Kawasaki disease (KD) is one of the most common systemic vasculitis in children under five years of age. The epidemiology of the disease is not well established in Mexico. The objective of this study was to describe the epidemiology, clinical features and treatment of patients with KD at the Hospital Infantil de Mexico Federico Gomez (HIMFG).
MethodsWe conducted a retrospective, descriptive and analytical study of patients diagnosed from January 2004 to December 2014 with KD at the HIMFG.
ResultsWe analyzed 204 cases of which 55% were male, with a median age of 32.5 months (6-120) and a rate of hospitalization of 96%. Twenty percent of patients presented incomplete KD. No differences in the somatometric measurements or vitals were reported. The most frequent symptoms were fever, conjunctivitis (89%), oral changes (84%), pharyngitis (88%) and strawberry tongue (83%). We found higher acute phase reactants in the classic presentation. Echocardiographic alterations were observed in 60 patients (29%) who presented ectasia (12%) and coronary aneurysms (11%). On the other hand, 169 (83%) patients received intravenous immunoglobulin (IVIG), 18 (9%) showed resistance to IVIG, 6 (3%) required corticosteroids, and 2 (1%) infliximab; all received acetylsalicylic acid.
ConclusionsThere were no important differences between classic and incomplete presentations. The incidence of cardiac alterations is less than previously reported in Mexico but similar to other countries.
La enfermedad de Kawasaki (EK) es una de las vasculitis sistémicas más comunes en niños menores de 5 años de edad. La epidemiología de la enfermedad no está bien establecida en México. El objetivo de este trabajo fue describir la epidemiología, características clínicas y tratamiento de los pacientes con EK atendidos en el Hospital Infantil de México Federico Gómez (HIMFG).
MétodosSe realizó un estudio retrospectivo, descriptivo y analítico de pacientes diagnosticados con EK en el HIMFG en el periodo comprendido entre enero de 2004 y diciembre de 2014.
ResultadosSe analizaron 204 casos, la mayoría de sexo masculino (55%), con mediana de edad de 32.5 meses (6-120) y una tasa de hospitalización del 96%. El 20% de los pacientes presentó EK incompleto. No se reportaron diferencias en la somatometría ni signos vitales. La sintomatología más frecuente fue fiebre, conjuntivitis (89%), cambios orales (84%), faringitis (88%) y lengua en fresa (83%). Se encontraron reactantes de fase aguda más elevados en las presentaciones clásicas. Se reportaron alteraciones ecocardiográficas en 60 pacientes (29%), de las cuales el 12% fueron ectasia y el 11% aneurismas coronarios. Por otro lado, 169 pacientes (83%) recibieron inmunoglobulina intravenosa (IGIV), 18 (9%) mostraron resistencia a IGIV, 6 (3%) requirieron corticosteroides y 2 (1%) infliximab; todos recibieron ácido acetilsalicílico.
ConclusionesNo se encontraron diferencias importantes entre las presentaciones clásicas e incompletas. La incidencia de alteraciones cardiacas es menor a la reportada previamente en México, pero similar a la de otros países.
Kawasaki disease (KD) is a systemic vasculitis frequently present in children under five years of age.1–3 It is a self-limited disease that affects predominantly medium caliber vessels, particularly the coronary arteries.
More than 45 years have passed since T. Kawasaki reported this condition; however, its etiology remains unknown. Some infectious agents have been suggested as triggers of the disease (Streptococcus, Staphylococcus, Propionibacterium sp., Yersinia sp., Epstein Barr virus, retrovirus, parainfluenza virus, Candida sp. and Lactobacillus sp., among others). However, the current trend is directed to bacterial superantigens related with the overproduction of proinflammatory cytokines, mononuclear cells, cytotoxic antibodies and activated T-cells. Other studies have found associated genetic alterations (ITPKC, CASP3, BLK, CD40), imbalance between helper (Th17) and regulatory T-cells, increase of IgA-producing plasma cells, concentrations of the components of the erythrocyte sedimentation rate (ESR), increased activity of matrix metalloproteinases and the hypothesis of the homeostasis protein system.1,4–7 These theories can explain very significant alterations, such as the presence of erythema at the site of BCG vaccine secondary to a polymorphism in the gene that encodes for the enzyme ITPKC.8
KD diagnosis is based on characteristic signs and symptoms. The classical criteria are a fever that lasts longer than five days, bilateral conjunctival injection, changes in the lips and oral cavity, polymorphic erythema, changes in extremities and non-purulent acute cervical lymphadenopathy. At least five of these symptoms must be present for the diagnosis of KD.9 Some patients do not meet the classic criteria and only present some of the main features. For this reason, diagnosis represents a challenge known as “incomplete KD.”10,11 The typical form of the disease has three phases: the acute phase, which lasts ten days and is characterized by high fever, adenopathies, erythema or peripheral edema, conjunctivitis and enanthema. The subacute phase lasts from 11 to 21 days and is characterized by thrombocytosis, desquamation, and resolution of the fever. Finally, the convalescence phase (from 21 to 60 days) during which clinical signs disappear gradually.12
The low suspicion favors the delay in the diagnosis of KD, which becomes a major problem that increases the risk of complications.13 Among these complications, the development of coronary artery aneurysms (CAA) has been observed in more than 25% of patients who are not treated14 compared with only 5% of those who received intravenous immunoglobulin (IVIG) during the first ten days of evolution.4
KD is almost unique to childhood. Half of the cases occur in children < 2 years of age; 80% in children < 4 years of age; and it is extremely rare in children > 12 years. It is 1.5 times more common in males and although the mortality rate is low, it increases in the first years of life, ranging between 1 and 4%.12,13
The global average incidence is 1-10/100,000 per year.12,13 However, although it has been reported in most ethnic groups, there is an overwhelming variability among different countries.15 Furthermore, it seems to be increasing in an important number of regions.16 Although the relationship between the incidence of KD and urbanization has been mentioned, there are still contradictory results in this regard.17–19
The differences between classical and incomplete presentations of KD and the risk of developing CAA are poorly defined, as well as the epidemiological behavior in Latin America.13 The objective of this study was to describe the clinical evolution of patients with KD at the Hospital Infantil de Mexico Federico Gomez (HIMFG).
2MethodsA retrospective study was conducted, which included patients < 18 years of age with KD (according to the criteria established by Dr. Tomisaku Kawasaki in 1967) who were evaluated by the Rheumatology Service (HIMFG) from January 2004 to December 2014. Data were gathered from the medical records of each patient. Patients with incomplete or illegible records, patients with a history of administration of IVIG 28 days before the onset of KD symptoms, or patients with severe infectious concomitant diseases two weeks before the onset of symptoms or a history of aneurysms or coronary ectasias at KD diagnosis were excluded from the study.
Sociodemographic data, clinical features, treatment, complications, development of CAA and mortality were collected and stored in the Statistical Product for the Social Science (SPSS) version 17.0 program database, which was used for subsequent statistical analysis. These data were represented with measures of central tendency and dispersion, and χ2 test, Fisher's exact test, Mann-Whitney U test and Student's t test were performed to analyze the differences between both types of presentation (classical or incomplete KD). The risk of development of CAA was analyzed as well as the logistic regression to complete the multivariate evaluation.
3ResultsDuring the period of the study, 204 cases were analyzed; 55% of the patients were male. A median age of 32.5 months (6-120 months), the rate of hospitalization of 96% and four days of stay (0-15 days) were found. Twenty percent of the patients presented incomplete KD. Patients had a mean of 1.5 prior medical consultations (0-6 consultations), and 76.5% received antibiotics during their evolution, mainly due to an airway infection diagnosis (67%). Significant differences between patients with the classical and incomplete presentations of KD, such as age (27 vs. 38 months, p=0.001), identification of KD on admission (100 vs. 90.5%, p=0.002) and the cause for the use of an antibiotic (p=0.003) were observed.
Normal weight- and height-for-age were reported in the majority of patients (12.9kg [6.7-42] and 91cm [69-156], respectively), while vital signs present were fever (39°C [37.8-41°C]), tachycardia (102/min [80-125/min]) and polypnea (22/min [18-45/min]). No significant differences in any vital signs or somatometry parameters between the classical and incomplete presentation of KD were observed.
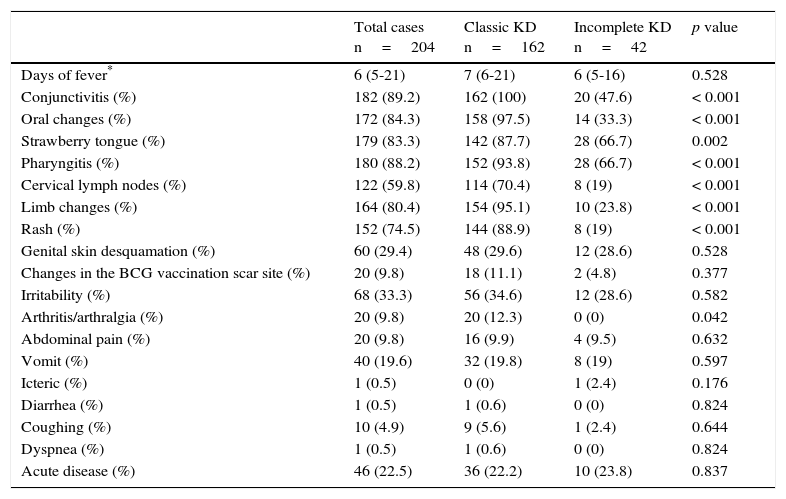
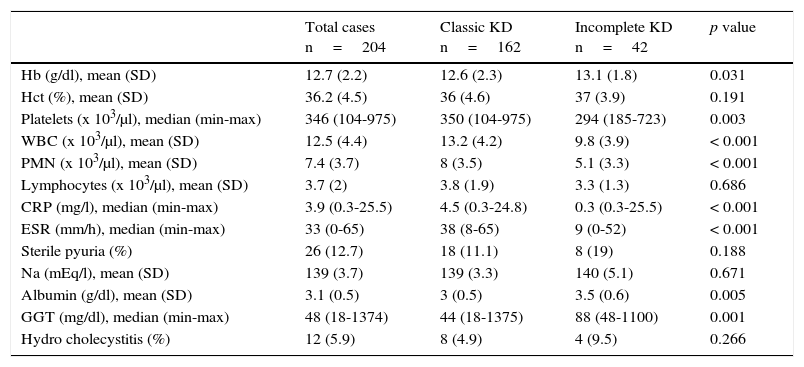
The most frequently observed signs and symptoms were fever, conjunctivitis (89.2%), oral changes (84.3%), pharyngitis (88.2%) and strawberry tongue (83.3%). Other less frequent findings, such as erythema and induration at the BCG vaccine scar site, were present in 9.8% of patients. The full description of these findings and the differences between the types of presentation are shown in Table 1. The most relevant laboratory results were the increase of white blood cell count and polymorphonuclear leukocyte count, and higher C-reactive protein (CRP) levels and erythrocyte sedimentation rate (ESR) (Table 2).
Clinical features of patients on admission.
| Total cases n=204 | Classic KD n=162 | Incomplete KD n=42 | p value | |
|---|---|---|---|---|
| Days of fever* | 6 (5-21) | 7 (6-21) | 6 (5-16) | 0.528 |
| Conjunctivitis (%) | 182 (89.2) | 162 (100) | 20 (47.6) | < 0.001 |
| Oral changes (%) | 172 (84.3) | 158 (97.5) | 14 (33.3) | < 0.001 |
| Strawberry tongue (%) | 179 (83.3) | 142 (87.7) | 28 (66.7) | 0.002 |
| Pharyngitis (%) | 180 (88.2) | 152 (93.8) | 28 (66.7) | < 0.001 |
| Cervical lymph nodes (%) | 122 (59.8) | 114 (70.4) | 8 (19) | < 0.001 |
| Limb changes (%) | 164 (80.4) | 154 (95.1) | 10 (23.8) | < 0.001 |
| Rash (%) | 152 (74.5) | 144 (88.9) | 8 (19) | < 0.001 |
| Genital skin desquamation (%) | 60 (29.4) | 48 (29.6) | 12 (28.6) | 0.528 |
| Changes in the BCG vaccination scar site (%) | 20 (9.8) | 18 (11.1) | 2 (4.8) | 0.377 |
| Irritability (%) | 68 (33.3) | 56 (34.6) | 12 (28.6) | 0.582 |
| Arthritis/arthralgia (%) | 20 (9.8) | 20 (12.3) | 0 (0) | 0.042 |
| Abdominal pain (%) | 20 (9.8) | 16 (9.9) | 4 (9.5) | 0.632 |
| Vomit (%) | 40 (19.6) | 32 (19.8) | 8 (19) | 0.597 |
| Icteric (%) | 1 (0.5) | 0 (0) | 1 (2.4) | 0.176 |
| Diarrhea (%) | 1 (0.5) | 1 (0.6) | 0 (0) | 0.824 |
| Coughing (%) | 10 (4.9) | 9 (5.6) | 1 (2.4) | 0.644 |
| Dyspnea (%) | 1 (0.5) | 1 (0.6) | 0 (0) | 0.824 |
| Acute disease (%) | 46 (22.5) | 36 (22.2) | 10 (23.8) | 0.837 |
Laboratory results of patients on admission.
| Total cases n=204 | Classic KD n=162 | Incomplete KD n=42 | p value | |
|---|---|---|---|---|
| Hb (g/dl), mean (SD) | 12.7 (2.2) | 12.6 (2.3) | 13.1 (1.8) | 0.031 |
| Hct (%), mean (SD) | 36.2 (4.5) | 36 (4.6) | 37 (3.9) | 0.191 |
| Platelets (x 103/μl), median (min-max) | 346 (104-975) | 350 (104-975) | 294 (185-723) | 0.003 |
| WBC (x 103/μl), mean (SD) | 12.5 (4.4) | 13.2 (4.2) | 9.8 (3.9) | < 0.001 |
| PMN (x 103/μl), mean (SD) | 7.4 (3.7) | 8 (3.5) | 5.1 (3.3) | < 0.001 |
| Lymphocytes (x 103/μl), mean (SD) | 3.7 (2) | 3.8 (1.9) | 3.3 (1.3) | 0.686 |
| CRP (mg/l), median (min-max) | 3.9 (0.3-25.5) | 4.5 (0.3-24.8) | 0.3 (0.3-25.5) | < 0.001 |
| ESR (mm/h), median (min-max) | 33 (0-65) | 38 (8-65) | 9 (0-52) | < 0.001 |
| Sterile pyuria (%) | 26 (12.7) | 18 (11.1) | 8 (19) | 0.188 |
| Na (mEq/l), mean (SD) | 139 (3.7) | 139 (3.3) | 140 (5.1) | 0.671 |
| Albumin (g/dl), mean (SD) | 3.1 (0.5) | 3 (0.5) | 3.5 (0.6) | 0.005 |
| GGT (mg/dl), median (min-max) | 48 (18-1374) | 44 (18-1375) | 88 (48-1100) | 0.001 |
| Hydro cholecystitis (%) | 12 (5.9) | 8 (4.9) | 4 (9.5) | 0.266 |
KD, Kawasaki disease; SD, standard deviation; Hb, hemoglobin; Hct, hematocrit; WBC, white blood cell count; PMN, polymorphonuclear leukocytes; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GGT, gamma-glutamyl transpeptidase.
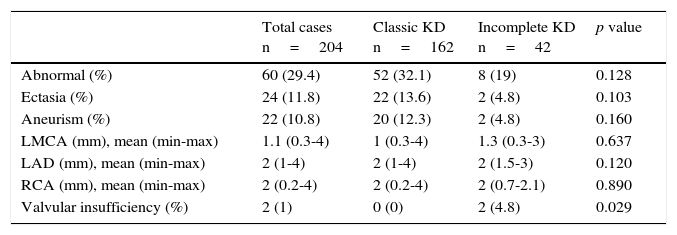
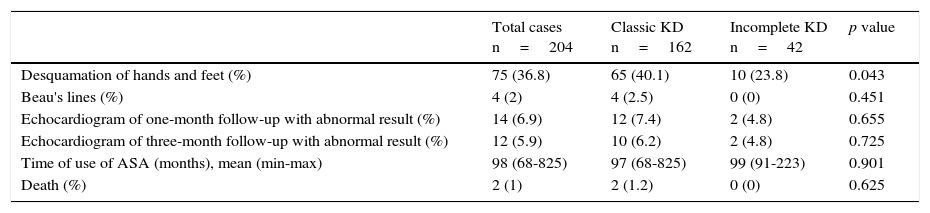
Echocardiographic alterations were found in 60 patients (29.4%): ectasia (11.8%) and coronary aneurysms (10.8%) (Table 3). On the other hand, 169 patients (82.8%) received intravenous immunoglobulin (IVIG) in a single dose (2g/kg); 18 patients with the incomplete KD (8.8%) showed more resistance to the treatment with IVIG (6.2 vs. 19%, p=0.027). Six patients (2.9%) required corticosteroids and two patients (1%) infliximab, with no significant differences between both groups. None of the patients developed any adverse event by IVIG. All patients received 80mg/kg/d of acetylsalicylic acid (ASA) until the fever was controlled. Subsequently, ASA was diminished to an antiplatelet dose (3-5mg/kg/d). During the follow-up, one-third of the patients presented skin desquamation of the limbs. Also, a low incidence of Beau's lines and a considerable improvement of coronary alterations were observed. Two patients died (Table 4).
Echocardiographic findings of patients on admission.
| Total cases n=204 | Classic KD n=162 | Incomplete KD n=42 | p value | |
|---|---|---|---|---|
| Abnormal (%) | 60 (29.4) | 52 (32.1) | 8 (19) | 0.128 |
| Ectasia (%) | 24 (11.8) | 22 (13.6) | 2 (4.8) | 0.103 |
| Aneurism (%) | 22 (10.8) | 20 (12.3) | 2 (4.8) | 0.160 |
| LMCA (mm), mean (min-max) | 1.1 (0.3-4) | 1 (0.3-4) | 1.3 (0.3-3) | 0.637 |
| LAD (mm), mean (min-max) | 2 (1-4) | 2 (1-4) | 2 (1.5-3) | 0.120 |
| RCA (mm), mean (min-max) | 2 (0.2-4) | 2 (0.2-4) | 2 (0.7-2.1) | 0.890 |
| Valvular insufficiency (%) | 2 (1) | 0 (0) | 2 (4.8) | 0.029 |
Only one patient with incomplete KD presented pericardial effusion.
KD, Kawasaki disease; LMCA, left main coronary artery; LAD, left anterior descending artery; RCA, right coronary artery.
Patients follow-up.
| Total cases n=204 | Classic KD n=162 | Incomplete KD n=42 | p value | |
|---|---|---|---|---|
| Desquamation of hands and feet (%) | 75 (36.8) | 65 (40.1) | 10 (23.8) | 0.043 |
| Beau's lines (%) | 4 (2) | 4 (2.5) | 0 (0) | 0.451 |
| Echocardiogram of one-month follow-up with abnormal result (%) | 14 (6.9) | 12 (7.4) | 2 (4.8) | 0.655 |
| Echocardiogram of three-month follow-up with abnormal result (%) | 12 (5.9) | 10 (6.2) | 2 (4.8) | 0.725 |
| Time of use of ASA (months), mean (min-max) | 98 (68-825) | 97 (68-825) | 99 (91-223) | 0.901 |
| Death (%) | 2 (1) | 2 (1.2) | 0 (0) | 0.625 |
KD, Kawasaki disease; ASA, acetylsalicylic acid.
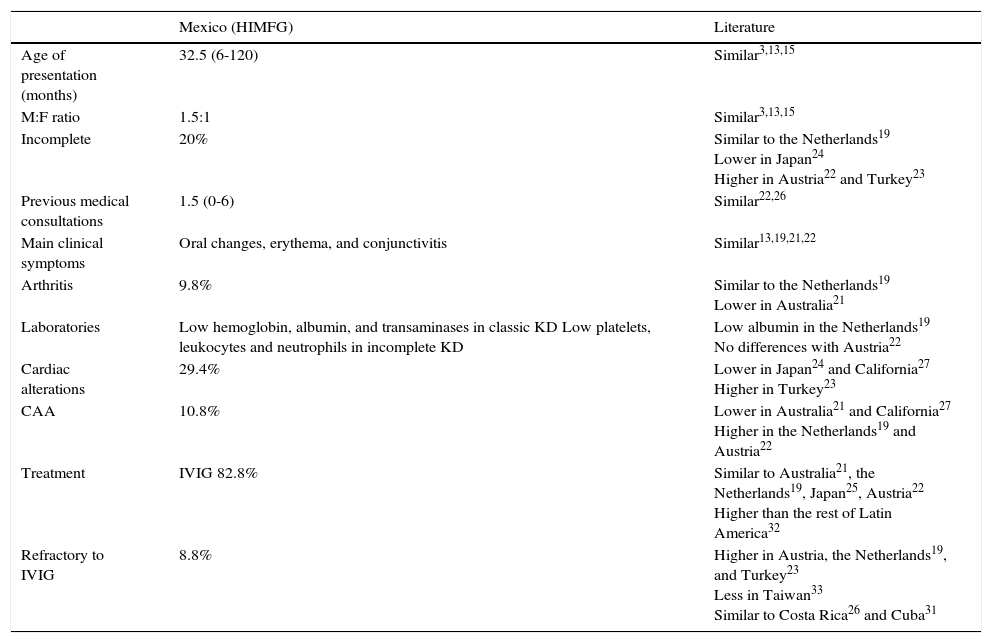
KD is one of the most frequent rheumatologic pathologies in the pediatric age. However, there is little information about this disease. This investigation constitutes the largest series in a pediatric hospital in Mexico with the aim of describing the main characteristics of KD. This information is intended to contribute to a better understanding of KD in Latin America. General demographic data such as age and gender are similar to those reported elsewhere.3,13,15 In Taiwan,20 the male/female ratio is 1.5 to 1.7, whereas in Australia21 and the Netherlands19 is 1.5. These data are slightly different from the 1.2 ratio observed in this study, which was similar to those in Austria,22 Turkey,23 and Japan.24 The age of onset of the patients was the same as in Turkey,23 Japan,24 the Netherlands,19 and Austria,22 but almost one year younger than in Australia,21 where a significant part of the population was of Aboriginal origin, which could explain this difference.
The incomplete form of presentation in this study (20.5%) was similar to that reported in the literature.3,13,15,25 However, differences with some studies from Australia (9.6%),21 Austria (25%),22 Turkey (36.7%)23 and Japan (1.8%),24 were observed, which could be related to ethnic characteristics of each population. In the Netherlands, a similar percentage was reported (22.3%),19 with a greater proportion of children under one year of age than those patients who presented the classical form, as in this report. These data show important regional differences, with a greater variability when compared with Asian populations.
Interestingly, a large number of consultations preceded the diagnosis of KD. Also, three-fourths of the patients received antibiotic therapy, primarily by the presence of upper airway infections. These data reflect the low suspicion index and an inappropriate use of antibiotics. However, although this phenomenon has not been reported consistently, it is similar to that reported by Binder et al. In Austria, in a 10-year series, they found that 78% of the patients received antibiotics before the diagnosis of KD.22 This management was more evident in patients with incomplete KD in both populations. This finding has been scarcely investigated in Latin American countries, although the use of antibiotics in 72% of the patients before establishing the diagnosis of KD has been reported in Costa Rica.26
Interestingly, KD was not considered as diagnostic reference in 43% of the patients, although this fact already had been reported in the literature, where a hidden disease in patients with an unknown origin fever was mentioned.22 This can be explained because, in general, pediatricians are not familiar with rheumatologic diseases.12
Clinical findings in this series agree with KD classic criteria reported in the literature.13,21 For example, changes in the oral mucosa and skin rash were the most frequent symptoms in Australia and the Netherlands, while lymphadenopathy was the least common.19,21 In addition to the skin rash, others studies have shown conjunctivitis as one of the most prevalent signs,22 which was also found in a large number of the patients in this study.
When the main clinical features between classic and incomplete KD were compared, significant differences were found in most of the symptoms (except the fever), which is explained by the definition of “incomplete.” Thus, most of these manifestations are observed in the classic presentation. However, the rest of the findings (common or atypical) were similar between the two groups, except for arthritis that was more common in classic KD. Although these differences have been scarcely reported, they agree with those described by Tacke et al., who found 10% of prevalence of artritis19 in contrast with the 25% reported in Australia.21 Regarding laboratory tests, significant differences were found only in the concentrations of albumin, which were lower in classic KD19, while no differences were identified by Binder et al. in Austria.22 In contrast, the results of this study showed lower concentrations of hemoglobin, albumin, and transaminases in the classic presentation, and a lower count of platelets, leukocytes, and neutrophils in incomplete KD. Interestingly, those patients with classic KD presented an increase in the acute phase reactants, which differs from other series.19,22
In this study, a higher incidence of echocardiographic alterations (29.4%) in comparison with those commonly reported in the literature were found: from 3.3% in Japan24 to 26.5% in Turkey.23 However, the presence of ectasia was similar to that described for Caucasian populations.16,21 On the other hand, 10.8% of the patients developed CAA, a greater frequency than that reported in Australia (6.8%)21 and less than the reported in the Netherlands (13.5%)19 and Austria (18%).22 In California, 19% of cardiac alterations and 5% of CAA27 were reported, while a recent report in Cleveland found CAA in 9.5% of the cases.28 A report of 214 patients with KD where 58% of the patients presented cardiac alterations (dilatation, ectasia, or CAA) was performed in Mexico,29 while Sotelo-Cruz conducted a review of small series and case reports, and reported the expansion of the coronary arteries in 32%.13 Both investigations showed a higher incidence of heart abnormalities than those found in this study and other publications. In Chile, 53% of patients with echocardiographic alterations were found, from which 9.4% were CAA30, while in one Cuban health facility 42.8% were reported,31 although both studies had fewer patients that the present study.
Most of the children treated in this series received IVIG during the acute phase. The reason why some patients were not given IVIG was that they came after the tenth day of evolution or because they had no disease activity data at the time of the evaluation. The use of IVIG was similar to that reported in Australia since 199021, and that registered in the Netherlands from 2008 to 2012.19 In contrast, 46% was reported in Latin America.32
On the other hand, the number of cases with IVIG resistance (8.8%) was lower than that reported in other health facilities.12,21,25 In Chile, 12.5% of the patients required two doses of IVGI,30 while in Cuba, 43%.31 In Australia, 0.03% of the cases received steroids, and 16% required a second dose of IVIG. In the Netherlands, 23.1% were treated again with IVIG and 5.5% with steroids; two patients were treated with infliximab, and one with anakinra, reporting 2.2% of cases with adverse effects to IVIG.19 Finally, in Turkey, 11.6% of the patients were refractory to IVIG.23 All these frequencies were higher than those found in this study. In Taiwan, 6.6% of CAA and 1.5% of refractoriness were reported. Furthermore, an analysis regarding the type of immunoglobulin manufacturing processes was conducted, and it was shown that the β-propiolactonation process was the most associated to treatment failure (RR 1.45).33
ASA was used in every patient initially at anti-inflammatory doses, following classical KD management recommendations, without any adverse events related to this drug. However, recent recommendations suggest an antiplatelet dose since the onset of the disease.4,34 No increase in the mortality rate was found concerning other series.12,21
In conclusion, data from this population were similar to those reported in the literature regarding age, sex, and frequency of presentation of incomplete KD. An important variability was found in comparison with Asian populations. Similar to other countries, there is a large number of patients referred with other diagnoses and treated with antibiotics before establishing KD diagnosis, which demonstrates a low suspicion of KD and, moreover, the need to extend the latter.
The most frequent clinical features were similar to those reported in the literature. However, laboratory results vary slightly from those found in other series, such as hemoglobin, albumin, and transaminase levels and leukocyte, neutrophil, and platelet count. The incidence of cardiac alterations was similar to those described in Caucasian populations and less than previous studies in Mexico. In contrast, the treatment was appropriate, with less refractory cases than other series but the same mortality as in other countries (Table 5).
Main differences between the studied patients and literature reports.
| Mexico (HIMFG) | Literature | |
|---|---|---|
| Age of presentation (months) | 32.5 (6-120) | Similar3,13,15 |
| M:F ratio | 1.5:1 | Similar3,13,15 |
| Incomplete | 20% | Similar to the Netherlands19 Lower in Japan24 Higher in Austria22 and Turkey23 |
| Previous medical consultations | 1.5 (0-6) | Similar22,26 |
| Main clinical symptoms | Oral changes, erythema, and conjunctivitis | Similar13,19,21,22 |
| Arthritis | 9.8% | Similar to the Netherlands19 Lower in Australia21 |
| Laboratories | Low hemoglobin, albumin, and transaminases in classic KD Low platelets, leukocytes and neutrophils in incomplete KD | Low albumin in the Netherlands19 No differences with Austria22 |
| Cardiac alterations | 29.4% | Lower in Japan24 and California27 Higher in Turkey23 |
| CAA | 10.8% | Lower in Australia21 and California27 Higher in the Netherlands19 and Austria22 |
| Treatment | IVIG 82.8% | Similar to Australia21, the Netherlands19, Japan25, Austria22 Higher than the rest of Latin America32 |
| Refractory to IVIG | 8.8% | Higher in Austria, the Netherlands19, and Turkey23 Less in Taiwan33 Similar to Costa Rica26 and Cuba31 |
HIMFG, Hospital Infantil de México Federico Gómez; M, male; F, female; CAA, coronary artery aneurysm; IVIG, intravenous immunoglobulin.
The present review has the limitation of the retrospective design, although it gathered the largest number of patients of the different series reported in Mexico until this day. However, it offers remarkable demographic features of the Mexican population. Clinical characteristics, treatment, and prognosis of these patients allow noting that the variability of KD implies the need for more studies to establish the foundations for the development of clinical guides supported with the experience observed in our field.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNone.
Conflict of interestThe authors declare no conflict of interests of any nature.
We would like to thank the administrative staff, nurses and pediatric residents who contributed to the attention of the patients included in this study.
Please cite this article as: García Rodríguez F, Flores Pineda ÁJ, Villarreal Treviño AV, Salinas Encinas DR, Lara Herrera PB, Maldonado Velázquez MR, et al. Enfermedad de Kawasaki en un hospital pediátrico en México. Bol Med Hosp Infant Mex. 2016;73:166–173.