Glomus tumours are neoplasms arising from cells of the neuromyoarterial glomus bodies, which almost always occur in a subungual location. A lung location is extremely rare, with few cases reported in the literature.
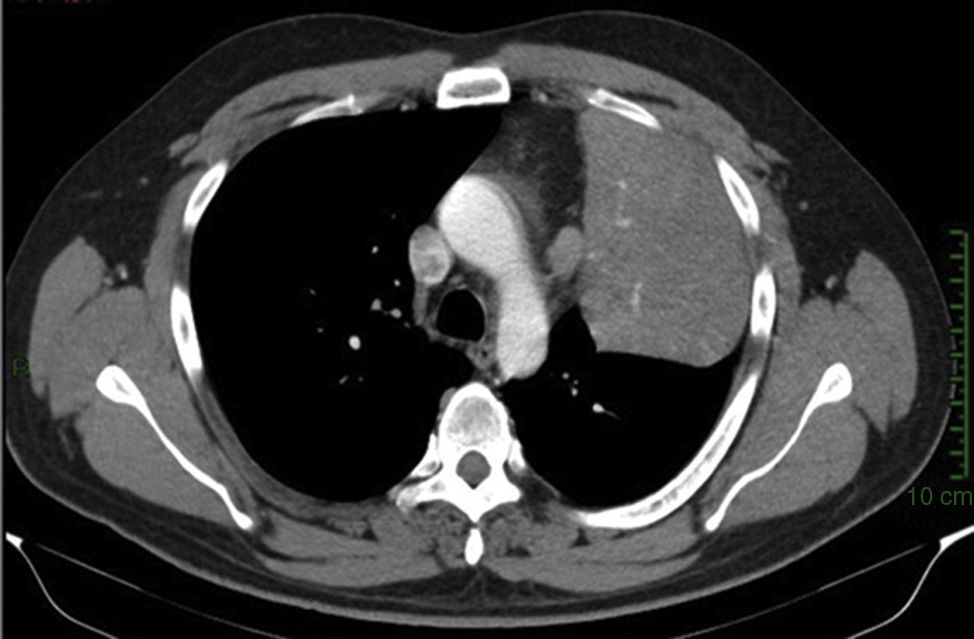
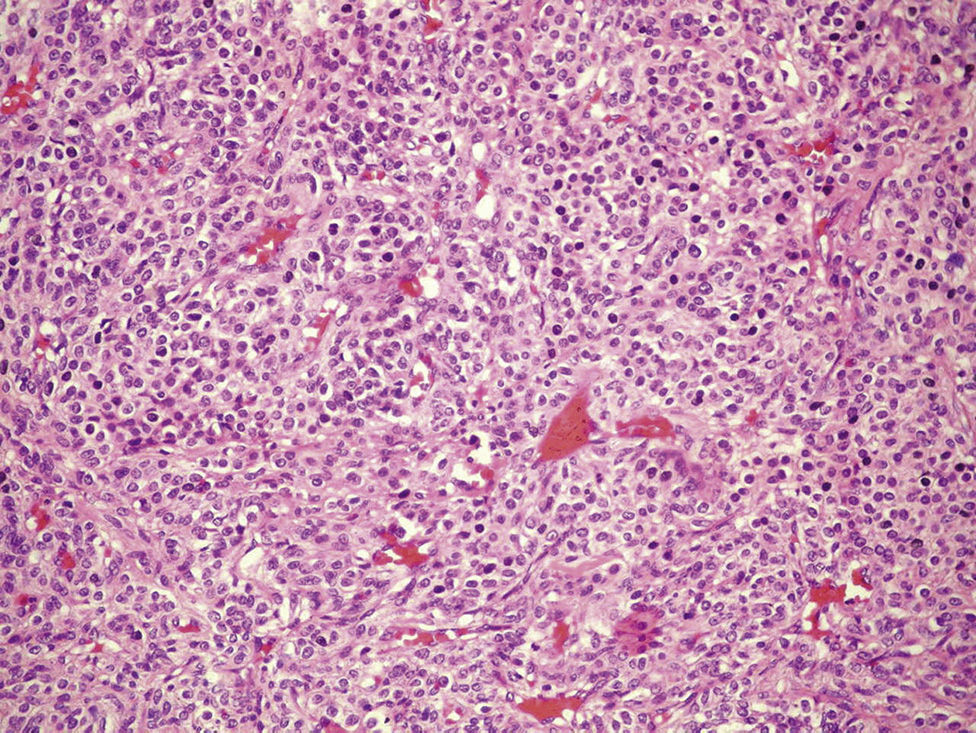
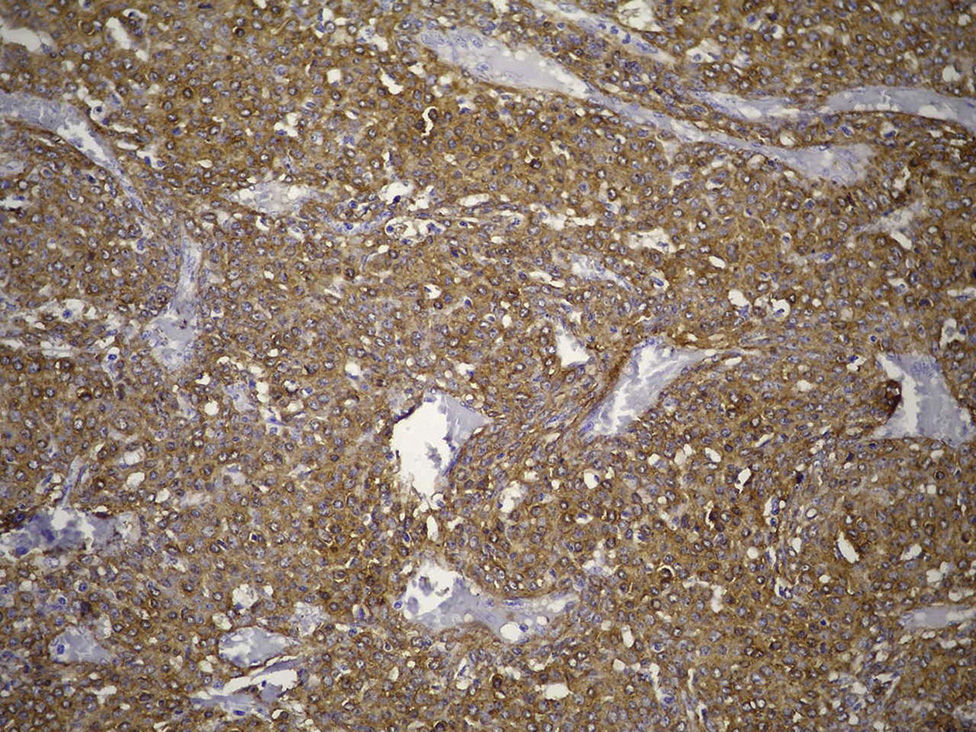
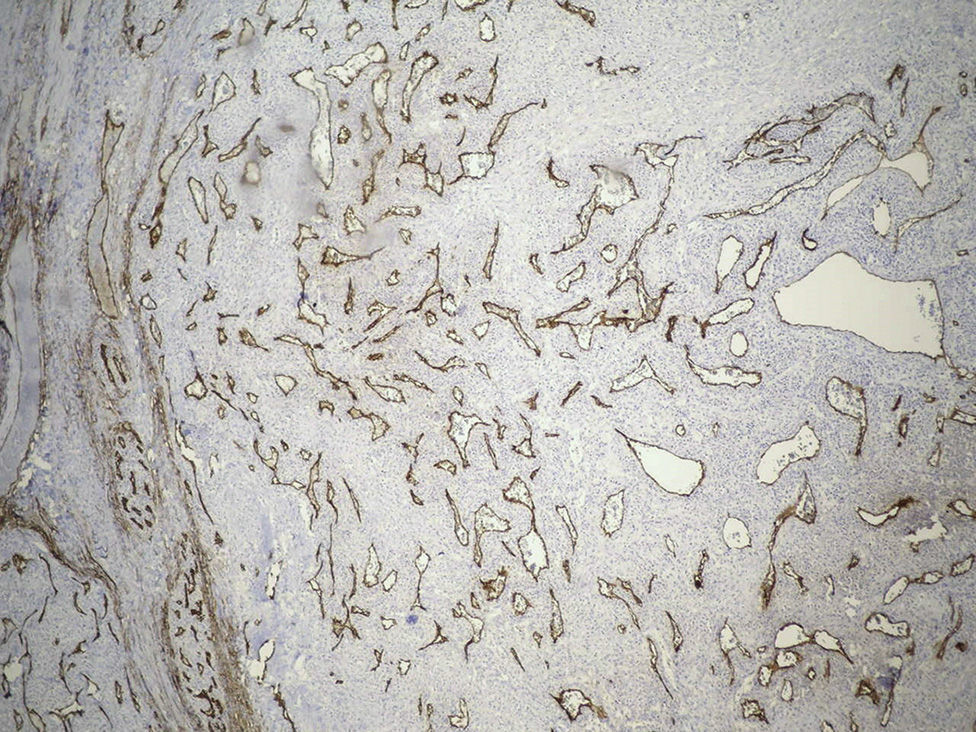
Clinical caseThe case is presented of a 33 year-old male, with non-productive cough, dyspnoea at rest, intermittent fever, and mild pain in rib cage. A chest radiograph showed a consolidation in the left lung, and computed tomography revealed a lesion in the hilum that extended to the bronchus of the lingula obstructing, and causing post-obstructive pneumonia. A biopsy was obtained by rigid bronchoscopy biopsy, which showed a well circumscribed tumour constituted by intermediate-sized cells, and abundant cytoplasm that are arranged in a pattern surrounding numerous thin-walled blood vessels, with no pleomorphism, significant mitotic activity or necrosis. Immunohistochemistry revealed diffuse positivity with smooth muscle actin, vimentin, caldesmon; focal reactivity with desmin and CD117, CD34 highlights the vascular pattern. Ki67 proliferation rate was 1%. Synaptophysin, EMA and cytokeratin cocktail were negative, making the diagnosis of glomus tumour.
ConclusionsGlomus tumours are rare neoplasms that usually appear in the dermis and subcutaneous tissue, where it is common to find glomus bodies. Occasionally glomus tumours can occur in extra-cutaneous sites such as the gastrointestinal tract, bone and respiratory system, with this case being a new case of rare lung location.
Los tumores glómicos son neoplasias derivadas de las células de los cuerpos glómicos neuromioarteriales, que casi siempre se presentan a nivel subungueal. La localización pulmonar es muy poco frecuente, con pocos casos reportados en la literatura médica.
Caso clínicoPaciente masculino de 33 años de edad, con tos no productiva, disnea en reposo, fiebre intermitente, y dolor leve en pared costal. La radiografía de tórax reveló consolidación en campo pulmonar izquierdo, y la tomografía computada evidenció una lesión en hilio, que se extendía hasta el bronquio de la língula obstruyéndolo, y causando neumonía post-obstructiva. A través de una broncoscopia rígida se obtuvo biopsia en la que se observó: neoplasia que en su mayoría estaba circunscrita; constituida por células de tamaño intermedio, núcleo oval, y citoplasma abundante que se disponen en un patrón sólido rodeando numerosos vasos sanguíneos de paredes delgadas, sin pleomorfismo, actividad mitótica significativa ni necrosis. La marcación inmunohistoquímica reveló positividad difusa con actina de músculo liso, vimentina, caldesmon; reactividad focal con desmina y CD117, CD34 resaltó la trama vascular de la lesión, el índice de proliferación Ki67 es de 1%. Los marcadores sinaptofisina, EMA y cóctel de citoqueratinas son negativos, haciéndose el diagnóstico de tumor glómico.
ConclusionesLos tumores glómicos, son neoplasias infrecuentes que usualmente se encuentran en la dermis y en el tejido celular subcutáneo, en donde es frecuente encontrar cuerpos glómicos. Ocasionalmente los tumores glómicos se pueden presentar en sitios extracutáneos como el tracto gastrointestinal, hueso, y aparato respiratorio; siendo éste un caso de localización pulmonar.
A glomus tumour is a benign neoplasia that arises from the glomus bodies in the skin or from subcutaneous cell tissue; they are usually located in the limbs.1–3 Extracutaneous manifestations are very rare, particularly in the viscera, where the glomus bodies are scarce or even absent. Manifestations in the lungs are infrequent, which is why these lesions are usually mistaken for other solid neoplasias, such as carcinoids, hamartomas, carcinomas and tumours from the Ewing's sarcoma family, and primitive neuroectodermal tumours.4
A case of a patient with a glomus tumour in his lung is reported, which was successfully treated by means of an upper left lobectomy.
The purpose of this study is to emphasise that when dealing with pulmonary nodules with rare lesions, it is essential to perform a comprehensive assessment of the patient through clinical, imaging and histopathological approaches to obtain an accurate diagnosis; only this way can an adequate treatment that ensures an excellent prognosis for the patient be offered. In addition, a review of the international scientific literature was performed.
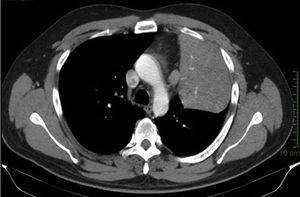
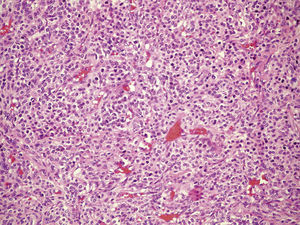
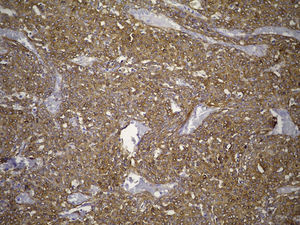
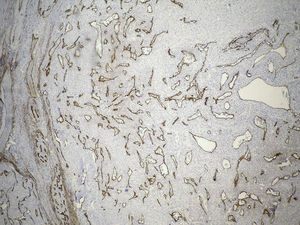
Clinical case33-Year-old male patient who consulted due to a six-month clinical condition that consisted of: non-productive cough, dyspnoea at rest, sporadic fever and mild pain in costal wall. During physical examination there was reduction of the breathing sounds in the upper half of the left hemithorax but no other relevant findings. Laboratory test results were normal. A chest X-ray showed solidification in the left pulmonary field and the axial computerised tomography scan revealed a space-occupying lesion of approximately 4×4×3.5, located in the upper left lobe, with preponderance at the hilum level, which extended to the lingular bronchus obstructing it and causing postobstructive pneumonia (Fig. 1). We performed a biopsy through a rigid bronchoscopy. A sample was added of haematoxylin–eosin staining (HE), which evidenced a neoplasia that was almost totally circumscribed, comprised of intermediate-size cells, oval nucleus and abundant cytoplasm. The cells had a solid pattern and were surrounded by numerous blood vessels with thin walls, without pleomorphism, significant mitotic activity or necrosis (Fig. 2). The immunohistochemical demarcation revealed diffused positivity with smooth-muscle actin, vimentin, caldesmon; focal reactivity with desmin, and CD117 (Fig. 3); CD34 highlighted the vascular markings of the lesion (Fig. 4), the Ki67 proliferation rate was 1%. Synaptophysin markers, EMA and cytokeratin cocktail tested all negative.
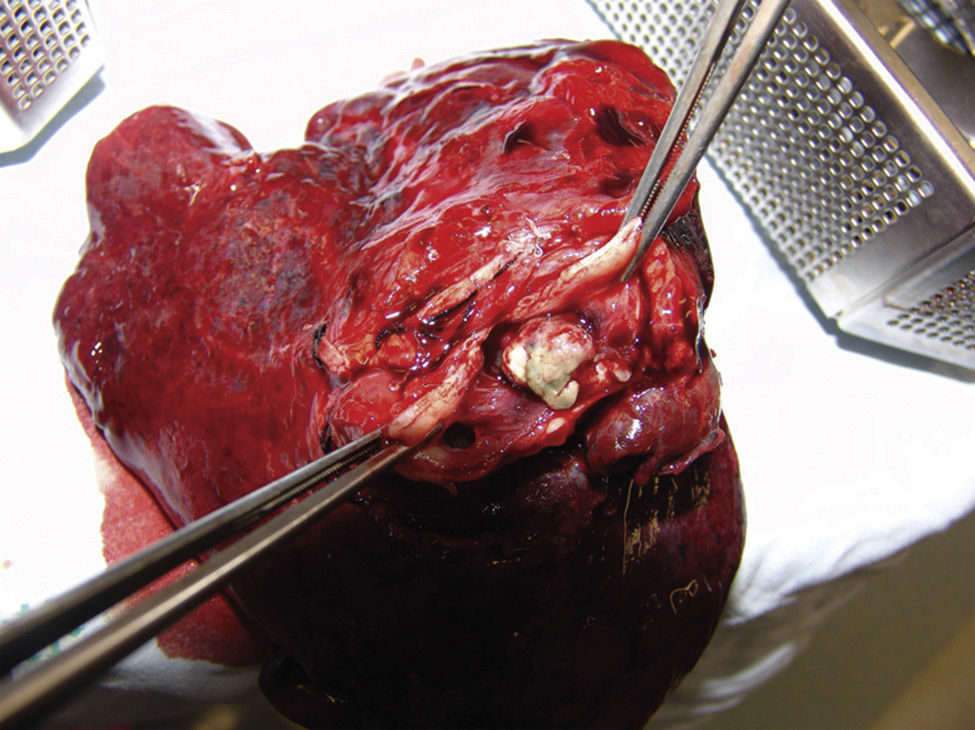
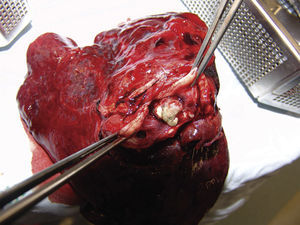
An upper left lobectomy was performed and the surgical piece weighed 210g and measured 10cm×9cm×6cm. A solid mass of 3cm×2cm×1cm was found 0.5cm from the bronchial margin; it was well-circumscribed, cream coloured with haemorrhagic areas, which obstructed the bronchial lumen by 40%. Surrounding it, the pulmonary parenchyma had dense areas, which were non-crepitant, and dilated bronchi with yellowish mucoid material inside the lumens (Fig. 5). The histopathological study of the specimen evidenced the same morphological features as the previously described biopsy. All findings were consistent with a pulmonary glomus tumour diagnosis.
After surgery, the patient progressed favourably with no respiratory symptoms. Three months afterwards, a control chest X-ray was performed, with no evidence of residual or recurrent tumour lesions, atelectasis or consolidation processes.
DiscussionGlomus tumours are perivascular neoplasias. In most cases they are benign and stem from smooth muscle cells modified by the glomus body, which is associated with temperature regulation. These tumours are comprised of capillaries surrounded by nests of uniform cells with abundant cytoplasm, and round or oval nucleus, with no significant pleomorphism (glomus cells). In addition, they contain a variable amount of smooth muscle.5 The morphological classification of these tumours is based on the percentage of components that make them up. The most common form is predominantly made up of glomus cells with a lower number of blood vessels and smooth muscle fibres. The second variant, called glomangioma, has a higher percentage of blood vessels, usually dilated, similar to a cavernous haemangioma, and it is usually less circumscribed than the most common form. The most rare variant, called glomangiomyoma, exhibits a transition from glomus cells to elongated smooth muscle cells.3,6
Tumour cells are reactive to vimentin, smooth-muscle actin and caldesmon, and test negative for cytokeratin markers, neuroendocrine and vascular markers; this immuno-marking profile was seen in our case. In addition, CD34 and reticulin staining highlighted the rich vascular markings and the distribution pattern in small cell nests, respectively.6 These tumours are also characterised by proliferation rates below 3%, which is consistent with the 1% Ki67 calculated in this case.
The manifestation of these tumours in the respiratory tract is considered an exotic event, with only a few cases reported in scientific literature.2,7,8 Inside the respiratory tract, the trachea is the most common place and the pulmonary parenchyma has been affected in only a few reports, including this one. The first case of pulmonary glomus tumour was described by Tang et al. in 1978,9 and today we consider that there have been approximately 25 reported cases, of which less than 20 cases correspond to benign glomus tumours.
Although some studies have described the average age of manifestation as 45 years,2,7 our youngest patient was 33. In addition, a trend affecting predominantly males has been described, with a men:women ratio that reaches 7:1.2 Our patient presented with respiratory symptoms, in contrast with the majority of the cases described, in which up to two-thirds of the patients were asymptomatic. The most common symptoms are pain and dyspnoea.2,7,10
Pulmonary glomus tumours can exhibit a central or peripheral manifestation. Chest X-rays usually show a classic nodular coin-shape lesion, or solitary nodule lesion, and they are rarely associated with obstructive alterations, as the ones described in our case.2,7,11 The axial computerised tomography scan and the magnetic resonance generally show an increase in the detection of contrast material at a peripheral level.12
The main differential diagnosis includes carcinoid tumour, solitary fibrous tumour and smooth muscle tumours.10,11 Carcinoid tumours, just like glomus tumours, can exhibit an organoid pattern, an increase in the vascularisation and uniform cells that are round with eosinophilic cytoplasm, but they usually test positive for cytokeratins and they always stain with chromogranin and synaptophysin, testing negative for smooth muscle markers. Tumours with hemagiopericytic patterns have numerous blood vessels with thin walls and deer-antler-like ramifications. This feature can also be seen in solitary fibrous tumours (previously hemangiopericytoma), but the latest shows a more fusiform cell component and is rarely positive for smooth-muscle actin; its CD34 and CD57 expression is variable. It is important to take this differential diagnosis into account, since solitary fibrous tumours have a worse prognosis than glomus tumours. Pulmonary leiomyomas are not usually a big problem for histological differential diagnosis, since the fusiform component that is arranged in intertwined bundles is very suggestive. However, the epithelioid variant can be similar to a glomus tumour, although the lack of the unique vascular marking can lead to the correct diagnosis.
As previously mentioned, glomus tumours are usually benign. Their prognosis is excellent and surgical removal is the treatment of choice6,8; just as in our case, in which the patient had excellent clinical progress after having a lobectomy. Malignant pulmonary glomus tumours are very rare; according to the series reported by Enziger3 and Gould et al.,13 we know the histopathological characteristics of this group of tumours. In 1990, Gould and his team13 proposed the terms locally infiltrative, glomangiosarcoma stemming from a benign glomus tumour, and De novo glomangiosarcoma as one of the first classifications of these tumours. Today, a malignant glomus tumour is considered a hypercellular tumour with round, pleomorphic and mitotically active cells, but there is no consensus on the morphological criteria that are necessary to categorise these lesions. Folpe et al.14 proposed an empirical classification of glomus tumours with atypical characteristics: malignant glomus tumour (glomangiosarcoma), single glomus tumour with nuclear atypia (symplastic glomus tumour), glomus tumour of uncertain malignant potential, and glomangiomatosis (histologically benign glomus tumour with a diffused growth pattern). According to this classification, a malignant glomus tumour should fulfil at least one of the following criteria: deep location and a size over 2cm, existence of atypical mitotic figures or a combination of a moderate to high nuclear grade and significant mitotic activity (five mitoses in 50 high power fields).
ConclusionWe report a case of a pulmonary glomus tumour with benign histological features, completely removed by means of an upper left lobectomy. Our report highlights the ubiquitous manifestation, histological features and unique immunohistochemical profile that aids in the differential diagnosis of other more frequent tumours in this location. Similarly, we accept it is important to report these cases with unusual locations, since their frequency can be underestimated, and more studies are needed to characterise their behaviour and predict their prognosis.
Conflict of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Baena-Del Valle JA, Murillo-Echeverri VE, Gaviria-Velásquez A, Celis-Mejía DM, Matute-Turizo G. Tumour glómico en pulmón: reporte de un caso y revisión bibliográfica. Cir Cir. 2015;83:319–323.