Bronchogenic cyst is a malformation of the ventral portion of the intestine, which is limited by bronchial epithelium and produces alterations in the development of the tracheobronchial tree. They may be single or multiple, and are usually confined to one lung or to the mediastinum, rarely to the neck, which is a subcutaneous tissue.
ObjectiveThe case of a 9 year old girl is reported, who presented with a clinical picture characterised by a slow-growing, asymptomatic tumour on the left side of the neck of 4 years onset. Chest X-ray, neck ultrasound and computed tomography of the neck and chest ruled out any other injury. A complete resection was performed, and the histopathological study confirmed the diagnosis of bronchogenic cyst.
ConclusionThe symptomatology of a bronchogenic cyst is due to the compression of the vascular, digestive or air structures, as well as its size, infection and location. The treatment of choice is a surgical resection, even when asymptomatic.
El quiste broncogénico es una malformación de la porción ventral del intestino primitivo, limitada por epitelio bronquial, que genera alteraciones en el desarrollo del árbol traqueo-bronquial. Pueden ser únicos o múltiples y generalmente están confinados a un pulmón o mediastino, rara vez a cuello y tejido celular subcutáneo.
ObjetivoSe reporta el caso de una paciente de 9 años de edad, con cuadro de 4 años de evolución caracterizado por tumour de crecimiento lento en el hemicuello izquierdo, asintomática. La radiografía de tórax, el ultrasonido de cuello y la tomografía de cuello y tórax permitieron descartar alguna otra lesión; se le realizó resección completa y el estudio histopatológico confirmo el diagnóstico de quiste broncogénico.
ConclusiónLa sintomatología de un quiste broncogénico se debe a la compresión de estructuras vasculares, digestivas o aéreas, así como de la infección del quiste, tamaño y lugar del mismo; el tratamiento de elección es la resección quirúrgica aun siendo asintomático.
Bronchogenic cyst is a malformation of the ventral portion of the primitive foregut, causing a bronchopulmonary alteration. This pathology presents between the fifth and sixteenth week of pregnancy, which is when the intestine divides into the dorsal portion, which develops into the oesophagus, and the ventral portion which develops into the pulmonary yolk sac, and the tracheo-bronchial tree. The cyst is therefore an ectopic pulmonary yolk sac which may or may not be associated with the tracheo-bronchial tree but which does not incorporate mesenchymal tissue.1,2 Regarding location, the cysts are divided into mediastinal and parenchymal. They may present singularly or be multiple, be asymptomatic or symptomatic depending on their proximity to the airway. When they are symptomatic they may appear with frequent airway infections, stridor, wheezing, dysphonia and difficulty breathing. When the lesion is not confined to its origin, it may occur in other locations and present in the neck, pericardium, paravertebral and subpleural regions.3 The cysts are generally asymptomatic and very few are diagnosed during the neonatal period3; the bronchogenic cyst has a ciliate column-shaped epithelium like the bronchials and may contain cartilage, muscles and mucous glands.3,4
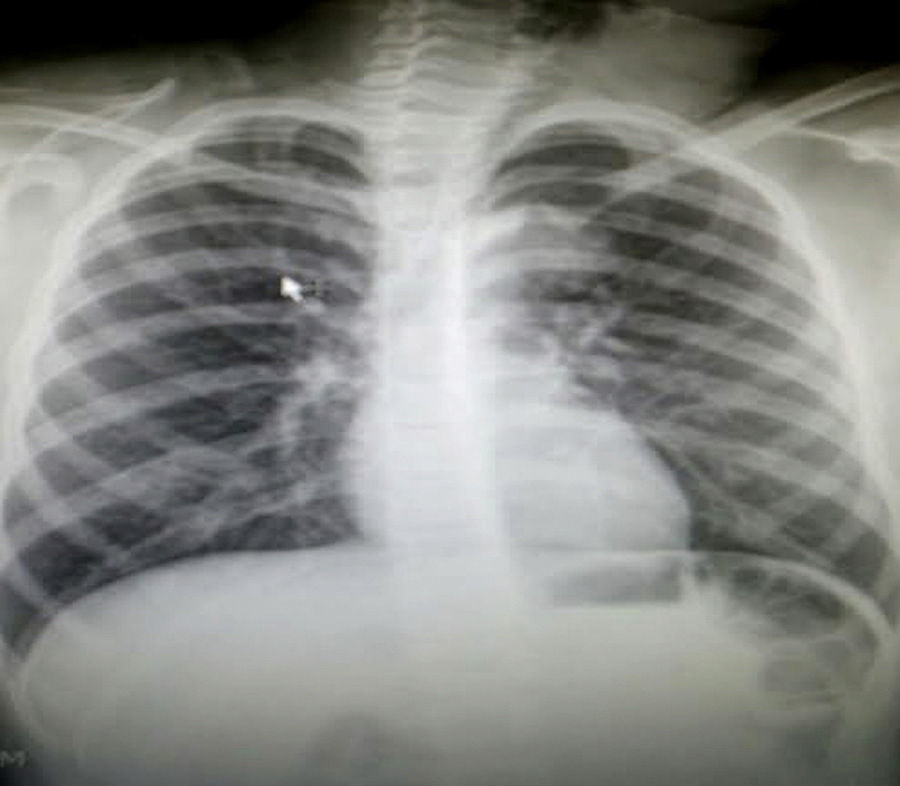
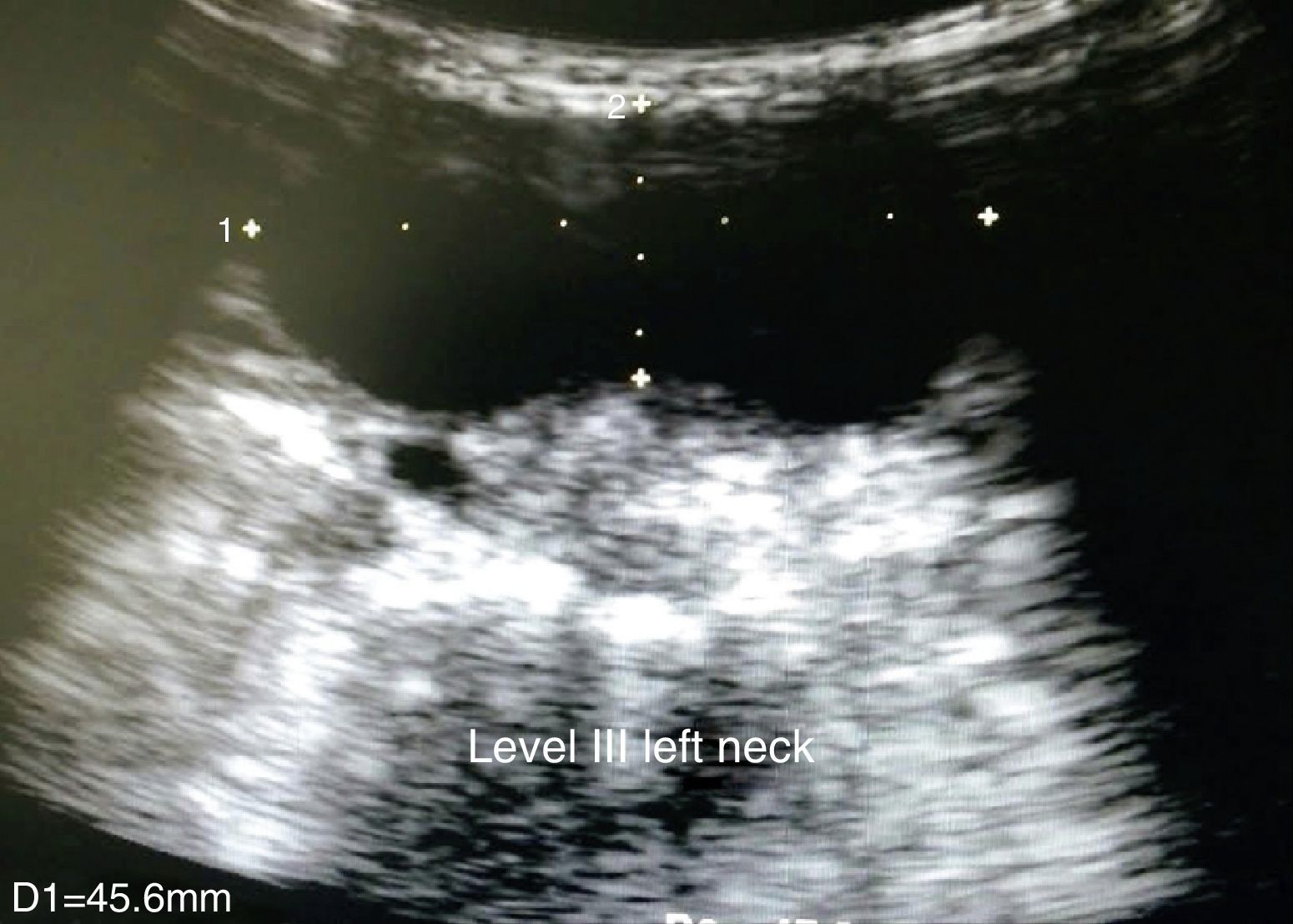
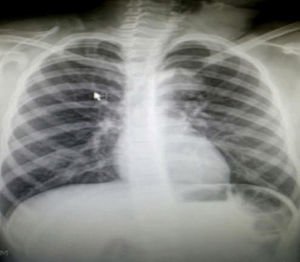
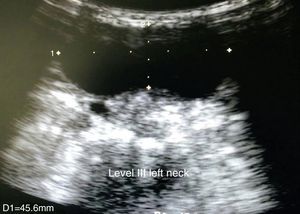
Clinical caseThe case of a 9 year old girl is reported. She was the result of a normal pregnancy, was born by caesarean section at 40 weeks, with an appropriate birth weight and height, APGAR rate of 8/9, with no alteration in her development. The patient does not suffer from any allergies. The patient developed the condition at 5 years of age, with increased volume on the left side of her neck. One year prior to going into hospital aspiration of the lesion was performed and the family member stated that it had been managed as a cystic hygroma, but did not have the pathologist's report. After aspiration of the cyst, the patient began to suffer from a runny nose, epiphora and itching on her palate. Frequent infections were denied, as were dyspnoea, dysphonia or disphagia. When an assessment was made, the patient was found to have appropriate colouring, adenoid facies, no dyspnoea nor stridor, appropriate hydration, with epiphora, hyaline rhinorrea and pharynx without discharge. The neck had lateralisation towards the right due to the increased 8cm diameter volume in the anterior triangle of the neck, area I–II, with a smooth surface, soft, well marked, with no changes in colouring, no attachments to deep layers, with 1cm adenomegalias in the bilateral jugular lymph node II, III area. All remaining examination showed normal results. Neck tomography showed a homogeneous mass, with thin walls, of 7cm×6cm, in front of the left sternocleidomastoid muscle; normal thyroid muscles, bilateral jugular cervical lymph nodes which were small and inflammatory and with a tomography report of cystic hygroma. The chest X-rays showed a central mediastinum, with no mediastinal lymph node growth nor masses (Fig. 1). Cervical ultrasound showed normal right thyroid muscles, left thyroid muscles which could not be assessed due to the mass. This was reported as being in the anterior triangle of the neck, of 59mm×48mm×28mm with a thin wall, heterogeneous, avascular, with normal submandibular gland and left parotid wall. The carotid and jugular veins were unaltered; bilateral lymph nodes IIA, IIB, III, IV, VA and VB tested positive, and were inflamed. The ultrasound reported a branchial cyst and cervical adenomegaly (Fig. 2).
An assessment was requested from the allergy department, which reported an allergic response to dust and treatment with 10mg montelukast per day was initiated, in addition to one 10mg loratadin tablet every 24h, mometasona nasal spray with 2 inhalers every 24h. Preoperative laboratory testing reported 7730 leucocytes, haemoglobin of 15.5g/dl, 400,000 platelets with no bands, 7% eosinophils, normal coagulation times, 50% eosinopils in nasal mucosa, Ig E 232 (normal at 60), 10 antistreptolysins, 0 C-reactive protein, rheumatoid factor 6, blood sedimentation rate of 13 (normal), glucose 93mg/dl, creatinine 0.7mg/dl, albumin 3.9g/dl, negative coproparasitoscopic study in a series of 3, normal urine test. Fine-needle aspiration biopsy was performed which obtained 10cm3 of transparent, mucoid, non foetid liquid. This was dispatched for testing and reported only non neoplasic cells.
The scheduled surgery took place, under general anaesthesia, with the patient in the Rosier position. A transverse incision was made; the upper and lower flaps were freed, the platysma muscle was impacted, the prethyroid muscles were freed to view the thyroid glands, which were of standard appearance and size; at left sternocleidomastoid muscle level we found a 8cm×6cm thin-walled cystic lesion, with clear liquid inside it; we also found a dilation of the internal jugular vein and pseudoaneurysm of the external jugular vein, in addition to longitudinal yellow platelets in the external jugular pseudoaneurysm region, lymph nodes under 1cm which were soft; the cyst was removed from the left jugular II area, resection ligation of the external jugular pseudo-aneurysm was performed and the biopsy was taken of the cervical lymph nodes. Soft palate drainage was used.
The patient was given food 2h after the surgical procedure had ended. Supplemental intermittent humidified oxygen was administered, micro nebulisation with steroids, antibiotics and analgesics, in addition to anti-allergic management.
The patient's clinical progress was favourable and she did not present with dyspnoea, dysphonia, stridor or dysphagia. As a result she was discharged the day after the procedure.
Histopathological study reported the 6cm×4cm×2cm cystic lesion, with a light coffee colour on the external surface and a dark coffee colour inside it. The wall was thin and smooth with white gelatinous material on the inside. There was dilation of the external jugular vein with no associated lesions. It was reported as a bronchogenic cyst.
The patient was assessed monthly and was found to be asymptomatic after 5 months, with correct cervical mobility, with no dysphagia or dysphonia and with an appropriate response to the anti-allergic management.
DiscussionIn accordance with Kumar et al., the bronchogenic cyst was first described by Blackader in 1911. However, it was not until 1948 when Maier performed the first surgical resection.5 The bronchogenic cyst is a remnant of the primitive foregut, secondary to trachea-bronchial malformations. In 2001,6 Bush described it as a congenital pulmonary malformation and that it represented from 14% to 22% of congenital pulmonary malformations and 10% of mediastinal malformations.
Bronchogenic cysts are generally communicated with the trachea-bronchial tree through an accessory bronchus or through the oesophagus. They may be single or multiple, asymptomatic or symptomatic and when they are found in the neck they may be associated with a vertebral malformation.3,6 Approximately from 65% to 86% are mediastinal (median and posterior mediastinum), and of these they may be adjacent to the distal third of the trachea or close to the main bronchus. From here they may subdivide into pericardiac (52%), paratracheal (19%), paraoesophagic (14%) and retrocardiac (9%), and the majority are on the right.3
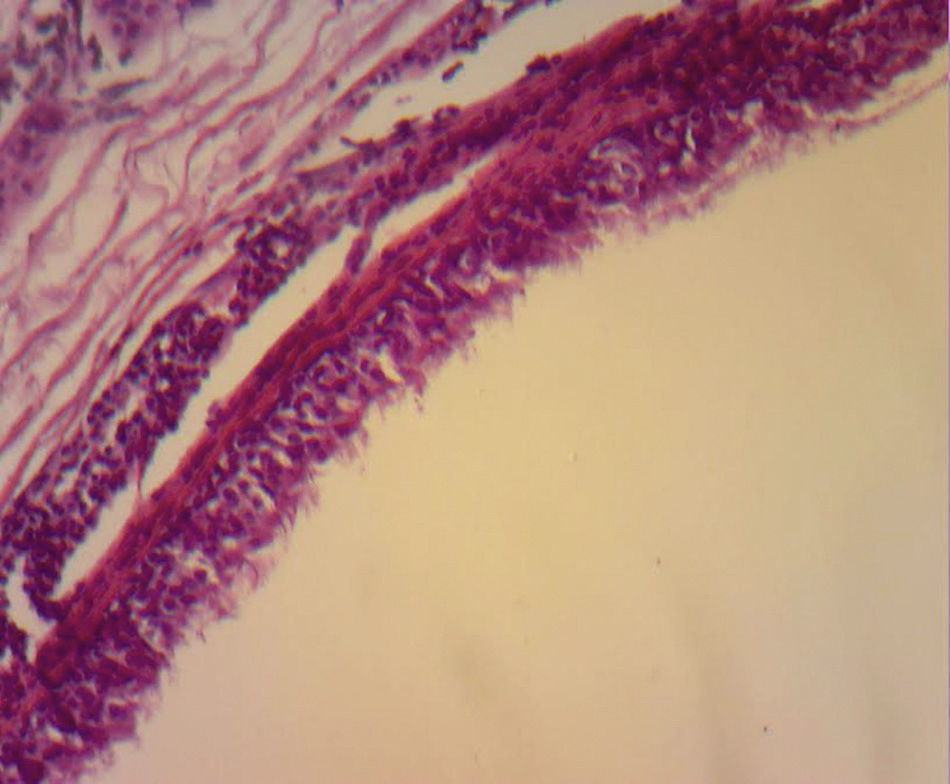
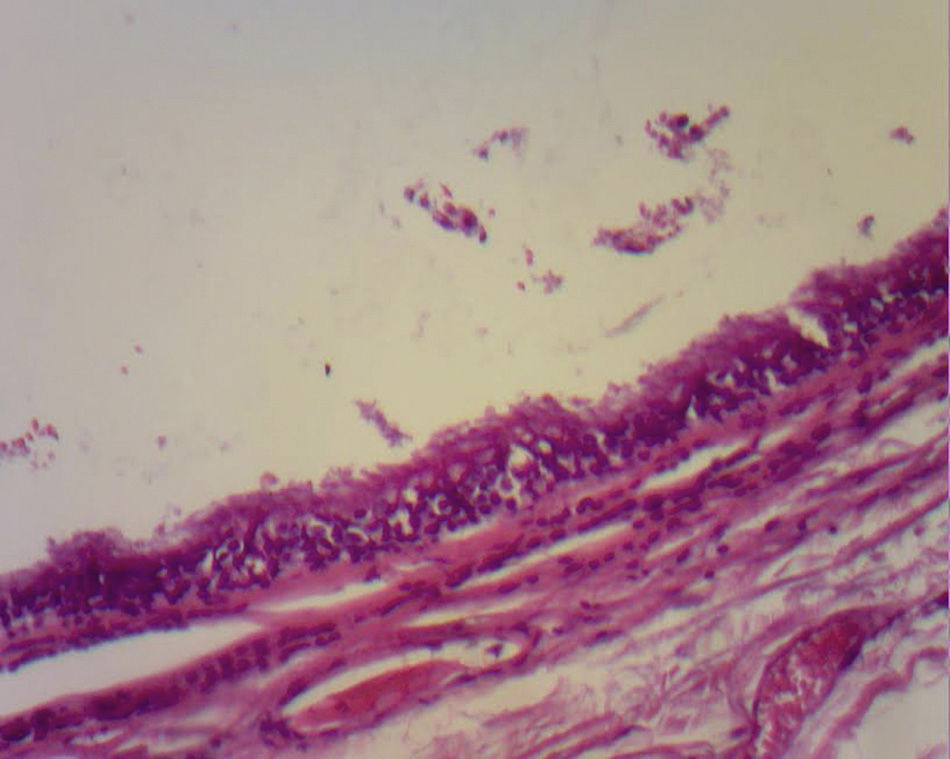
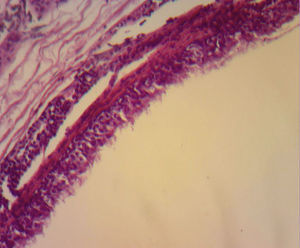
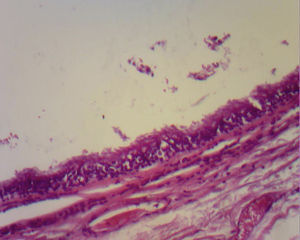
The majority are asymptomatic, but when they present symptoms this is due to communication with the trachea, compression of the oesophagus (dysphagia), to recurrent infections (75% of cases), haemoptysis as the only symptom, fever, coughing, cardiac arrhythmias, right cardiac failure, recurrent pericarditis or compression of the superior vena cava.7 Infrequent locations are: neck, pericardium, abdominal cavity and subcutaneous regions. Although they are more frequent in the mediastinum, occasionally they are not visible with chest teleradiography, but may be appreciated as a mediastinal or hyliary tumour; the intrapulmonary muscles are more frequently seen in inferior lobules (75% of cases). When it is not possible to see the lesion, computerised tomography of the neck or chest shows the presence of lesions in 50% of cases and these have defined sides, thin walls and a density of liquid8 (##–20 and +20UH); in the other 50% of cases there is a mucoid density. Depending on size, this may or may not compress structures such as the oesophagus and trachea, or affect atelectases. It is also possible to perform MRI. The cysts show up as hypointense lesions in T1 and hyperintense lesions in T2.8 Histologically, the bronchogenic cysts present a thin wall (when not infected), with ciliated columnar epithelium, similar to that covering the bronchials. Furthermore, they may contain cartilage, muscles, nerve tissue or bronchial glands, which contain a clear or mucous liquid3,9 (Figs. 3 and 4).
Bronchogenic cysts should be differentiated from other median and posterior mediastinum masses (Bush, 2001), including goitre, teratomas, tumours (thymic, germinal and neurogenic) and pulmonary tumours, or diaphragmatic hernias, lymphagiomas, hemangiomas, oesophagic duplication, pericardium cysts, or enteric cysts.
Treatment even in asymptomatic patients is surgical to prevent complications such as pulmonary infections, compression of structures and malignant transformation. There is a 100% mortality rate without surgical treatment.10
ResultsWhen a neonatal or paediatric patient presents with recurrent respiratory symptoms, such as coughing, stridor, pneumonias, or wheezing, the possibility of a malformation of the trachea-bronchial tree must always be considered. It has been stated that they are predominantly thoracic (mediastinal and pulmonary), but they may also present in the neck, the cellular subcutaneous tissue, or they may be parasternal. Clinical symptoms, chest X-rays, tomography of the neck or chest, and ultrasound imaging of the neck (depending on location), allows us to identify the lesion, its spread, and differentiate it from other pulmonary lesions, and even diagnose it in high resolution foetal ultrasound imaging.11 The definitive diagnosis is histopathological on demonstration that the walls are covered in ciliated columnar epithelium (bronchial)3,9 with a clear liquid on the inside or mucous, which may even be greenish and in the majority of case the culture tests negative.12,13 When infected, the isolated microorganisms are generally Pseudomona aeruginosa, Klebsiella pneumoniae, Aspergillus fumigatus and Streptococcus viridans.14
ConclusionsThe importance of the presentation of this case study originates in the cervical tumours which present at a young age and are not always cystic hygromas. Both the presence of frequent airway infections and obstructive symptoms which are either digestive or respiratory should alert us to the possible presence of a bronchogenic cyst. Although the majority of them are confined to the chest, the presence of a tumour in the neck, subcutaneous, muscular, should suggest a bronchogentic cyst and therefore initiate treatment in the early stages, when the lesion is smaller, since in 2% of cases there is bleeding, infection, obstruction of the airway or digestive system or an adenocarcinoma.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Nolasco-de la Rosa AL, Nuñez-Trenado LA, Román-Guzmán E, Chávez-Villicaña CE. Quiste broncogénico en cuello. Reporte de un caso y revisión de la bibliografía. Cirugía y Cirujanos. 2016;84:235–239.