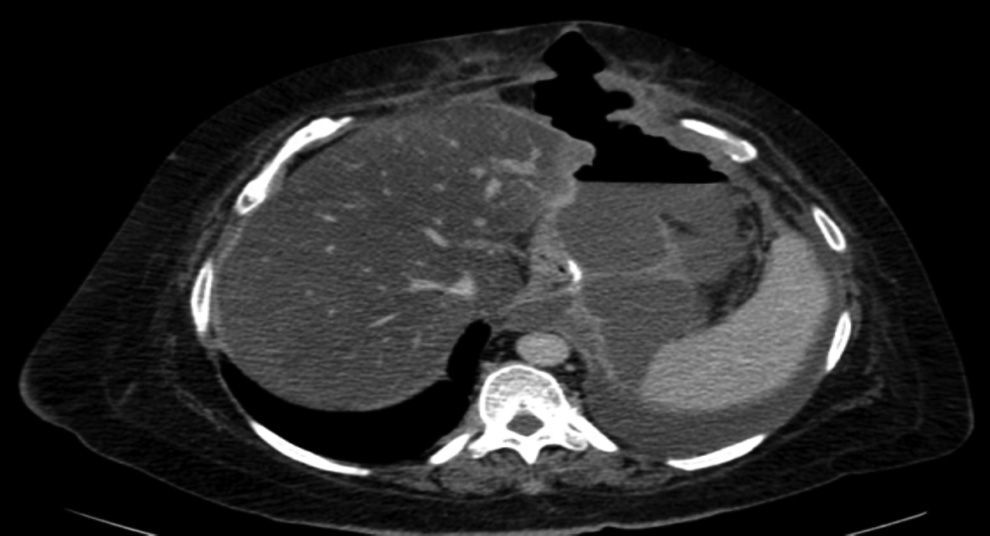
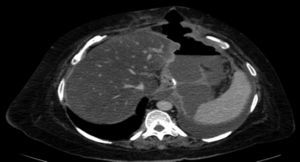
The complications in the postoperative period were 3 fistulae (4.1%), one total stenosis (1.4%) and one case of postoperative bleeding (1.4%) with no signs of haemodynamic repercussions. The 3 fistulae were diagnosed during outpatient follow-up by the clinical symptoms and imaging tests (computed tomography) (Fig. 3). All were resolved satisfactorily, one with conservative treatment and the placement of an endoprosthesis and the other 2 with surgical treatment after failed attempts to place an endoprosthesis; one underwent a simple laparoscopic closure and the other was reconverted to gastric bypass. The stenosis required conversion to gastric bypass. The bleeding was resolved by surgical review and haemostasis of the short blood vessels. All the complications presented during the first 25 interventions of the learning curve. The 73 patients completed the 12-months’ follow-up and none of them died throughout this entire period.
DiscussionLaparoscopic surgery has represented such surgical progress over recent years that it is difficult to imagine this operation performed without the use of minimally invasive techniques. The first laparoscopic sleeve gastrectomy was performed by Gagner and Patterson5 as part of a duodenal switch. However, in recent years this operation has become established as single treatment for morbid obesity, due to its good outcomes and the low incidence of complications in comparison with other surgical techiques.7,8
To complete their training bariatric surgeons require advanced training in laparoscopic surgery.9 Despite the fact that laparoscopic sleeve gastrectomy is considered by some to be a simple technique, we should remember that its complications can endanger the life of the patient, and are generally more difficult to resolve than those that appear after the mixed techniques. Some authors consider that there is a long learning curve (more than 100 cases) during which the intra- and postoperative morbidity is higher.10–12 Due to the inherent conditions of the patient and the difficulty of the surgical technique, there are 2 fields of learning required for laparoscopic surgery: first, it is necessary to have experience in the treatment of obese patients, and second, it is essential to have experience in advanced reconstructive laparoscopic surgery techniques.13
One of the complications that bariatric surgeons most fear after laparoscopic sleeve gastrectomy is the appearance of a fistula in the proximal third, both due to the morbidity it involves and the difficulty in resolving it. The incidence of fistula after this surgery varies between 0 and 7%.14–16 Baker et al.17 argue that there are many causes of fistulae on the staple line, but these can be placed into 2 categories: mechanical/tissue and ischaemic causes. In both, the intraluminal pressure exceeds the resistance of the tissue and the suture line, giving rise to the fistula. Traditional ischaemic fistulae usually appear between the 5th and 6th postoperative day, when the healing process of the wall is between the inflammation stage and fibrosis. When the cause is mechanical/tissue, it is usually discovered earlier, in the first 2 days after surgery. This supports the use of reinforcement materials which, although they do not act on the ischaemic cause of fistulae, in theory might reduce the risk of mechanical failure.18 Baltasar et al.,19 like our group, protect the section line with a continuous sero-serous suture that inverts the staples, controls bleeding and attempts to reduce the number of leaks, without increasing the operation costs (Fig. 2). They have recently described their technique using an omental patch together with invagination, the idea being to reduce the number of leaks and improve stability and emptying by reducing the partial torsion that some residual tubes suffer.20
In all cases, the 3 fistulae presented by the patients in our study occurred after discharge from hospital (after 72h) and therefore mechanical failure can be ruled out as the cause. The 3 cases presented symptoms of fever and dyspnoea, and no case presented abdominal pain. Therefore, we should consider, that patients who attend after discharge from hospital with fever or dyspnoea in the postoperative period after laparoscopic sleeve gastrectomy, might have a fistula and emergency computed tomography should be performed. With the advent of the new endocutters with tri-staple technology, the amount of fistulae appears to have decreased. In fact, they have not presented at all since we used them in our group (48 patients).
Throughout our series, the total rate of complications was 6.85%, lower than the 17% reported in other series.21 However, it was similar to that of a long Spanish multi-centre cohort which included 540 patients and that which was accepted in a consensus document of the American Society for Metabolic and Bariatric Surgery.7,22 These complications appeared during the first 25 learning curve interventions, all in females, and only one of the fistulae and postoperative bleeding presented in superobese patients.
Deitel and Greeinstein23 propose the use of the excess BMI loss percentage to evaluate outcomes, so that an excellent outcome would be if it were in excess of 65%, a good outcome if it were between 50% and 65%, and failure if less than 50%. In our series, the mean excess BMI loss percentage was 67.92%, in line with the data in the literature.7,24,25 Furthermore, it is important to highlight that the reduction in BMI, in the excess BMI loss percentage and in the weight loss percentage were not dependent on the time in the learning curve of our series.
There are still many points of controversy in laparoscopic sleeve gastrectomy which create a range of possibilities on which there is no consensus,26 such as (a) the size of the bougie used as a calibrator, (b) the distance from the pylorus to the first section line, (c) reinforcement of the staple line, and (d) the routine use of intraoperative leak testing, all of these are issues that are the subject of constant debate amongst the authors of other series and should be assessed in randomised prospective studies.
A large percentage of the patients of this study presented comorbidities. Diabetes mellitus, high blood pressure, dyslipidaemia, obstructive sleep apnoea syndrome, hypoventilation-obesity syndrome and osteoarthritis remitted in at least 70%, and the case of obstructive sleep apnoea syndrome and hypoventilation-obesity syndrome disappeared entirely. This demonstrates that laparoscopic sleeve gastrectomy is a technique which not only influences weight loss but also improves complications.27–29 The mechanisms responsible for this association are outside the scope of this study.
With regard to the mean transit volumes taken after the first month and at 12 months after the operation, 69.46 and 117.58cm3 respectively, statistically significant differences were observed both at a general patient level and by sex. Vidal et al.30 give mean volumes both at one month and at 12 months that are similar to those we present in our study. It is true that despite increasing the volume of the gastric sleeve, the patients continued to maintain their weight loss at their check one year later. Long-term follow-up would be helpful in order to evaluate both the patients’ weight loss and the proportional increase in gastric volume, and to confirm whether this equation continues to be maintained or whether, by contrast, these figures come to a halt or, even might facilitate further weight gain.
To conclude, laparoscopic sleeve gastrectomy is an effective and safe technique for the treatment of morbid obesity in reference centres with experience in bariatric surgery. It is also associated with a significant remission of the comorbidities associated with morbid obesity, although the pathophysiological mechanism is not completely understood.
The fact that it has been considered erroneously to be a simple procedure has encouraged a large number of surgeons to perform laparoscopic sleeve gastrectomy. However, it is not free from complications that present in a low percentage and are dependent on the learning curve.
If these findings were to be confirmed in longer patient series, laparoscopic sleeve gastrectomy would be the treatment of choice for morbid obesity and should preferably be undertaken in reference centres.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: García-Díaz JJ, Ferrer-Márquez M, Moreno-Serrano A, Barreto-Rios R, Alarcón-Rodríguez R, Ferrer-Ayza M. Resultados, controversias, y volumen gástrico después de la gastrectomía vertical laparoscópica en el tratamiento de la obesidad. Cir Cir. 2016;84:369–375.