Abdominal wall actinomycosis is a rare disease associated with the use of intrauterine device and as a complication of abdominal surgery. Diagnosis is difficult because it is unusual and behaves like a malignant neoplasm.
AimA case report is presented of a patient who had used an intrauterine device for 4 years and developed a stony tumour in the abdominal wall associated with a set of symptoms that, clinically and radiologically, was simulating a peritoneal carcinomatosis associated with paraneoplastic syndrome, even in the course of an exploratory laparotomy.
Clinical caseThe patient attended our hospital with a 2-month history of abdominal pain and symptoms that mimic a paraneoplastic syndrome. The diagnosis of abdominal actinomycosis was suspected by the finding of the microorganism in cervical cytology together with other cultures and Actinomyces negative in pathological studies, confirming the suspicion of a complete cure with empirical treatment with penicillin.
ConclusionsActinomycosis should be considered in patients with pelvic mass or abdominal wall mass that mimics a malignancy. Antibiotic therapy is the first treatment choice and makes a more invasive surgical management unnecessary.
La actinomicosis de pared abdominal es un cuadro clínico poco frecuente, asociado al uso de dispositivo intrauterino, o como complicación de cirugía abdominal. Su diagnóstico es difícil por ser poco habitual y comportarse como una neoplasia maligna.
ObjetivosPresentamos el caso de una paciente portadora de DIU desde hacía cuatro años que presentaba un tumor pétreo en pared abdominal asociada a un conjunto de síntomas que, clínica y radiológicamente, simulaba una carcinomatosis peritoneal asociada a síndrome paraneoplásico, incluso en el curso de una laparotomía exploradora.
Caso clínicoLa paciente acudió a nuestro hospital con un cuadro de dos meses de evolución con dolor abdominal y síntomas que simulaban un síndrome paraneoplásico. El diagnóstico de sospecha se realizó por el hallazgo del microorganismo en una citología cervical con el resto de cultivos y estudios anatomopatológicos negativos para Actinomyces, confirmándose por la curación completa con el tratamiento empírico con penicilina.
ConclusionesLa actinomicosis debe ser sospechada en pacientes con tumores pélvicos o de pared abdominal que simulan procesos malignos. El tratamiento antibiótico es el de elección y hace innecesario el manejo quirúrgico más agresivo.
Infection by Actinomyces is a slow progression chronic bacterial disease caused by Gram-positive, anaerobic, non-spore-forming germs typically colonising the mouth, colon and vagina.1 This infection occurs in immunocompetent patients, with anatomical barriers as disruptive gateway, which slowly allow the access of the commensal bacteria of the mucosa to the deep tissues by adjacency, causing the formation of sole or multiple abscesses surrounded by fibrosis granulation tissue, which makes the surface hard, simulating neoplasm involvement.2 The final diagnosis is reached with proof of sulphur granules in pus or histological sections of a surgical sample.
Actinomycosis has been called “the great mimicker” in clinical practice. There are multiple cases in medical literature of pelvic actinomycosis mimicking malignant neoplasms,3,4 leading to an entirely different management of the disease. The proper treatment is penicillin, with surgical drainage of abscesses in the event of therapeutic failure.2
We present the case of a patient who had an copper intrauterine device (IUD) for 4 years, with a stone tumour in abdominal wall associated to a set of symptoms which, clinically and radiologically, mimicked a peritoneal carcinomatosis associated to paraneoplastic syndrome, even in the course of an exploratory laparotomy.
Clinical caseWe present the case of a patient, 49 years old, admitted to the emergency department at our hospital who had continuous hypogastric pain for a month associated to 12kg weight loss, anorexia, nausea and vomiting, with no rhythm alteration or fever. She mentioned a history of eight voluntary abortions and being a carrier of a copper intrauterine device (IUD) for 4 years, withdrawn 2 months previously during a gynaecological examination.
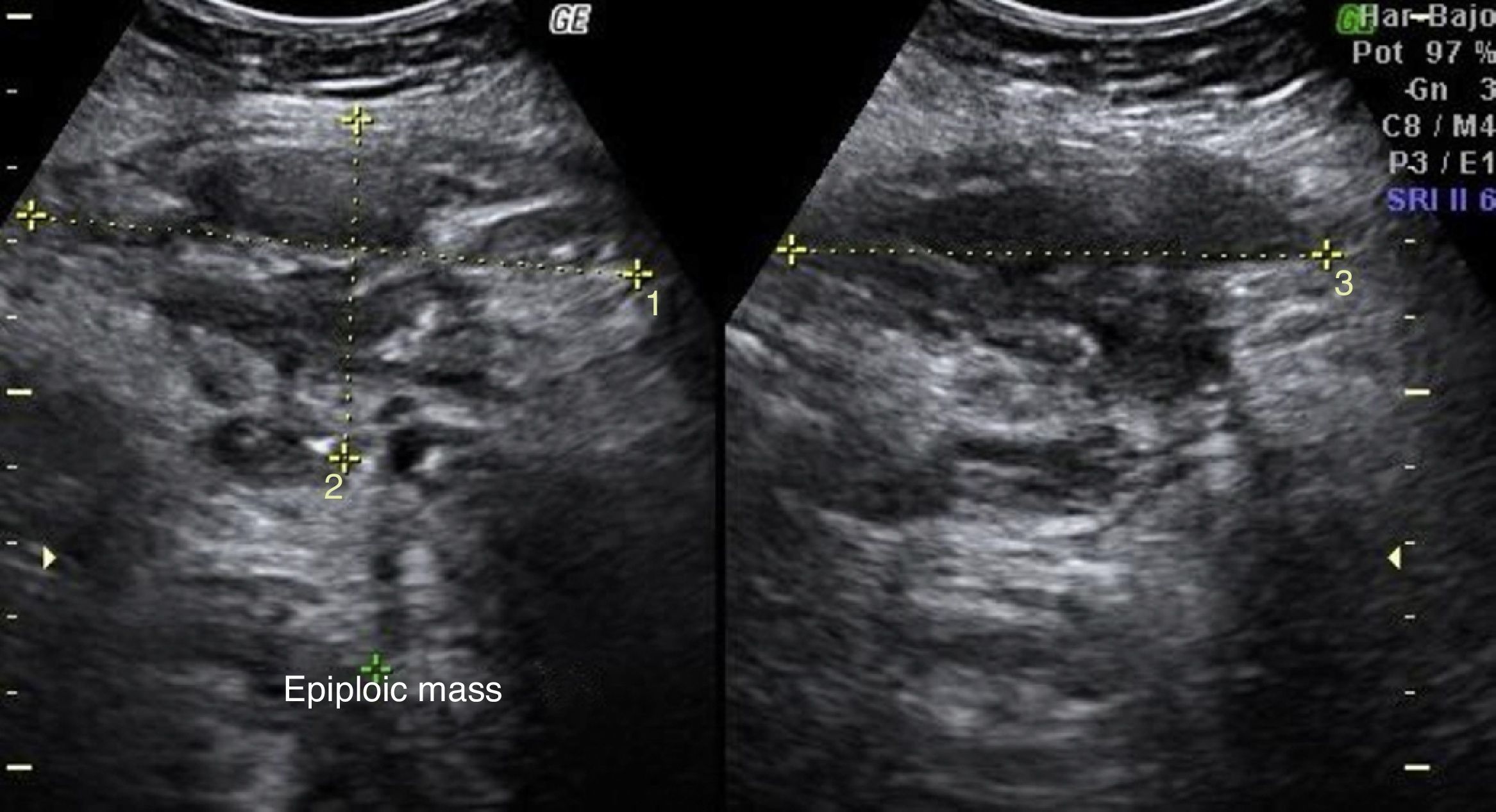
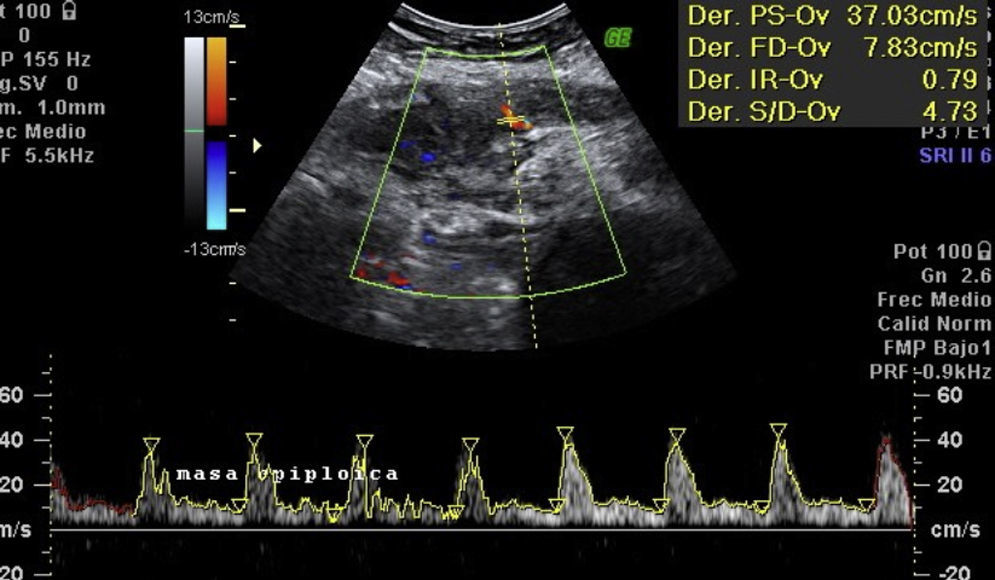
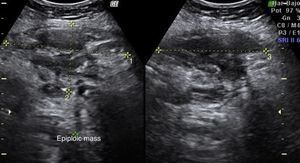
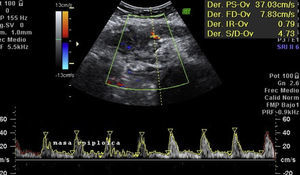
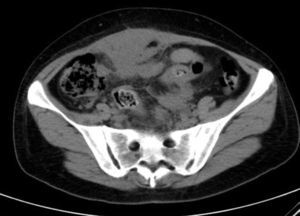
Laboratory results upon admission to the emergency department proved severe anaemia (haemoglobin 8.2g/dl), leukocytes 17.6/mm3 with left shift, platelets 546/mm3, prothrombin time 13.8, prothrombin activity 70, normal biochemistry. Tumour markers Ca 125 and Ca 19.9 are negative. The vaginal and abdominal ultrasound scan reports normal anteverted position of the uterus, poorly delimited, with a 3cm fibroid in the right edge; in the left ovary, heterogeneous and irregular image, solid-cystic, 51mm×43mm×67mm, with large vascularisation and high resistance flows, suggesting inflammatory process; right ovary apparently normal although difficult to evaluate. In the abdominal wall, a tumour is described towards the right iliac fossa, 81mm×45mm×71mm with large vascularisation and characteristics similar to the left adnexal tumour, interpreted as peritoneal carcinomatosis in the context of left ovarian tumour suspected of malignancy. No free fluid in pouch of Douglas (Figs. 1 and 2).
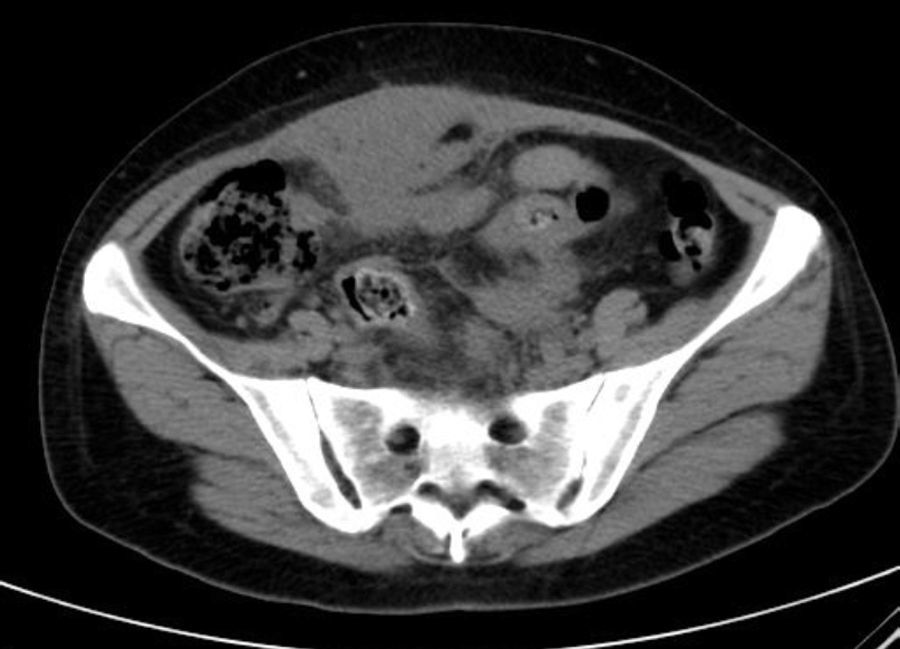
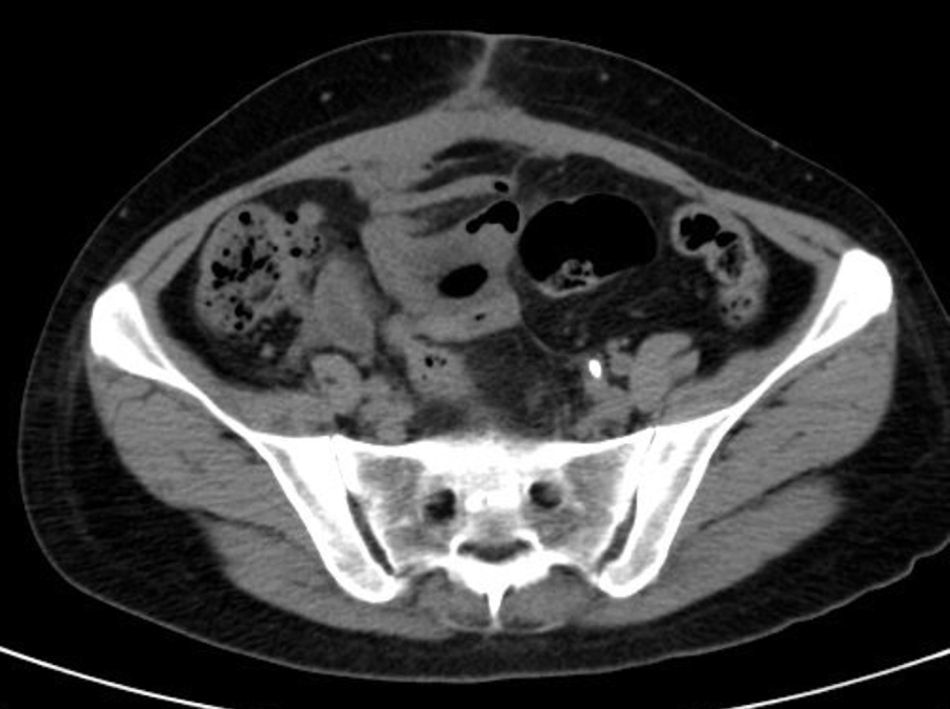
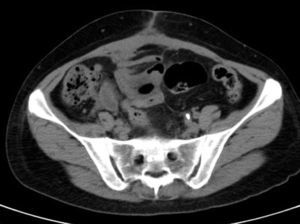
She is admitted for examination with this suspected diagnosis. During examination, fever spikes of up to 38.5°C and very bad condition in general is detected. After 6 days of admission, a computerised axial tomography is performed, reporting extensive density areas, irregular soft parts obliterating fat planes of the pelvic region, including hypodense areas suggesting fluid collection in the left periuterine and periadnexal regions, with involved uterus and adnexal regions; said involvement has multifocal contact with the rectosigmoideal region, with slight associated wall thickening; several areas of loops contiguous to pelvic involvement, with potential secondary involvement, with no significant retrograde distension suggesting obstructive repercussion. Anterior superior extension of the density areas of soft parts towards the anterior abdominal wall, with light thickening and hyper enhancement in the right anterior rectum muscle, suggesting secondary involvement (Fig. 3).
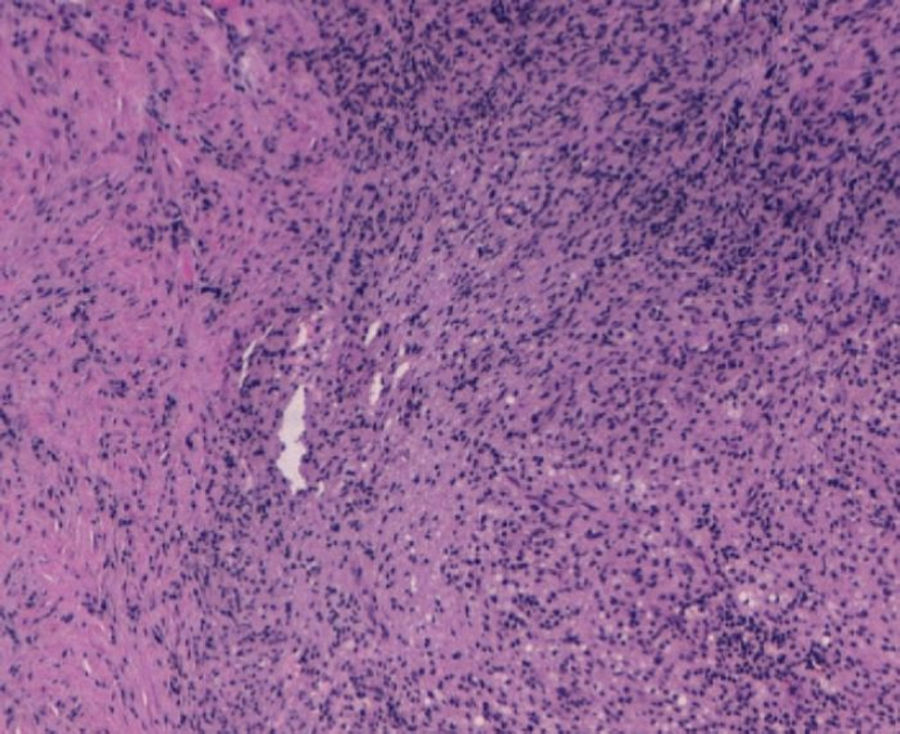
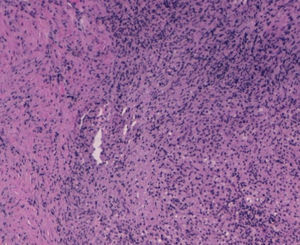
With the suspected diagnosis based on the computed axial tomography of extensive involvement in the pelvic region of an inflammatory-infectious nature, with probable gynaecological dependence, it is decided to perform an exploratory laparotomy. Prior to intervention, catheterisation of the uterus is performed due to compromised ureter due to the inflammation, more evident in the left side, with acute left obstructive nephropathy. During the laparotomy, a tumour of stone consistency approximately 8cm×10cm and 5cm thick is observed, affecting all layers of the abdominal wall, from the periumbilical region to the right iliac fossa, including colon and a loop of small bowel in the peritoneal face. In the left ovary another tumour of approximately 6cm×7cm, with similar characteristics is visualised, intimately in contact with left uterine wall and rectosigmoid. With no other implants in the rest of the peritoneal cavity. Abdominal wall biopsies are taken in the area appearing as infiltrated by the tumour with a result in the intraoperative biopsy indicating “inflammatory” and “malignant cells of mesothelial or small cell origin”. Scarce non-malodorous purulent material is sent to microbiology, obtained from the fascia (Fig. 4).
On the belief that it is a peritoneal carcinomatosis of unknown primary origin, and the great difficulties involved in ressecting the tumour, which infiltrates the entire abdominal wall, it is decided to end the intervention with no hysterectomy or adnexectomy, and gather multiple biopsies.
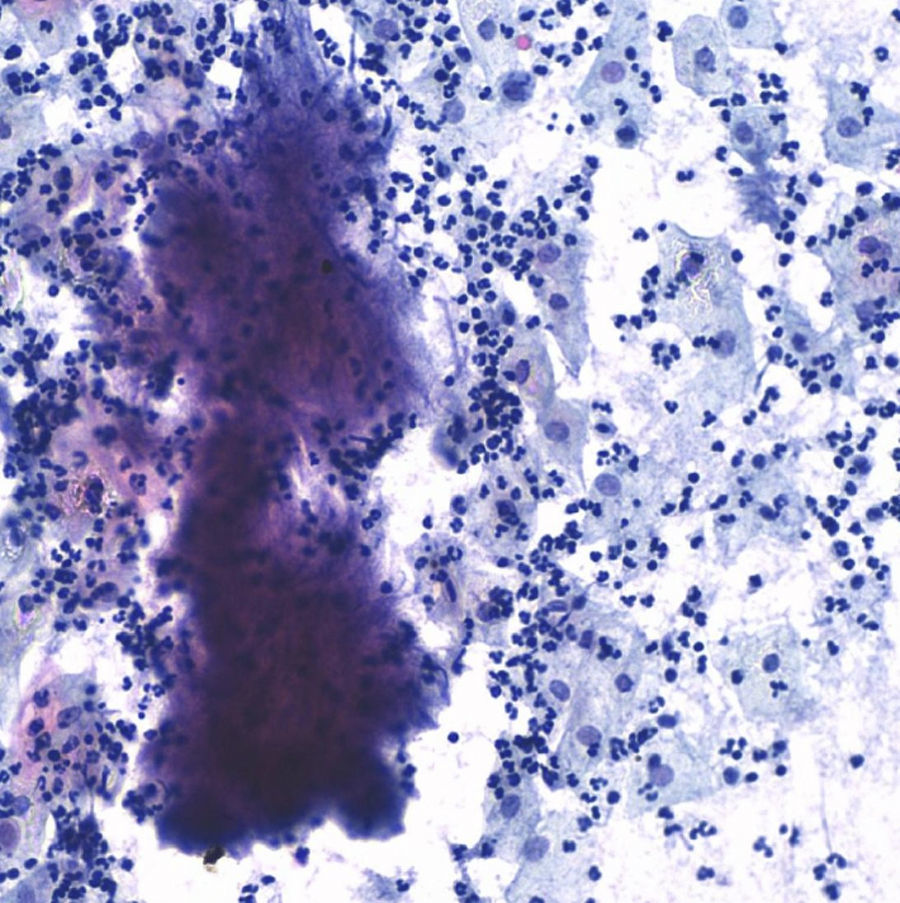
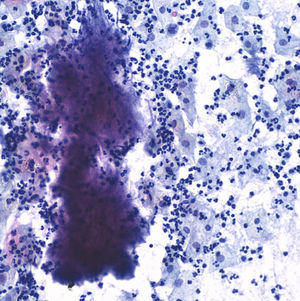
The postoperative stage evolves torpidly, with fever spikes and pseudo-obstruction symptoms, which lead to antibiotic intravenous treatment with amoxicillin–clavulanic acid, which lowered the hyperthermia. Four days after the intervention the result of the cervicovaginal cytology taken 15 days before comes back with a result of “negative of intraepithelial lesion, bacterial flora compatible with Actinomyces” (Fig. 5).
After a review of the bibliography, several cases similar to ours were found in medical literature, with large pelvic or abdominal wall tumours mimicking malignant processes, which were infections caused by Actinomyces. Waiting for the results of the biopsies and cultures taken during the intervention, the antibiotic treatment is replaced with intravenous penicillin G sodium 3,000,000/4h. Evolution from that moment on is better, slow but progressively intestinal peristalsis is recovered, the patient begins to tolerate food, fever and abdominal pain go away, laboratory results improve, and 12 days after the intervention, in the vaginal ultrasound scan the left adnexal tumour was reduced to 4cm, and the abdominal wall to 4cm×7cm, while the computerised axial tomography shows a decrease in the irregular occupation of soft parts in the pelvic region, and regression of symptoms suggesting nephropathy in the left kidney.
The results of 12 biopsies taken are negative for malignancy in their final reading, and are described as “necrosis and acute inflammation with histiocytes” with no findings of Actinomyces. Vaginal cultures, with endometrial aspiration and peritoneal culture also come back negative, finding only slow and scarce growth of Peptostreptococcus species in peritoneal fluid sensitive to penicillin and amoxicillin.
Given the spectacular clinical improvement since the treatment with penicillin began, having ruled out malignant cells in final biopsies and with the only finding of Actinomyces in the cervical–vaginal cytology, the condition is considered a pelvic actinomycosis and it is decided to continue treatment with intravenous penicillin for 1 month, and oral amoxicillin for 6 months. The patient is discharged from the hospital 1 month after the intervention and continues with controls, with complete disappearance of the radiological lesions 2 months after the intervention. Nine months later, the patient remains asymptomatic, with no ultrasound scan evidence of abdominal tumours and negative cervical cytology for Actinomyces (Fig. 6).
DiscussionActinomycosis is a suppurative granulomatous chronic disease caused by a bacterium called Actinomyces, the most frequent being Actinomyces israelii, habitual commensal of the oropharynges, digestive tract and female genitalia. Human beings are the only reservoir for Actinomyces, there is no person-to-person transmission, nor animal-to-person transmission of the agent.5 The establishment of the disease may require the presence of other bacteria acting as co-pathogens,6 as in our case with the finding of Peptostreptococcus in the peritoneal culture.
Traditionally, pelvic actinomycosis was considered as secondary to an intra-abdominal infection, such as appendicitis. Currently the association of actinomycosis with the use of IUD has become the recognised origin of the abdominal-pelvic disease in up to 81% of cases,7 increasing the frequency of the colonisation exponentially upon insertion of the IUD, especially after 4 years.8,9 Approximately 25% of IUDs are colonised by Actinomyces species and out of these, 2–4% develop serious infections eventually.10
An ultrasound scan may be useful in the diagnosis when the infection is advanced and with pelvic abscesses, but there are many times when images can simulate neoplastic processes. A computerised axial tomography in this case has more resolution and can confirm the non-malignant nature of the process, avoiding unnecessary surgeries.11 In our case the clinical and ultrasound scan examination assumed a neoplastic origin, while the computerised axial tomography reported an infectious process.
The Actinomyces culture has several limitations: it requires obtaining pus or tissues that have to be transported in anaerobe culture mean and processed immediately, and may still come back negative in up to 76% of cases.12 Sulphur granules are seen in pus in only 50% of cases13 and the diagnosis is made preoperatively in less than 10% of patients due to the low index of suspicion, its unusual presentation and the difficulty in the culture of Actinomyces.14
Given the intense fibrosis and scarce vascularisation of actinomycotic abscesses, the infection has to be given a prolonged treatment with antibiotics,6 which is why most authors recommend 6–12 months. The antibiotic of choice is penicillin, at 10–20million U/day intravenous doses during 4–6 weeks, followed by penicillin orally with doses of 30mg/kg/day,7,15 or amoxicillin.8 The exact treatment regime must be individualised according to the location of the infection, the severity of the disease, and the response of the patient to the treatment, and clinical and radiological controls are necessary to confirm the resolution of the case.14 Thus, in our case we decided to continue only 6 months due to the spectacular initial improvement of the clinical condition with penicillin, and the absence of radiological lesions 3 months after treatment.
The need to complete the antibiotic treatment with the surgical drainage of abscesses is controverted. Although there are authors who defend the resection of all the affected tissue,15 this requires very aggressive and mutilating surgeries in many cases, with a possibility of very serious complications, which may be avoided if the antibiotic treatment is effective, as in our case. First the possibility of a new intervention for the resection of the abdominal tumour and potential hysterectomy with double adnexectomy was considered, this idea was discarded when full remission of the lesions was proved in imaging tests. In any case, the surgery itself is not curative, which is why the prolonged use of antibiotics is always required.9
ConclusionPelvic and abdominal wall actinomycosis associated to the use of IUD may simulate a neoplastic disease, and it is therefore frequently treated surgically. However, if there is preoperative suspicion of actinomycosis diagnosis, it may be treated satisfactory only with antibiotics.
Conflict of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Rojas Pérez-Ezquerra B, Guardia-Dodorico L, Arribas-Marco T, Ania-Lahuerta A, González Ballano I, Chipana-Salinas M, et al. Actinomicosis de pared abdominal. A propósito de un caso. Cir Cir. 2015; 83: 141–145.