The desmoplastic small round cell tumour is a rare and aggressive intra-abdominal neoplasia, with only 200 cases reported, and a higher incidence in men and predilection for the second decade of life. Histologically it is characterised by the presence of small nests of undifferentiated tumour cells, wrapped in fibrous desmoplastic stroma.
Clinical caseA 24-year-old male started with abdominal pain of 4 weeks onset in the right upper quadrant, colic type, sporadic, self-limiting and accompanied by early satiety, decreased appetite, and involuntary weight loss of 10kg in 3 months. At the time of admission the abdomen was globular, with decreased peristalsis, soft, and depressible. Computed tomography of the abdomen showed multiple enlarged lymph nodes in the abdominal-pelvic cavity. A laparotomy was performed, with a subsequent omentum resection due to the presence of multiple tumours, which microscopically were characterised by groups of small, round, blue cells, separated by a desmoplastic stroma. The immunohistochemistry was positive for desmin (>75%), epithelial membrane antigen (>75%), CD99 (>50%), and S100 (25%), concluding with an abdominal tumour of small, round, blue cells as a diagnosis. Chemotherapy treatment was initiated based on IMAP plus GM-CSF.
ConclusionsThe desmoplastic small round cell tumour is a rare neoplasia, with diagnostic complexity and a lethal course. Its clinical presentation is unspecific. Histologically, it is classified as an aggressive soft tissue sarcoma that shares similar characteristics with the family of the small and blue cells tumours.
El tumor intraabdominal desmoplásico de células pequeñas y redondas es una entidad rara y agresiva con solo 200 casos reportados, con una incidencia mayor en varones y predilección por la segunda década de la vida. Histológicamente se caracteriza por la presencia de nidos de células tumorales pequeñas e indiferenciadas, envueltas en estroma fibroso y desmoplásico.
Caso clínicoVarón de 24 años, que en las 4 semanas previas comienza con dolor abdominal en hipocondrio derecho, tipo cólico, esporádico, autolimitado, acompañado de saciedad temprana, hiporexia y pérdida involuntaria de 10kg de peso en 3 meses. A su ingreso se encuentra abdomen globoso, con peristalsis disminuida, blando, depresible. La tomografía computada de abdomen evidencia múltiples adenomegalias en la cavidad abdominopélvica. Se realiza laparotomía exploradora con la consecuente resección del omento por la presencia de múltiples tumoraciones, las cuales microscópicamente se caracterizaban por grupos de células redondas, pequeñas y azules separadas por un estroma desmoplásico. La inmunohistoquímica reveló positividad para desmina (>75%), antígeno de membrana epitelial (>75%), CD99 (>50%) y S100 (25%), por lo que se diagnostica tumor desmoplásico abdominal de células redondas, pequeñas y azules. Se inició tratamiento quimioterapéutico con base en esquema IMAP más GM-CSF.
ConclusionesEl tumor intraabdominal desmoplásico de células pequeñas y redondas es un tumor poco frecuente, de complejidad diagnóstica y de curso letal. Clínicamente presenta manifestaciones inespecíficas. Histológicamente se clasifica como un sarcoma agresivo de tejidos blandos, que comparte características similares con la familia de tumores de células pequeñas y azules.
The intra-abdominal desmoplastic small round cell tumour is a very rare aggressive neoplasm, with approximately 200 cases reported since it was first described in 1989 by Gerald and Rosai,1 who described that it has a higher incidence in the male gender, with a 2–10:1 ratio and it was more frequent during the twenties in 80% of the cases (range 4–52 years).1–9
It mainly affects serous membranes, particularly those of the peritoneum, and of these lesions, 62% are located in the abdomen and the remaining 36% in the pelvis; there are cases of primary tumours located in the testicles, scrotum, pleura, posterior fossa, petrous portion of the temporal bone, eye orbit and abdominal organs. Most of the cases are advanced stage, large tumours without apparent organic origin, which may be accompanied by large tumour implants located in the peritoneum.2–6,8
Histologically, it is characterised by the presence of small and undifferentiated tumour cell nests, embedded in a fibrous and desmoplastic stroma. It is a sarcoma (of round cells) which is genetically characterised by the expression of a reciprocal translocation of the gene t(11;22)(p13;q11 or q12), resulting from a fusion of the Ewing sarcoma gene and chromosome 22 of the Wilms tumour (WT1), mainly located in the intra-abdominal region.2,3,5,6,8–11
The objective of this report is to present a typical case of a desmoplastic small round cell tumour.
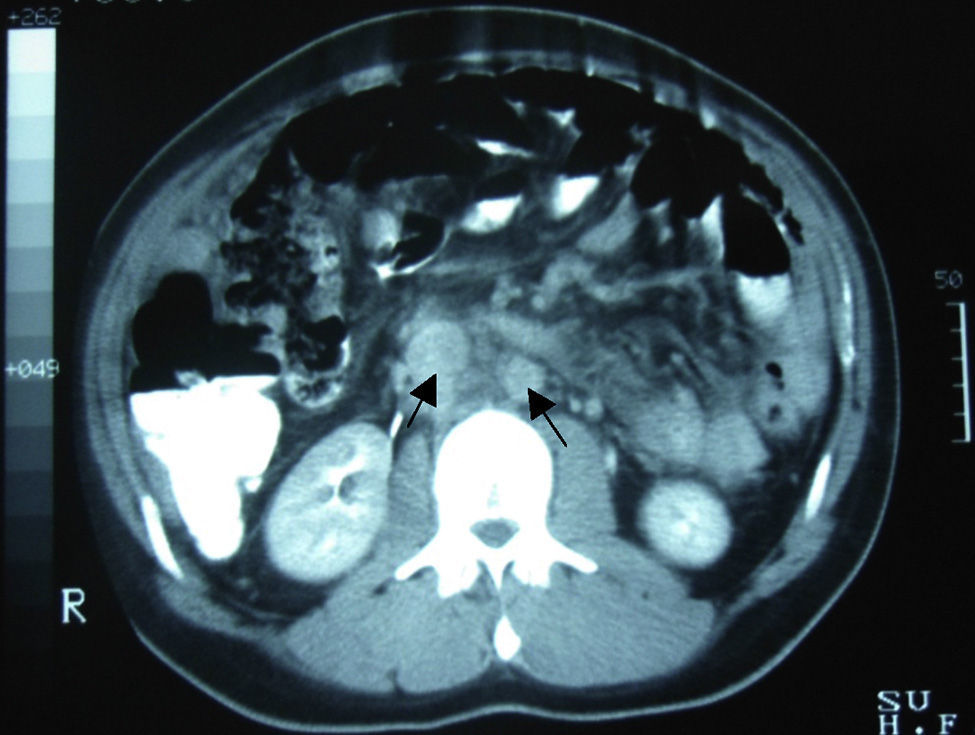
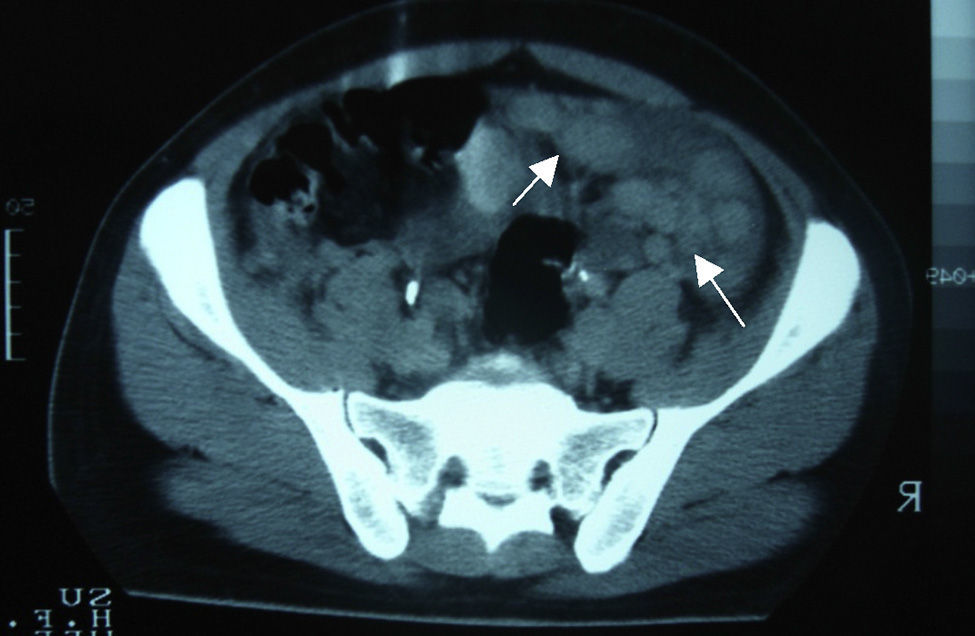
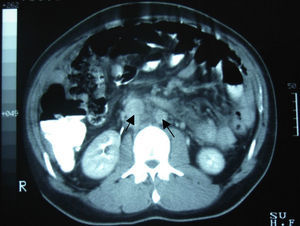
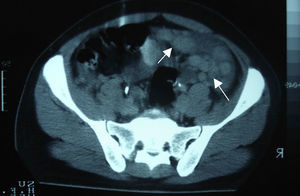
Clinical caseA 24-year-old male patient, with no history of surgeries, trauma, autoimmune diseases or medication intake. He presented symptoms 4 weeks before his admission, including sudden colic, sporadic, 10-minute long abdominal pain in the right hypochondrium, accompanied by early satiety, hyporexia and involuntary loss of 10kg of weight in the last 3 months. The patient denied having fever, asthenia, adynamia, as well as other relevant history. On admission, he was found to be haemodynamically stable, without fever and without relevant pathological data. He presented a soft, depressible, globus abdomen, with diminished peristalsis, without pain on palpation and without visceromegaly. Alpha-fetoprotein of 4.28IU/ml, CA-125 of 110IU/ml and lactic dehydrogenase of 1025IU/l were observed. The abdominal computed tomography showed the presence of multiple nodular-to-ovoid lesions in the abdominopelvic cavity, of increased density, which had no apparent organic origin, the largest of which was 6cm×4cm, and were well defined. These findings suggested peritoneal carcinomatosis with metastatic retroperitoneal adenopathies (Figs. 1 and 2).
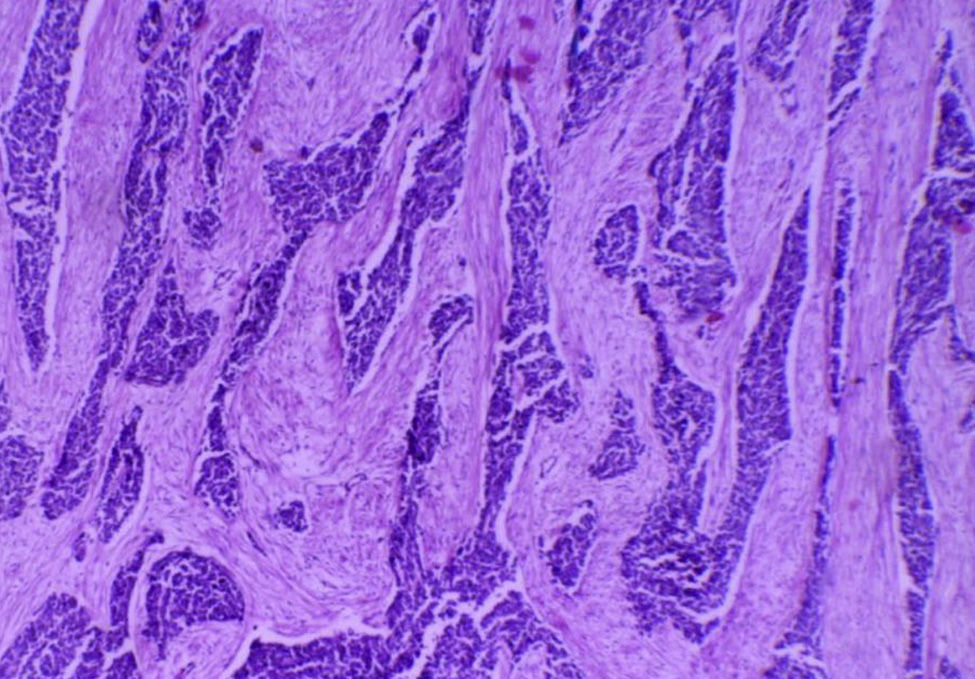
An exploratory laparotomy showed the presence of ascites of approximately 1000×1cc, exhibiting a haemorrhagic, hypercellular smear with reactive mesothelial cells, as well as the presence of neoplastic cells with a tendency to form moriform and papillary structures with marked atypia. A tumour at the level of the right hepatic lobule of approximately 17cm×14cm, indurated and with well-defined borders, was detected on palpation. The presence of retroperitoneal, para-aortic and parahilar adenopathies was also observed. However, no lymph nodes were biopsied. There was no evidence of any other macroscopic extension to diaphragms or pelvic structures. The presence of multiple, solid and irregular nodulations was observed in the omentum. Thus, a total omentectomy was performed (Fig. 3) as surgical treatment and diagnosis, which was sub-optimal due to the advanced stage of the disease.
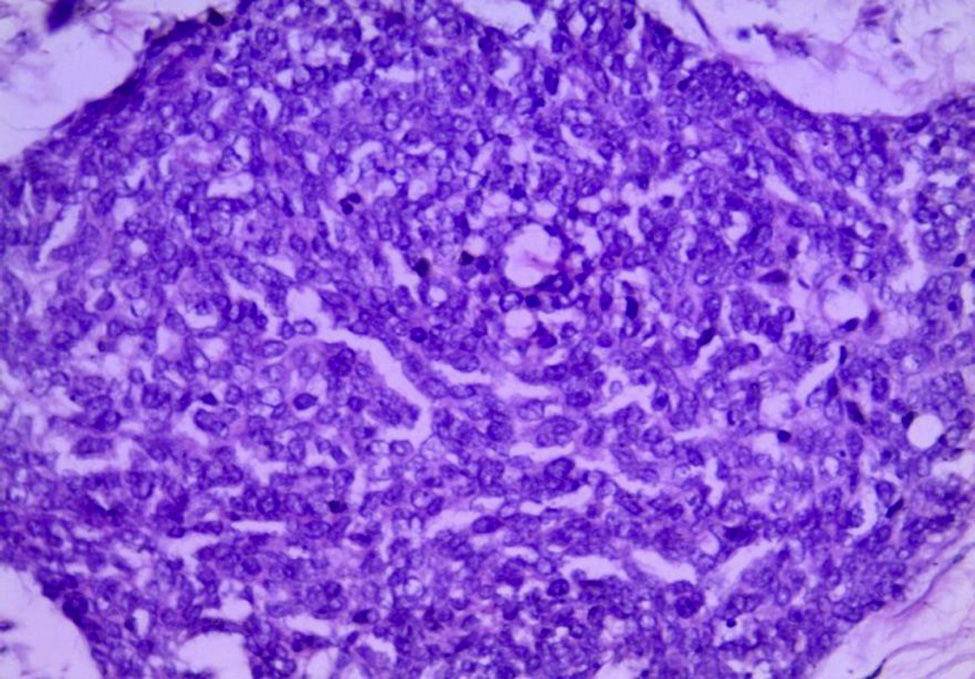
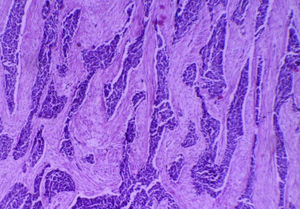
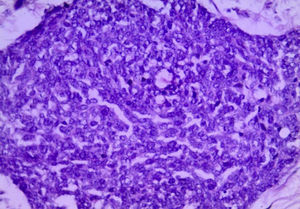
For the anatomopathological analysis, a brown-violet coloured, highly vascularised sample of 52cm×22cm×7cm with multiple nodular tumours was collected. The largest of these tumours was 6cm×4cm×4cm, and the smallest was 0.5cm×0.5cm×0.5cm. The nodules presented a smooth and vascularised external surface, with solid consistency when cut, a homogeneous surface and white-yellowish colour (Fig. 4). Microscopically, the lesions showed the presence of small-blue-round cell groups, which formed nests and cords with solid areas surrounded by a desmoplastic stroma and a large number of blood vessels and high lymphatic permeation. The cells were uniform, with little cytoplasm, ill-defined borders, with a large number of mitosis, arranged in a rosteoid, pseudoglandular and picket-fence form (Figs. 5 and 6). The immunohistochemistry analysis was positive for desmin (+++ or >75%), epithelial membrane antigen (+++), cytokeratin (+++), CD99 (++ or >50%) and S100 (+ or ≤25%). It complied with clinical, histological and immunohistochemical criteria for a diagnosis of abdominal small-blue-round cell tumour.
Medical treatment involved a chemotherapy scheme of IMAP (vincristine, doxorubicin, cyclophosphamide, ifosfamide and etoposide), plus a granulocyte-macrophage colony-stimulating factor, which was only used for palliative purposes. During the twelfth cycle, the patient presented dyspnoea, epistaxis, pancytopoenia and increased nitrogen compounds. Thus, it was necessary to discontinue chemotherapy due to secondary medullary aplasia. The patient died 10 days after this clinical course.
DiscussionThe desmoplastic small round cell tumour is very rare and aggressive, and belongs to the family of neoplasms known as small-blue-round cell tumours, which include non-Hodgkin lymphomas, neuroblastoma, rhabdomyosarcoma, Ewing sarcoma, Wilms tumour and peripheral primary neuroectodermal tumour.2,4,5,9,12,13
Its histogenesis is uncertain, but it has been associated with the mesothelium, called mesothelioblastoma, based on its frequent relation with serous surfaces, immunoreactivity for WT1 and expression of epithelial and mesenchymal markers.2,5,6,11
The most common symptomatology is the presence of abdominal pain, distension and palpable abdominal tumours. In 50% of the cases, it may present clinical symptoms compatible with intestinal pseudo-obstruction, night diaphoresis, weight loss, haematuria and pleural effusion.4,12,14
Approximately 30% of the cases present ascites and, in the cytological study, show a population with loss of cohesivity, morphologically round, oval or spiculated cells, with little cytoplasm, irregular nuclear membrane, granular chromatin and inconspicuous nucleolus. Presley et al.12 described that morphology may be modified after treatment with chemotherapy, which causes loss of cohesivity, karyomegaly and absence of mitosis.3,6,12
Among the studies conducted for its diagnosis, the abdominopelvic computed tomography generally involves one or multiple tumours of lobulated and well-defined borders, with hyperdense and heterogeneous soft tissue, with hypodensity areas related to necrosis and haemorrhage foci, located in the intraperitoneal region, without apparent abdominal organic origin. About 50% may present adenomegalies and 20% may present calcifications. Bellah et al.15 reported that lesions were located in the vesical rectum and/or urinary rectum in 82% of the cases.3–5,15
The magnetic resonance imaging showed a tumour with multiple intraperitoneal neoplasms with intermediate signal in potential sequences on T1, an increase on T2 and little intravenous contrast capture.5,14 Radiologically, no adequate specificity has been demonstrated to support the diagnosis, so the clinical/pathological correlation is necessary.
Histologically, it presents a pattern of small, round, oval and/or spindled cells, with hyperchromatic nuclei, little cytoplasm, a large number of mitosis, arranged in nests, cords, lines, sheets, trabecula and well-defined solid areas, embedded in an desmoplastic stroma. There are less frequent morphological patterns, such as papillary, glandular and cribriform patterns with clear, fusiform, pleomorphic, rhabdoid and basaloid cells forming rosettes or pseudorosettes.2,5,6,9,14 In our case, the cell arrangement was conventional, and included the formation of rosettes, peripheral picket-fence form and central necrosis nests.
Immunophenotypically, it is characterised by a pattern of expression for epithelial, mesenchymal and neuronal markers. This tumour is positive for desmin (100%), AE1-AE3 (100%), epithelial membrane antigen (100%), cytokeratin (100%) and neuron-specific enolase (100%); S100 and CD99 are expressed as partially positive,2–5,10,11 as it occurred with our patient.
In 1991, it was first genetically described with a fusion of EWS genes from the Ewing sarcoma of exon 7 and exon 8 of the gene WT1 of Wilms tumour. This reciprocal translocation characterised as t(11:22)(p13;q11) or (p13;q12) may be detected by means of a reverse transcription polymerase chain reaction (PCR-RT) with a 93% sensitivity and 100% specificity, or by means of hybridisation using the Southern-Blot analysis with a 97% sensitivity.2,4–6,8–12
Therapeutic options are limited. Surgical cytoreduction with primary partial or complete tumour removal is preferred. This is only possible in 60% of the cases and it is associated with a better long-term prognosis. These tumours show better results when a combined treatment is used. Hassan et al. demonstrated a 3-year survival of 58% in patients who received chemotherapy, radiotherapy and cytoreductive surgery, compared to those who received no treatment (0%). The exclusive treatment with chemotherapy has been associated with a higher rate of toxicity, which worsens patients’ prognosis and life expectancy.3,5
Since this is a very rare tumour, there is no established chemotherapy scheme of choice. However, there are several recommended protocols, and IMAP plus a granulocyte-macrophage colony-stimulating factor is the most commonly used treatment,5,16,17 followed by other schemes, such as IMAP,11 PAVEP (cyclophosphamide, etoposide, doxorubicin and cisplatin) and PEVEP (cyclophosphamide, etoposide, epirubicin and cisplatin).13,17
The most frequent long-term complication is intestinal obstruction, which occurs in approximately one-third of patients. The clinical course is aggressive, with multiple local recurrences and distant metastases in the lungs, liver and lymph nodes.5,6
Most cases are detected in advanced stages. Patients present an average life of less than 3 years, and fewer than 15% of patients present a 5-year life expectancy.2–4,6,7
In relation to the differential diagnosis based on imaging studies, the following items should be taken into account: mesothelioma, carcinoid tumour, peritoneal carcinomatosis, peritoneal leiomyomatosis, intraperitoneal desmoid tumour, peritoneal lymphomatosis, peritoneal sarcoma and peritoneal tuberculosis.4
Histopathologically, the differential diagnosis is broad, so the morphological and immunohistochemical patterns must be taken into account. In paediatric patients, these include: Ewing sarcoma, rhabdomyosarcoma, neuroblastoma, small-cell lymphoma, anaplastic synovial sarcoma and Wilms tumour. On the other hand, in adults, the following aspects should be considered: lymphomas, small-cell carcinoma, Merkel-cell carcinoma, neuroendocrine carcinoma and mesothelioma, among others.2,7,13
ConclusionsThe intra-abdominal desmoplastic small round cell tumour is a very rare tumour, with diagnostic complexity and lethal course. Clinically, it presents non-specific manifestations. Histologically, it is classified as a soft-tissue aggressive sarcoma that shares the same characteristics as the family of small-blue cell tumours.
Conflict of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Briseño-Hernández AA, Quezada-López DR, Corona-Cobián LE, Castañeda-Chávez A, Duarte-Ojeda AT, Macías-Amezcua MD. Tumor intraabdominal desmoplásico de células pequeñas y redondas. Cir Cir. 2015;83:243–8.