In the pathophysiology of spinal cord injury, the secondary biological processes involving changes in gene expression become more important day a day. Within these changes, the expression of different microRNAs has been involved in some of the pathophysiological processes of spinal cord injury.
There are several studies that describe the transient expression of microRNA in spinal cord injury, some of them related to inflammation and apoptosis and others to functional recovery and regeneration.
MicroRNA may be a potential target for the treatment of spinal cord injury, modifying the processes of inflammation, oxidation, apoptosis, functional recovery and regeneration.
It is necessary to continue the study of microRNAs in spinal cord injury, as well as the identification of their target genes and signalling mechanisms involved in its neurological effects. With this, the ultimate goal is the development of effective and safe therapeutic and diagnostic strategies for patients with spinal cord injury.
Dentro de la fisiopatología de la lesión medular, cada día se da más importancia a los procesos biológicos secundarios, con la participación de cambios en la expresión genética. Dentro de estos cambios, la expresión de diferentes microARNha sido involucrada en algunos de los procesos fisiopatológicos de la lesión medular.
Existen diversos estudios que describen la expresión temporal de microARN en la lesión medular, algunos de ellos relacionados con la inflamación y la apoptosis, y otros con la recuperación funcional y la regeneración.
Estos microARN pueden ser blancos potenciales para el tratamiento de la lesión medular, al intervenir en los procesos de inflamación, oxidación y apoptosis, y en la recuperación funcional y regeneración.
Resulta necesario continuar con el estudio de los microARN en la lesión medular, así como la identificación de sus genes blanco y los mecanismos de señalización que participan en sus efectos neurológicos. Con esto, el objetivo final será el desarrollo de estrategias terapéuticas y diagnósticas eficaces y seguras para los pacientes con lesión medular.
The crucial role played by the spinal cord in a wide range of physiological functions is clearly demonstrated by the deficit seen after a spinal cord injury, and by the medical conditions which develop in the various subsequent stages. This injury usually results in devastating neurological changes and disability, which causes not only the loss of sensory and motor capacity (paraplegia or tetraplegia), but also other common problems associated with spinal cord injury such as: urinary complications, gastrointestinal problems, cardiorespiratory dysfunction and musculoskeletal deformities.1,2 On a global level, it principally affects men between the ages of 18–32, with an estimated incidence of 23 cases per million, of traumatic spinal injury (1,79,312 cases per year), with a bimodal peak incidence in young adults (20–29 for men and 15–19 for women), and elderly adults (men over 70 and women over 60).2
There are 2 main damage mechanisms in spinal cord injury, namely: primary mechanical damage and secondary damage caused by numerous biological processes which include extensive temporary changes in genetic expression.3,4 Secondary damage includes inflammation, oxidation and apoptosis,3 and as a consequence, the formation of a glial scar which acts as both a physical and a molecular barrier to axonal regeneration.5 However, it has been demonstrated that astrogliosis also plays a key role, as it restores subsequent homeostasis to the spinal cord injury, by promoting repair of the haematoencephalic barrier and limiting the infiltration of inflammatory cells.6,7 The beneficial effect of astrogliosis has been found in early, hypertrophic stages, while later, hyperplastic stages result in the formation of a dense scar which hinders axonal regeneration.8,9
Furthermore, it has been demonstrated that apoptosis not only affects the neurons but also other spinal cord cells, such as the microglial cells.6 The loss of oligodendrocytes in the white matter tracts continues weeks after spinal cord injury, and might contribute to progressive demyelation after the injury.10,11
It has been demonstrated that a change in the expression of various genes plays a major role in the pathogenesis of secondary spinal cord injury.3,4 However, little is known about the mechanisms which regulate changes in the expression of these genes. Micro RNAs are attractive candidates as “upstream” regulators of the secondary progression of spinal cord damage, as they can regulate complete series of genes post-translationally.11–13 Various forms of micro RNA have been found in the central nervous system of mammals, which includes the brain and the spinal cord, where they play an essential role in neurodevelopment and seem to be important mediators of neuronal plasticity.14,15 Some micro RNAs have even been involved in severe neurological diseases such as Tourette's and Fragile X syndrome.16 Some studies have suggested the possibility that micro RNAs are involved in neurodegeneration.17,18 However, as yet the role of micro RNAs in spinal cord injury has been little studied,15 despite the fact that the expression of a great many micro RNAs has been demonstrated in the spinal cords of adult mice.13
There follows a brief review of the relationship between spinal cord injury and micro RNAs.
Expression of micro RNA in spinal cord injuryTemporary expression of micro RNA in spinal cord injuryLiu et al.15 carried out an experiment in rats with traumatic spinal cord injury to establish the temporary expression of micro RNAs. The authors managed to demonstrate that a rat's spinal cord contains approximately 77% (269) of the micro RNA identified in mature rats (350), which suggests that the spinal cord is a rich source of micro RNA. Of these, 97 changed in a statistically significant way after the spinal cord injury. Thus, 60 were expressed in moderate, high and very high levels, and were classified into 5 different groups. Groups A and C contained upregulated micro RNAs, group D contained downregulated micro RNAs and groups B and E contained micro RNA upregulated 4hours after the injury, and subsequently downregulated 1 and 7 days after the injury. The remaining 37 micro RNAs expressed at a low level and were inhibited after the injury.
As decompression of the spinal cord is the main surgical option, Ziu and co-authors19 carried out a study to analyse the spatial and temporary expression of the levels of different micro RNAs and their relationship with the duration of compression. These researchers managed to demonstrate that, depending on the compression time, the temporary expression of some micro RNAs is different. In particular, miR-107 over-regulates in a prolonged compression model, whereas its levels do not vary in a short compression model. They found that the expression of miR-148 over-regulated 3 and 6hours after the injury in prolonged compression, and after 6hours in short compression. Finally they found that miR-210 over-regulates after 3, 6 and 24hours in the prolonged compression model and only after 24hours when the compression is of short duration.
Micro RNAs associated with inflammation and apoptosisIn turn, Sahni and co-authors9 carried out an experiment on mice with spinal cord injury to establish the role of bone morphogenetic proteins (BMPs) and their receptors in astrogliosis secondary to spinal cord injury. These authors managed to demonstrate a significant increase in BMP4 levels 4, 7, and 15 days after the injury. They also found a modest increase in BMP7 levels 4 days after injury, and a return to the baseline after 7 days. Together with these findings, they demonstrated an increase in the transcription of BMP receptor 1A (BMPR1a) and of glial fibrillary acidic protein (GFAP) 4 and 7 days after the injury. The BMP signalling route involves SMAD proteins, and it has been demonstrated that these proteins control the post-transcriptional processing of micro RNA-21 (miR-21).17 In relation to this, Sahni and co-authors9 studied the behaviour of this micro RNA in their mice. They managed to demonstrate that BMPR1a signalling regulates the cytoplasmatic processing of miR-21 in a negative way, such that the final processed product of this micro RNA is usually inhibited in spinal cord injury. Subsequently, Bhalala et al.20 performed an experiment in transgenic mice in order to understand the role of miR-21 in astrocytic response after spinal injury. In order to do this, they generated mice with over-expression of the primary transcript of miR-21 in astrocytes with a micro RNA sponge designed to inhibit the function of miR-21. The authors demonstrated that after traumatic spinal cord injury, the over-expression of miR-21 in astrocytes attenuates the hypertrophic response, while the expression of the sponge increases the hypertrophic phenotype, even in chronic stages. They also found that the inhibited function of miR-21 is accompanied by an increase in axonal density in the site of the injury. With these results, the authors managed to demonstrate a new effect of miR-21 in the regulation of astrocytic hypertrophy and the progression of glial scarring after spinal cord injury.
Also as part of the inflammatory process, Izumi and co-authors21 focussed on the expression of miR-223 and managed to demonstrate a high expression of this micro RNA 12hours after spinal cord injury. Moreover, their data indicate that miR-223 can regulate neutrophils in the acute stage of spinal cord injury.
With regard to apoptosis, Liu et al.22 in another study, demonstrated a significant change in the expression of some proapoptotic micro RNAs in rats with spinal cord injury; in fact, they found an increase in the expression of Let-7a and miR-16 10 days after the injury, and a reduction in the levels of miR-15b.
Micro RNAs associated with functional recovery and regenerationRegeneration refers to the ability to reproduce precise replicas of lost structures, a phenomenon which is observed in some species of vertebrates, such as salamanders, axolotls and zebrafish, but lost in humans. The molecular mechanisms which enable this process remain unknown.23 The role of micro RNAs has been investigated in the regeneration of the spinal cord after injury in some of these species.
Sehm and co-authors23 performed an experiment on axolotls to determine the role of miR-196 in the regeneration of the tail, after it had been amputated. These authors demonstrated a major increase in the expression of miR-196 in the first 14 days after the amputation, which was not maintained subsequently. Furthermore, the inhibition of miR-196 caused significant defects in the regeneration. These data suggest that this micro RNA plays a key role in the first stages of regeneration of an amputated tail in axolotls.
With this same species, Díaz Quiroz and co-authors24 found that miR-125b is necessary for the functional recovery of spinal cord injuries. In fact, these authors demonstrated that the reduction of miR-125b in axolotls to similar levels of those of rats, inhibits regeneration by means of the over-regulation of an “axonal repellent” gene, Sema4D, which induces the formation of a reminiscent glial scar.
Yu et al.25 for their part, studied the role of miR-133b in functional recovery after spinal cord injury. To do this, they used a spinal cord injury model in a zebrafish, and demonstrated an upregulation of miR-133b in the neurons that presented regeneration data after the section of the spinal cord and the inhibition of miR-133b which resulted in altered motor recovery, as well as decreased axonal regeneration.
Potential targets of micro RNA in spinal cord injuryInflammation, oxidation and apoptosisLiu et al.15 analysed the role of micro RNAs after spinal cord injury by investigating potential targets. By bioinformatic analysis they demonstrated that these targets are genes which code for components involved in various pathophysiological processes such as inflammation, oxidation and apoptosis. Diverse inflammatory genes are potential targets for micro RNAs downregulated after spinal cord injury, such as: miR-122, miR-181a, miR-411, miR-99a, miR-34a, miR-30c, miR-384-5p and miR-30b-5p. On the other hand, several anti-inflammatory anti-oxidant genes are potential targets for RNA micros which have been upregulated after spinal cord injury, such as: miR-221, miR-1, miR-206, miR-152 and miR-214. Some apoptotic genes are potential targets for micro RNAs which have been downregulated after spinal cord injury, such as: miR-127, iR-181a, miR-411, miR-34a and miR-384-5p.
These findings suggest that abnormal expression of micro RNAs after a traumatic spinal cord injury might contribute towards the pathogenesis of secondary damage, and therefore they could be potential targets for therapeutic interventions after spinal cord injury.
Sahni and co-authors,9 for their part, demonstrated that miR-21 negatively regulates the reactive hypertrophy of astrocytes in spinal cord injury. However, they did not manage to determine the targets of miR-21 which might affect the size of these cells, and therefore they suggest that further studies should be carried out with this objective, and to find possible targets for therapeutic intervention.
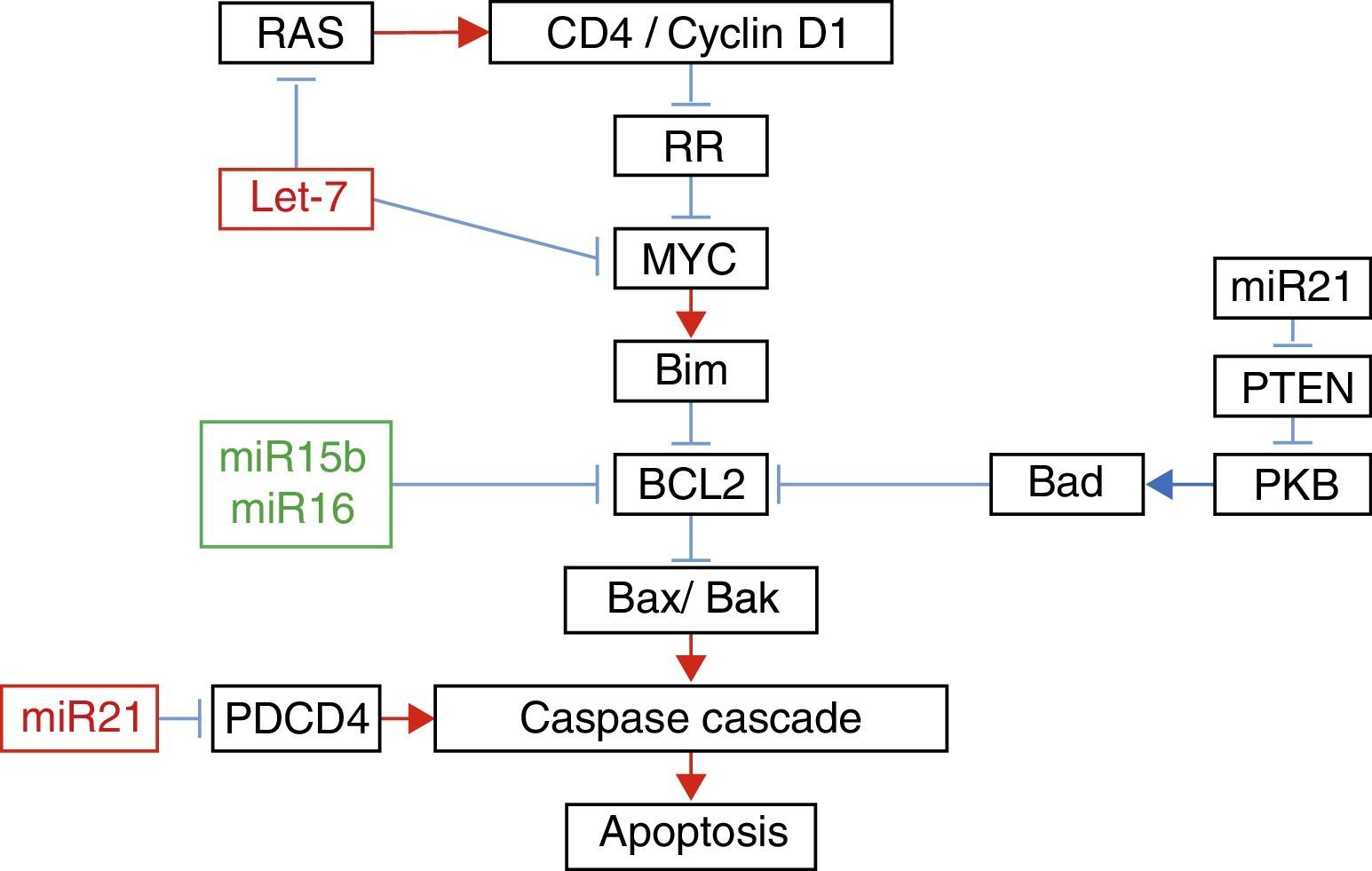
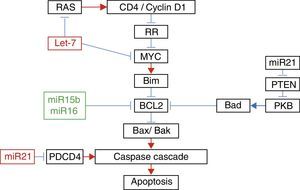
In their study of micro RNAs associated with apoptosis, Liu et al.22 demonstrated that the increase in the expression of Let-7a was accompanied by an increase in the expression of RAS and MYC 10 days after the injury; however 31 days afterwards, the expression of RAS and MYC returned to baseline levels, despite the fact that the expression of Let-7a remained elevated. The increase in the expression of miR-16 was associated with the increase in the expression of Bcl-2 (Fig. 1).
Relationship between the different micro RNAs with their target genes in apoptosis.
Adapted with the permission of Liu et al.22
Furthermore, it has been demonstrated that exercise after spinal cord injury maintains muscle mass in paralysed limbs,26 stabilises rhythmic firing patterns in the spinal motor neurons,27 stimulates anatomical and biochemical plasticity in the spinal cord28 and produces increased levels of neurotrophic factors in muscle and the spinal cord.29,30 This is why Liu et al.22 studied the effect of exercise on the expression of some micro RNAs. These authors managed to demonstrate that 5 days of exercise after spinal cord injury were accompanied by a significant increase in the expression of miR-21, known for its anti-apoptotic effect,31 and a significant decrease in the expression of miR-15b. The increase in the expression of miR-21 was accompanied by a decrease in the expression of PTEN messenger RNA and PDC4. It is known that the inhibition of these proteins is associated with decreased apoptosis in cancer cells by inhibition of PKB.22 With regard to the decrease in the expression of miR-15b, a paradoxical increase in the expression of Bcl-2 associated with an increase of Bcl-2 was found; these authors found a significant decrease in the expression of mRNA of caspases 7 and 9. Finally, despite the fact that they did not encounter any changes in the expression of Let-7a, these authors demonstrated that exercise significantly decreased the expression of RAS. All these changes in the expression of micro RNA with exercise might explain some of its beneficial effects.
Functional recovery and regenerationSehm and co-authors23 studied the potential targets of miR-196 in relation to tail regeneration in the axolotl. These authors showed that this micro RNA acts directly on the Pax7 gene by downregulating levels of this protein, which affects cell division during regeneration and produces a phenotype with a small tail. The mechanism of this protein to produce this phenotype was by using a feedback loop with the proteins BMP4 and Msx1, known for their control in cell proliferation in the spinal cord.
Díaz Quiroz and co-authors,24 after identifying miR-125 as a major factor in the creation of an environment enabling regeneration, proved the effect of increased levels of miR-125b in rats after spinal cord injury. In order to do so, they injected a synthetic miR-125b into the site of the injury 7 days after the trauma, and thus demonstrated decreased levels of Sema4D and the formation of glial scarring, as well as a positive effect on regeneration with significantly improved locomotion in some animals.
In their study on the role of miR-133b in functional recover after spinal cord injury, Yu et al.25 demonstrated that this micro RNA is important in the regeneration of the spinal cord of the adult zebrafish by reducing the levels of the protein RhoA, a small GTPase. The activation patterns of this protein have been studied after spinal cord injury, and the role of its activation on apoptosis in the central nervous system.32 Both the myelin-derived growth inhibitor proteins and tumour necrosis factor (TNF) directly activate Rho. P75NTR (neurotrophin receptor p75) activates Rho in the absence of neurotrophin binding. The inactivation of Rho by C3-05 (RhoA antagonist) after spinal cord injury blocks the elevation of p75NTR protein levels and inhibits apoptosis. The inactivation of Rho with C3-05 prevents apoptosis and stimulates regeneration.
MiR-133b produces this reduction by its direct interaction with RhoA Messenger RNA. This is an important finding, as it has been demonstrated that the inactivation of this GTPase results in rapid recovery of locomotion and progressive recovery of coordination between front and rear limbs in adult mice.33
Possible therapeutic interventionsThe role of micro RNAs in spinal cord injury needs to be explored further; however, every day there is more evidence to suggest that these micro RNAs represent a new class of therapeutic target.34–36 The micro RNA in the central nervous system decreases protein levels by post-transcriptional regulation.6,37 Thus the inhibition of a micro RNA linked to a particular disease can remove the block of expression of a therapeutic protein. Conversely, the administration of a mimetic micro RNA can stimulate a population of endogenous micro RNA which in turn represses a harmful gene.38 If its small size and the current knowledge of the biogenesis of micro RNAs is exploited, some modified RNAs could be used temporarily as pre-processed micro RNAs or as anti-micro RNA oligonucleotides.39
Anti-micro RNA oligonucleotides are reverse-chain complementary oligonucleotides. Their stability, binding affinity, and specificity have been improved by chemical modification. The most common modifications to oligonucleotides are locked nucleic acids (LNA), 2′-O-methyl, and phosphorothioate skeletons. Oligonucleotides with 2′-O-methyl modification have been demonstrated to be effective inhibitors in various cell lines and in cultivated primary neurons.40–42 Recently, oligonucleotides modified with LNA and phosphorothioate against miR-122 given intravenously to mice and non-human primates has resulted in a lasting and effective reduction in plasma cholesterol, with no apparent toxicity.43 Oligonucleotides modified with LNA against miR-122 (SPC3649) are currently being analysed in a Phase I study on hepatitis C virus infection, and will be the first therapeutic target with micro RNA in humans.44
The imitators of micro RNA are small oligonucleotides, generally double chain, and chemically modified which can be used to downregulate specific target proteins. The double chain structure is necessary for efficient recognition and binding to RISC. One of the chains is mature micro RNA, and the complementary chain forms a complex with the mature micro RNA sequence.36 Despite the fact that these imitators are frequently used in research in cultures, there are still no in vivo data to demonstrate the efficacy of micro RNA imitators.45
Many challenges are yet to be overcome before micro RNAs are used as therapeutic targets, namely: their difficult administration, possible effects on other systems and ensuring their safety. Nonetheless, the strategy for handing micro RNAs in vivo to regulate pathological processes is becoming a feasible therapeutic approach. In the future, a greater understanding of biogenesis and the function of micro RNAs will doubtless promote the development of micro RNA-based therapies.
ConclusionsSpinal cord injury is a major cause of disability for which there is no successful treatment as yet. A study of micro RNAs in this pathology has been started, which has shown their importance in controlling inflammation, oxidation, apoptosis, proliferation and regeneration. It is necessary to continue studying micro RNAs in spinal cord injury, and to identify their target genes and the signalling mechanisms which participate in their neurological effects. Thus the final objective will be to develop effective and safe therapeutic and diagnostic strategies for patients with spinal cord injury.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Quinzaños-Fresnedo J, Sahagún-Olmos RC. Micro ARN y su papel en la fisiopatología de la lesión medular; un paso más hacia la medicina neurorregenerativa. Cir Cir. 2015;83:442–447.