Histamine is a chemical mediator, released predominantly by tissue mast cells, circulating basophils, and neurons, which are activated in response to various immunological and non-immunological stimuli. Histamine has to bind to specific receptors to exert its physiological and pathophysiological functions. Endogenous histamine is the main mediator of the immediate allergic response, which moreover, performs other multiple functions, including regulation of gastric secretion, neurotransmission in the central nervous system, and immunomodulatory activity. The involvement of histamine in various disorders and the importance of receptors in the clinical features have relevant implications in clinical practice. Anti-H1 antihistamines contrast the histamine-dependent effects, mainly concerning nasal symptoms and cutaneous itching and wheal. Antihistamines are among the most prescribed drugs in pediatric care. This review updates the practical use of antihistamines in children and adolescents.
Histamine is an important mediator of inflammation and is largely released by tissue mast cells, circulating basophils, and neurons as a result of both immunological and non-immunological stimuli, such as allergenic, inflammatory, toxic, chemical, and iatrogenic agents.
Discovered in the early 1900s by Sir Henry Dale, histamine is produced in the cytoplasm from the decarboxylation of histidine via the enzyme histidine decarboxylase; it has a short-term action (1–10min) and is rapidly degraded to imidazole acetic acid. Endogenous histamine is the main mediator of the immediate allergic response, participates in the regulation of gastric secretion (through the action of H2 receptors), and has immunomodulating activity.1 Moreover, histamine plays an important role as a neurotransmitter in the central nervous system (CNS), where it is catabolized by the enzyme histamine N-methyltransferase to tele-methylhistamine.2
Histamine performs its actions by binding to specific receptors on the membrane of various cells, such as mast cells, endothelial cells of the vessels, cells of sensitive nerve fibers, and bronchial smooth muscles, resulting in different effects depending on the site and type of receptor with which it interacts. To date, four types of histamine receptors have been discovered: H1, H2, H3, and H4, which belong to the superfamily of G protein-coupled receptors (GPCRs).3 The receptor activation causes various biological effects, including vasodilation, increased vascular permeability, itchiness, smooth muscle contraction, spasm of coronary arteries, and regulation of the sleep–wake rhythm.4 The current review will focus exclusively on H1 receptor and anti-H1 antihistamines.
The H1 receptor for histamine is in a dynamic equilibrium between two isoforms: active and passive. However, this receptor shows spontaneous basal activity via the nuclear factor κB (NF-κB).
NF-κB is a transcription factor involved in inflammation that has obtained a great interest as a drug target for the treatment of various allergic conditions. Histamine H1 receptor activates NF-κB in both a constitutive and agonist-dependent manner.5
Anti-H1 antihistamines cause an imbalance in favor of the isoform characterized by inactivity; they behave, in practice, as inverse agonists. An inverse agonist is a ligand that binds to the same receptor-binding site as an agonist and not only antagonizes the effects of an agonist but, moreover, exerts the opposite effect by suppressing spontaneous receptor signaling.6 As a result, they are capable of shifting the receptor balance from the biochemically active form to an inactive form. In this way, they also reduce the activation of NF-κB and thereby may reduce the synthesis of pro-inflammatory cytokines, cell adhesion molecules, and chemotactic factors.1 Antihistamines are, therefore, a cornerstone in the treatment of histamine-mediated diseases.7
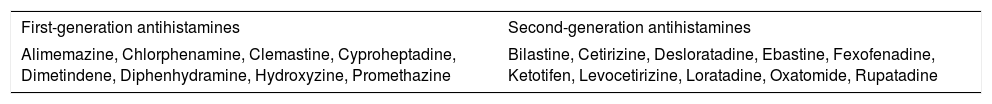
Classification and pharmacological properties of anti-H1 antihistaminesAnti-H1 antihistamines are functionally classified into two groups, namely first- and second-generation molecules (Table 1). First-generation anti-H1 antihistamines (e.g., clemastine, diphenhydramine, promethazine) can be administered by injection, orally, or topically, including dermatological, intranasal, and ocular formulation.7
Most common first- and second-generation oral antihistamines in alphabetic order.
| First-generation antihistamines | Second-generation antihistamines |
|---|---|
| Alimemazine, Chlorphenamine, Clemastine, Cyproheptadine, Dimetindene, Diphenhydramine, Hydroxyzine, Promethazine | Bilastine, Cetirizine, Desloratadine, Ebastine, Fexofenadine, Ketotifen, Levocetirizine, Loratadine, Oxatomide, Rupatadine |
After oral administration, most of them are well absorbed from the gastrointestinal tract; they bind to plasma proteins (70–97%) and are then metabolized by the liver and mostly excreted in the urine within 24h of intake. The therapeutic effect begins to appear within 30–60min, peaks within 1–3h, and usually persists for 4–6h. Some preparations, however, have a more prolonged effect (e.g., chlorpheniramine, hydroxyzine), with a half-life of over 20h in the adult, less time in the child, which metabolizes these drugs more quickly.1 The action mechanism is to bind to the H1 receptor, which in the first-generation molecules represents the prevalent or perhaps the only activity of the drug; it is perfected and expanded in the second-generation molecules.
In addition, for the second-generation molecules, there are different formulations, such as for oral use (e.g., drops, syrup, tablets) and for topical nasal, ocular, and cutaneous use. After oral administration, the plasma peak is earlier with cetirizine (30–60min) and later with loratadine (45–60min), terfenadine (1–2h), fexofenadine (1–3h), astemizole (1–3h), and bilastine (1–4h). The elimination half-life is extremely variable, from 24h for loratadine, desloratadine, cetirizine, and levocetirizine, up to 18 days for astemizole.1,3,4 In children, the cetirizine half-life is reduced due to increased hepatic metabolism; this practice entails the need for a twice-daily administration.8 The duration of the pharmacological effect of all these drugs presents a marked variability, and is much longer than the plasma half-life, being linked to the volume of distribution of the drug as well as to the action of the metabolites that also remain in active form for long periods. The binding of these drugs to plasma proteins is generally high (88–98%). From a clinical point of view, the therapeutic effect is, therefore, prolonged. The inhibition of the cutaneous response to histamine (histamine hives) persists for 12–24h after a single dose of loratadine and cetirizine9; the inhibitory effect on the skin response can endure for 7–10 days depending on the type of molecule taken. For this reason, antihistaminic therapy should be suspended before the skin prick test. Most second-generation antihistamines are metabolized in the liver by the cytochrome P450 system. The intake of some antihistamines, such as terfenadine or astemizole, together with drugs capable of inhibiting this system (e.g., anti-fungal agents such as ketoconazole or macrolide antibiotics) or some foods (e.g., grapefruit, pomelo, raspberry) can cause an abnormal accumulation of these agents and their metabolites in the body, with consequent risk of adverse reaction, even serious, especially at the cardiac level (e.g., tachyarrhythmias, torsade de pointes, or prolongation of the QT interval until ventricular fibrillation). These effects are not the result of the action on the H1 receptor but arise from the direct blockage of the potassium channels that control the cardiac repolarization phase. For this reason, terfenadine and astemizole were withdrawn from clinical use in many countries.
Other molecules, such as loratadine and desloratadine, are instead metabolized by more enzyme systems over the hepatic cytochrome P450 system, thus limiting the potential resultant clinical interactions. It is unlikely that multiple systems will be simultaneously inhibited with consequent accumulation of the molecule.10 Fexofenadine is minimally metabolized by the organism; its main route of elimination is by biliary excretion (approximately 80%), while −10% of the ingested dose is eliminated unchanged in the urine.1 About 60–70% of cetirizine and levocetirizine are eliminated via the urinary tract, with only 10% eliminated hepatically.11 Elimination is mainly fecal for astemizole, and fecal and urinary for loratadine.12 Furthermore, the binding of the new antihistamines with the H1 receptor is more stable and persistent; thus, the administrations can be more delayed over time and, for some molecules such as loratadine, desloratadine, cetirizine, levocetirizine, bilastine, fexofenadine and rupatadine, it is possible to use once-daily administration.3 These characteristics are particularly advantageous in clinical practice, as the reduced frequency of daily drug administrations consequently entails improvement of tolerability and compliance by patients and prolonged activity.11 It should also be noted that new antihistamines hold little or no risk of “pharmacological addiction” (tachyphylaxis) following long-lasting administration.3
Along with the antihistaminic effects, some second-generation drugs (e.g., loratadine, desloratadine, cetirizine, levocetirizine, ebastine, fexofenadine) were believed to have potential anti-inflammatory properties in the following manners: (1) reduction of the production of pro-inflammatory cytokines (e.g., IL-4 and IL-13, chemokines) and the release of both histamine and other pre-formed or neo-formed mediators by activated mastocytes and basophils; (2) reduction of the recruitment of eosinophils in the late phase of allergic reaction or the phase of tissue damage and chronicization of the allergic inflammation; and (3) reduced expression of adhesion molecules on epithelial cells and/or endothelium, thus inhibiting cellular trafficking.12,13 However, the anti-inflammatory activity of antihistamines has probably to be reduced because these effects would seem evident only in vitro at very high concentrations. Therefore, the anti-inflammatory activity of antihistamines administered at therapeutic doses seems to be scarcely relevant in clinical practice.
Side-effectsCurrently, the clinical use of first-generation molecules is markedly limited by some of their pharmacological characteristics. Due to their high lipo-solubility, first-generation anti-H1 antihistamines can easily cross the blood–brain barrier (BBB) and induce sedation, drowsiness, decreased attention and reaction times, which comprise their most common side-effects.14 In this regard, a study about cerebral histamine H1 receptor occupancy (H1RO) using positron emission tomography (PET) has shown that the most penetrating antihistamines in the brain are chlorphenamine, ketotifen and hydroxyzine.15
First-generation anti-H1 antihistamines also exert a poorly selective action on H1 receptors because they can also interact with non-histamine receptors (especially serotonergic, cholinergic, and α-adrenergic receptors). As a consequence, the other main side-effects include dry mouth, nausea, vomiting, diarrhea, constipation, pollakiuria, dysuria, urinary retention, increased appetite, and tachycardia.14 Cyproheptadine and ketotifen can increase appetite and cause weight gain, which does not occur with other antihistamines (except sporadically by astemizole), mainly acting on serotoninergic pathways.16 Because of the unfavorable risk/benefit ratio due to relevant side-effects, first-generation antihistamines, if possible, should no longer be used in the treatment of rhinitis and urticaria.14 As regards the safety of these drugs, warnings have been issued, more recently in 2015, by the European Medicines Agency (EMA) on the use of first-generation anti-H1 for children under two years of age, especially for hydroxyzine. That drug is associated with a low but definite risk of QT tract prolongation and torsade de pointes, conditions that can lead to an abnormal rhythm until cardiac arrest.
For this reason, its use must be limited to the minimum effective dose for the shortest possible duration; it is also imperative to avoid its use in patients who present risk factors for heart rhythm disorders. Regarding the pediatric age, the maximum daily dose of hydroxyzine should not exceed 2mg/kg (maximum 50mg/day) in children weighing less than 40kg.17 Given their low lipo-solubility, the second-generation molecules have a reduced capacity to cross the BBB. These molecules are also able to bind to P-glycoprotein, which acts as a transporter at the BBB and can actively “expel” these molecules from the BBB.
Among the second-generation antihistamines, cetirizine appears to be most frequently associated with a greater incidence of drowsiness in comparison to placebo, although to a lesser extent than that observed with the use of first-generation antihistamines.18 However, this effect is less relevant in comparison with adult patients.
Antihistamine overdose remains a risk, especially in children. Historically, diphenhydramine has been involved in episodes of overdose poisoning (some fatal), especially in children, partly because many preparations are sold without a prescription (over-the-counter products).19 The most serious effects of overdose are attributable to neurological or cardiac alterations; for example, convulsions that are followed (at high dosages) by states of coma, which are sometimes irreversible.14 Some second-generation antihistamines, such as ebastine, or mizolastine can cause a prolongation of the QT tract. Considering this potential risk, particular attention should be paid to the simultaneous administration of other drugs that can also prolong the QT tract, such as macrolides, which are among the classes of drugs most frequently prescribed in pediatric populations.10
Therapeutic indications of anti-H1 antihistaminesThe main indications of anti-H1 antihistamines are allergic manifestations with prevalent exudative and irritative neurogenic features; however, the efficacy of antihistamines varies in different pathologies depending on the prominence of histamine's contribution to the clinical symptomatology and local health agency rules.11,18 Second-generation antihistamines should be preferred since they are endowed with few sedative properties. Treatment with a second-generation anti-H1 antihistamine (e.g., loratadine) in children suffering from allergic rhinitis did not impair scholar performance, unlike treatment with a first-generation molecule (e.g., diphenhydramine).14 Second-generation anti-H1 antihistamines also have the advantage, due to their pharmacological characteristics, of being used not only in the treatment of an acute episode but also in the long-term treatment of allergic diseases.20
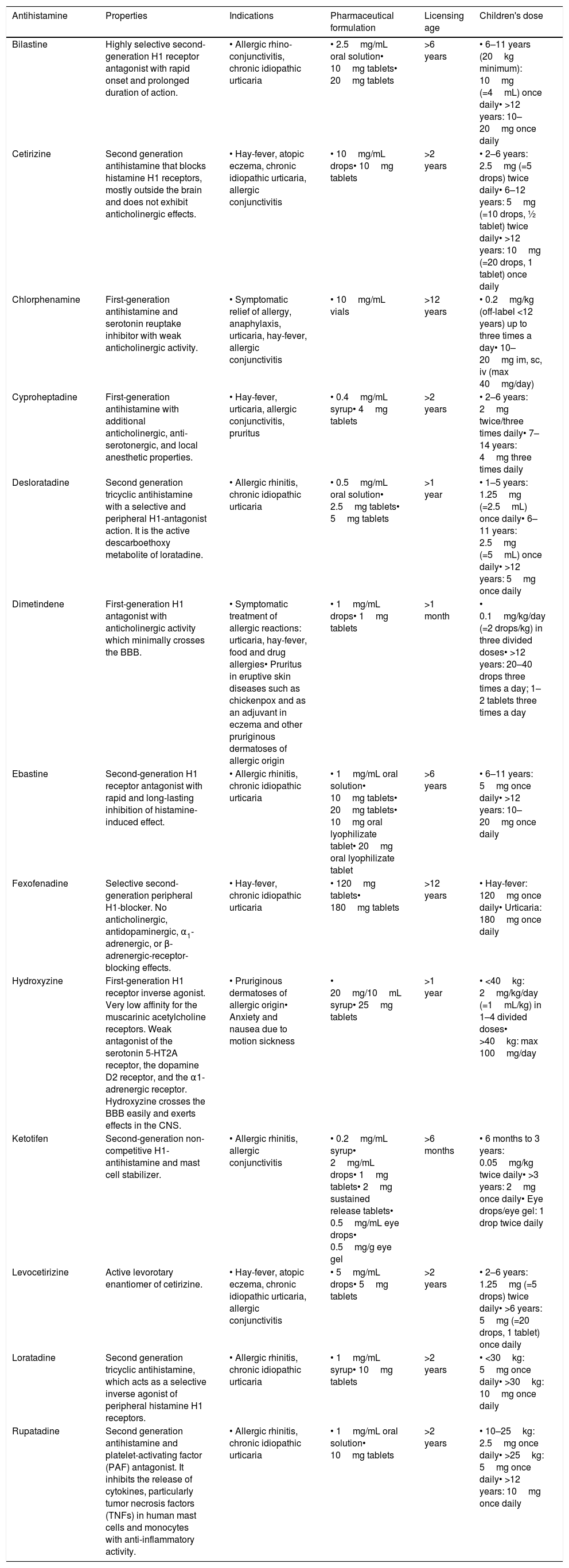
The oral route is the main way of administration, while the parenteral route, which is only possible with some first-generation molecules, is reserved for the prevention or treatment of serious and rare eventualities (e.g., episodes of anaphylaxis, blood transfusions, adverse drug reactions). The topical route is reserved for eyes, nose, or cutaneous disease (eye drops, spray, cream, gel). The topical dermal route, despite having indications of insect bites and pruritic dermatitis, should be used with great caution as it commonly induces photosensitivity.21 A summary of the most common antihistamines is available in Table 2.
Summary of the most common antihistamines in alphabetic order.
| Antihistamine | Properties | Indications | Pharmaceutical formulation | Licensing age | Children's dose |
|---|---|---|---|---|---|
| Bilastine | Highly selective second-generation H1 receptor antagonist with rapid onset and prolonged duration of action. | • Allergic rhino-conjunctivitis, chronic idiopathic urticaria | • 2.5mg/mL oral solution• 10mg tablets• 20mg tablets | >6 years | • 6–11 years (20kg minimum): 10mg (=4mL) once daily• >12 years: 10–20mg once daily |
| Cetirizine | Second generation antihistamine that blocks histamine H1 receptors, mostly outside the brain and does not exhibit anticholinergic effects. | • Hay-fever, atopic eczema, chronic idiopathic urticaria, allergic conjunctivitis | • 10mg/mL drops• 10mg tablets | >2 years | • 2–6 years: 2.5mg (=5 drops) twice daily• 6–12 years: 5mg (=10 drops, ½ tablet) twice daily• >12 years: 10mg (=20 drops, 1 tablet) once daily |
| Chlorphenamine | First-generation antihistamine and serotonin reuptake inhibitor with weak anticholinergic activity. | • Symptomatic relief of allergy, anaphylaxis, urticaria, hay-fever, allergic conjunctivitis | • 10mg/mL vials | >12 years | • 0.2mg/kg (off-label <12 years) up to three times a day• 10–20mg im, sc, iv (max 40mg/day) |
| Cyproheptadine | First-generation antihistamine with additional anticholinergic, anti-serotonergic, and local anesthetic properties. | • Hay-fever, urticaria, allergic conjunctivitis, pruritus | • 0.4mg/mL syrup• 4mg tablets | >2 years | • 2–6 years: 2mg twice/three times daily• 7–14 years: 4mg three times daily |
| Desloratadine | Second generation tricyclic antihistamine with a selective and peripheral H1-antagonist action. It is the active descarboethoxy metabolite of loratadine. | • Allergic rhinitis, chronic idiopathic urticaria | • 0.5mg/mL oral solution• 2.5mg tablets• 5mg tablets | >1 year | • 1–5 years: 1.25mg (=2.5mL) once daily• 6–11 years: 2.5mg (=5mL) once daily• >12 years: 5mg once daily |
| Dimetindene | First-generation H1 antagonist with anticholinergic activity which minimally crosses the BBB. | • Symptomatic treatment of allergic reactions: urticaria, hay-fever, food and drug allergies• Pruritus in eruptive skin diseases such as chickenpox and as an adjuvant in eczema and other pruriginous dermatoses of allergic origin | • 1mg/mL drops• 1mg tablets | >1 month | • 0.1mg/kg/day (=2 drops/kg) in three divided doses• >12 years: 20–40 drops three times a day; 1–2 tablets three times a day |
| Ebastine | Second-generation H1 receptor antagonist with rapid and long-lasting inhibition of histamine-induced effect. | • Allergic rhinitis, chronic idiopathic urticaria | • 1mg/mL oral solution• 10mg tablets• 20mg tablets• 10mg oral lyophilizate tablet• 20mg oral lyophilizate tablet | >6 years | • 6–11 years: 5mg once daily• >12 years: 10–20mg once daily |
| Fexofenadine | Selective second-generation peripheral H1-blocker. No anticholinergic, antidopaminergic, α1-adrenergic, or β-adrenergic-receptor-blocking effects. | • Hay-fever, chronic idiopathic urticaria | • 120mg tablets• 180mg tablets | >12 years | • Hay-fever: 120mg once daily• Urticaria: 180mg once daily |
| Hydroxyzine | First-generation H1 receptor inverse agonist. Very low affinity for the muscarinic acetylcholine receptors. Weak antagonist of the serotonin 5-HT2A receptor, the dopamine D2 receptor, and the α1-adrenergic receptor. Hydroxyzine crosses the BBB easily and exerts effects in the CNS. | • Pruriginous dermatoses of allergic origin• Anxiety and nausea due to motion sickness | • 20mg/10mL syrup• 25mg tablets | >1 year | • <40kg: 2mg/kg/day (=1mL/kg) in 1–4 divided doses• >40kg: max 100mg/day |
| Ketotifen | Second-generation non-competitive H1-antihistamine and mast cell stabilizer. | • Allergic rhinitis, allergic conjunctivitis | • 0.2mg/mL syrup• 2mg/mL drops• 1mg tablets• 2mg sustained release tablets• 0.5mg/mL eye drops• 0.5mg/g eye gel | >6 months | • 6 months to 3 years: 0.05mg/kg twice daily• >3 years: 2mg once daily• Eye drops/eye gel: 1 drop twice daily |
| Levocetirizine | Active levorotary enantiomer of cetirizine. | • Hay-fever, atopic eczema, chronic idiopathic urticaria, allergic conjunctivitis | • 5mg/mL drops• 5mg tablets | >2 years | • 2–6 years: 1.25mg (=5 drops) twice daily• >6 years: 5mg (=20 drops, 1 tablet) once daily |
| Loratadine | Second generation tricyclic antihistamine, which acts as a selective inverse agonist of peripheral histamine H1 receptors. | • Allergic rhinitis, chronic idiopathic urticaria | • 1mg/mL syrup• 10mg tablets | >2 years | • <30kg: 5mg once daily• >30kg: 10mg once daily |
| Rupatadine | Second generation antihistamine and platelet-activating factor (PAF) antagonist. It inhibits the release of cytokines, particularly tumor necrosis factors (TNFs) in human mast cells and monocytes with anti-inflammatory activity. | • Allergic rhinitis, chronic idiopathic urticaria | • 1mg/mL oral solution• 10mg tablets | >2 years | • 10–25kg: 2.5mg once daily• >25kg: 5mg once daily• >12 years: 10mg once daily |
Allergic rhinitis with or without concomitant ocular involvement is a clinical indication for the use of such drugs and for impairment of scholar learning that occurs in affected and untreated children and adolescents.22 Histamine is the mediator released during the early phase of the allergic reaction and causes itching, sneezing, and watery rhinorrhea; the late phase is clinically dominated by the presence of nasal obstruction, which is linked more to inflammatory cell infiltration and the action of other mediators (e.g., prostaglandins, leukotrienes) than to a purely vasodilatory action from histamine. For this reason, allergic inflammation is less favorably affected by the action of antihistamines but more sensitive to corticosteroids.23
As regards antihistaminic therapy, allergic rhinitis and its impact on asthma (ARIA) guidelines recommend using second-generation oral molecules for both intermittent and persistent allergic rhinitis; these molecules represent the most suitable preparations in the treatment of allergic rhinitis. In this regard, it has been reported that some antihistamines, including azelastine, cetirizine, desloratadine, fexofenadine exert a relevant antiallergic activity able to reduce nasal inflammation and consequently nasal obstruction.24–28 Such drugs can be used as needed if symptoms are occasional. In rhinitis from seasonal allergens, treatment with anti-H1 antihistamines could be initiated before allergen exposure and must then be continued for the entire duration of the pollination.29 A close link has been reported between allergic inflammation and nasal airflow in patients with pollen allergy.30 In perennial allergic rhinitis, the treatment should instead be based on clinical symptoms and has the dual purpose of controlling the persistent inflammation by reducing the inflammatory mucosal infiltrate and the expression of adhesion molecules.31
Among antihistamines, bilastine was recently approved for children aged 6–11 years. This license is the consequence of the results of studies included in the bilastine Pediatric Investigation Plan, approved by the EMA Pediatric Committee, for children aged 2–12 years.32
In even younger children, among the newest antihistamines, rupatadine 1mg/mL oral solution has been shown to be safe and effective in a cohort of 44 children with allergic rhinitis.33
Topical preparations (nasal and ocular) have good clinical efficacy and high tolerability, even if they act only at the site of administration.34 The rapid and prolonged action permits only two daily administrations, with the advantage of obtaining high concentrations of the drug at the target organ, diminishing the risk of systemic side-effects. The most common topical antihistamines are astemizole, olopatadine, emedastine, and levocabastine.35
The effect of antihistamines in vasomotor rhinitis and non-allergic rhinitis with eosinophils (NARES) is modest.36,37 In adolescents, the use of topical nasal azelastine is considered the first-line treatment. Moreover, a combination of an intranasal corticosteroid (fluticasone) and azelastine demonstrated good efficacy, there was evidence that this combination was superior to either intranasal corticosteroids or topical intranasal antihistamines alone.38
UrticariaUrticaria is another important indication for the use of antihistamines, which have been proven to have unquestionable efficacy.39 Anti-H1 antihistamines are effective in reducing itching and the number, size, and duration of cutaneous manifestations (e.g., wheals, erythema) in patients with both acute and chronic urticaria. In both cases, the current European guidelines recommend the use of second-generation molecules for their tolerability and safety profile, which allows easy modification of their use and dose over time. Among these, the most tested drugs were: loratadine, desloratadine, fexofenadine, cetirizine, levocetirizine, rupatadine, and bilastine.40 Bilastine was recently tested for safety and tolerability in 6–11-year-children with chronic urticarial showing with great outcomes also in terms of efficacy.41
A double-blind, randomized, parallel-group, multicenter, placebo-controlled study involving 257 children aged 2–11 years with chronic spontaneous urticaria showed that rupatadine is well tolerated and improves quality of life over six weeks.42
In chronic spontaneous urticaria, complex pathogenesis is generally more difficult to treat; in the absence of response at standard doses, it is recommended to gradually increase the dose of second-generation anti-H1 antihistamines (up to four times the standard dose) and to eventually introduce another drug (e.g., omalizumab, ciclosporin).40,43
Atopic dermatitisThe use of anti-H1 antihistamines in the management of atopic dermatitis (AD) remains a controversial topic. The presence of histamine in AD skin lesions has been, in the past, the main rationale for their use. These compounds, however, only partially control skin itching, whose intensity mainly depends on the severity of dermatitis.44 The itching characteristic of dermatitis has a rather complex pathogenesis, which is not only attributable to the release of histamine, but also to the involvement of several other mediators able to evoke pruritus, such as proteases, gastrin-releasing peptide, substance P, and IL-31. It has also been recently shown that the predominant component of pruritus is mediated by PAR-2 receptors present on keratinocytes and other skin cells, and is activated by protease.33 Moreover, complex neural pathways are involved with the profound relationship between neural and mental mechanisms, mainly concerning the cognitive and behavioral aspects of itching.45 As a result, anxiety and sleeplessness are frequently associated with AD.46 As first-generation antihistamines frequently induce sedation, this is the main reason for their popular use in AD management in clinical practice.47 In this regard, minimizing itchiness is an important element in the management of AD to reduce discomfort, improve quality of life, and prevent scratching injuries that may result in impetigo. The release of auto-allergens by keratinocytes following scratching is also believed to contribute to a vicious circle in patients with eczema. Pruritus is substantially controlled with careful skin care and the use of creams with anti-inflammatory activity (e.g., steroids, topical calcineurin inhibitors). The use of anti-H1 antihistamines has, therefore, an adjunctive role in controlling itching.48 The National Institute for Health and Care Excellence (NICE) guidelines for treating atopic eczema suggest a one-month trial of a non-sedating antihistamine in children with severe itching; if successful, this treatment may be continued while symptoms persist, but this regimen should be reviewed every three months.49 Finally, the topical use of antihistamines is not recommended in patients with AD due to the risk of absorption and contact allergy.50
AsthmaEpidemiological, pathophysiological, and clinical evidence demonstrates the existence of a tightly linked relationship between the upper and lower airways, which are often considered a single entity (united airways).51,52 During allergic rhinitis and asthma, the upper and lower airways are affected by a common inflammatory process that can be sustained and amplified by interconnected mechanisms.53 Allergic rhinitis and non-specific vasomotor rhinitis are some of the most important risk factors for the onset of asthma and, subsequently, an important aggravating factor. In this regard, it has been reported that early impairment of lung function may predict the asthma development in AR patients.54 Combined treatment with nasal steroids and antihistamines in children with allergic rhinitis has been shown to significantly improve asthma symptoms. In this context, therapy with anti-H1 antihistamines confers an additional benefit in controlling asthma symptoms in individuals affected by concomitant allergic rhinitis and bronchial asthma.55 However, it has to be outlined that antihistamines have no indication for asthma, but they can be used in AR patients with comorbid asthma.56
AnaphylaxisAdrenaline given by injection is the first-line and life-saving treatment in case of an anaphylactic reaction. As part of the international guidelines for the management of anaphylaxis, the administration of systemic antihistamines by injection is part of emergency interventions in addition to adrenaline and steroids, although some guidelines discourage their use as they reduce vigilance and may have vasodilatory effects (from first-generation antihistamines) when administered in an intravenous bolus. Instead, there is an advantage for antagonists of H2-receptor as they contrast histamine-dependent vasodilation, but they should be administered per parenteral route.57
Oral antihistamines, on the other hand, can control certain symptoms (e.g., rhinitis, urticaria) once the acute phase of anaphylaxis has passed. In this context, a greater role can have molecules endowed with the ability to antagonize the PAF (e.g., rupatadine), in consideration of the role played by this molecule in anaphylaxis.58
Other diseasesSome clinical trials support the use of anti-H1 antihistamines for the control of pruritus and skin lesions in mastocytosis,59 in contact dermatitis, in case of allergic reactions to insect bites, and an allergy to the venom of Hymenoptera.60 These drugs are also used for the control of pruritus during varicella.61 No efficacy is demonstrated by the preventive use of antihistamines in recurrent respiratory infections and the therapeutic management of the common cold in non-atopic subjects.62 Finally, some first-generation anti-H1 antihistamines (diphenhydramine, doxepin, doxylamine, pyrilamine) are used at low doses for the prophylaxis and treatment of insomnia, while others (diphenhydramine, hydroxyzine, promethazine) can be administered, in combination with other drugs, for sedation and analgesia and the prophylaxis of motion sickness.11
ConclusionsAnti-H1 antihistamines are frequently used in children and adolescents to treat allergic diseases. The effectiveness of second-generation antihistamines has been well studied, and they should be preferred to minimize adverse effects and to take advantage of their antiallergic activity. The choice of the preferred molecule should be based and individualized on the clinical and pharmacological characteristics of each subject.
Conflicts of interestThe authors declare no conflicts of interest.
FundingThe authors did not receive any funding for the research.