To know the necessary information to reproduce the results found in the literature on active surveillance (AS) in prostate cancer (PCa) in our own center so that the information would be objective and correctly given to the patients. We have aimed to study the percentage of candidates for AS chosen in our setting, and the data on infrastaging, subgrading and prediction of insignificant PCa, debugging the predictive value of clinical variables to improve our selection criteria and finally to analyze the results of our patients enrolled in AS.
Materials and methodsA retro- and prospective review of our databases was performed. A one-year period was analyzed to know AS candidates. Analysis of our radical prostatectomy specimens for infrastaging, subgrading and prediction of insignificant PCa (Epstein's criteria) was made as well as a uni/multivariate analysis of clinical variables in patients with insignificant PCa in the specimen. A prospective validation was performed with overall survival and survival free of active treatment (SFAT) as endpoints in patients enrolled in AS.
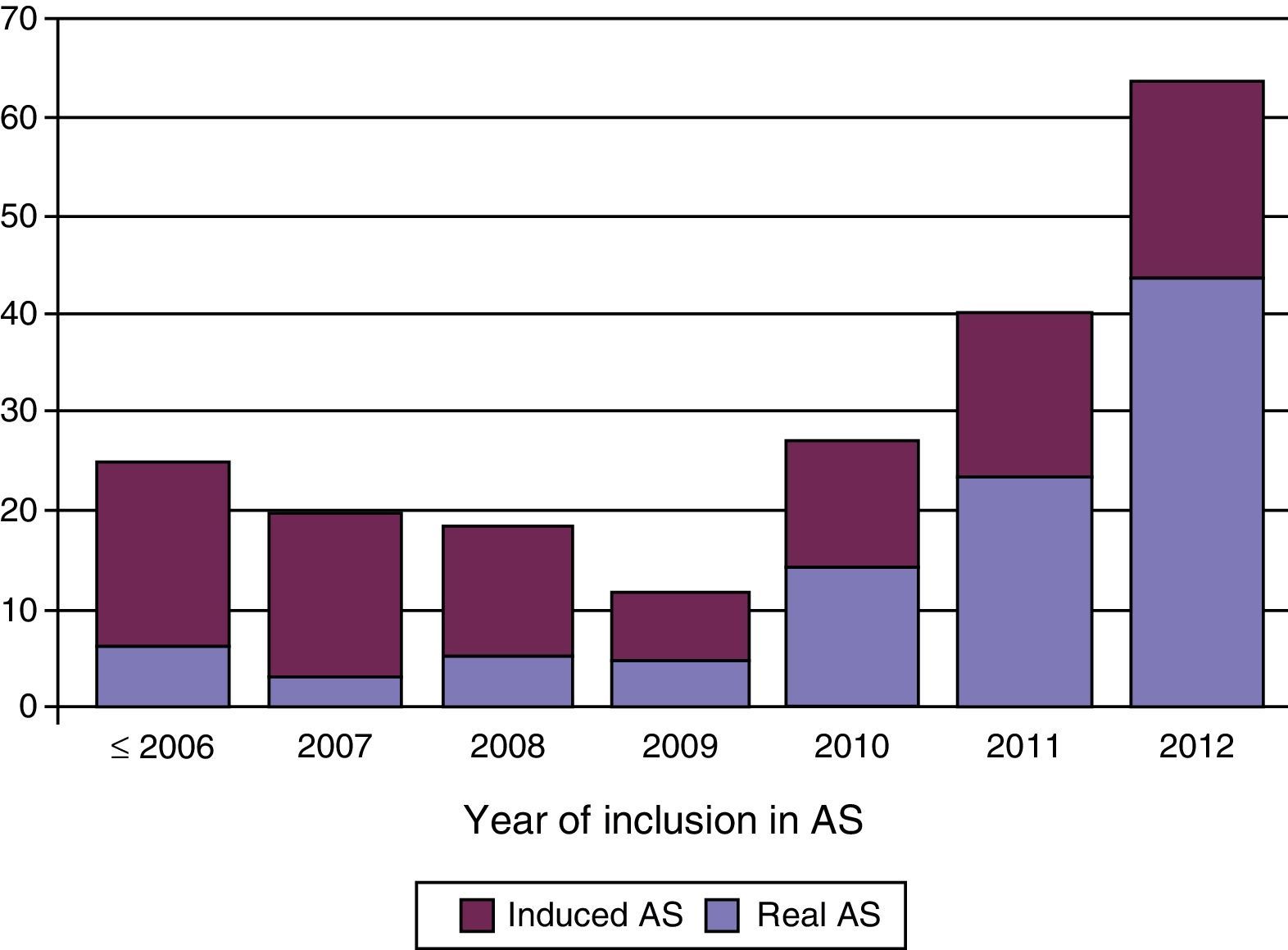
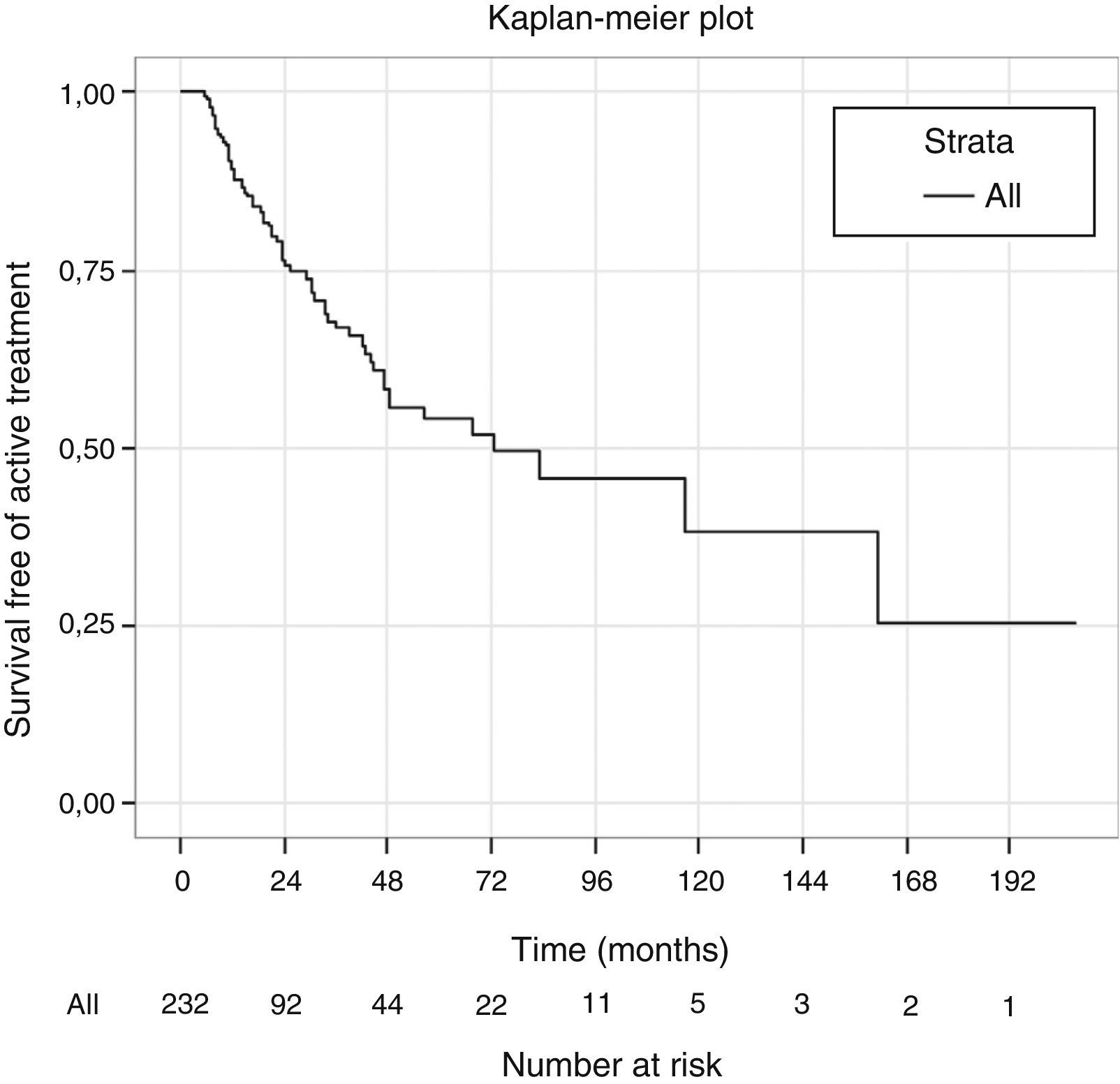
ResultsBetween October-2010 and October-2011, 44.7% of our PCa were candidates for AS, but only 11.2% chose it. The percentages found for infrastaging, subgrading and prediction of insignificant PCa were 14%, 31.4% and 55.7%, respectively. However, only just 6 patients (6.97%) had ≥pT3a+Gleason ≥7+volume >0.5cc PCa. The multivariate analysis showed that PSA density and number of affected cores were independent predictors of insignificant PCa. With a mean follow-up of 36±39months, 63 out of 232 patients enrolled in AS went on to active treatment (27.1%), with only 13 due to anxiety without pathologic progression. Median time of SFAT was 72.7 months (CI 95% 30.9–114.4). SFAT at 24 months was 76.4% (69.7–83.1%) and at 48 months 58.1% (48.8–67.4%). Only 10 patients died (4.3%), 9 due to causes different of PCa. Estimated overall survival at 5 years was 92.8% (CI 95% 86.7–98.9%).
ConclusionsIt should be mandatory to have the exact knowledge of the local data of each center in order to objectively inform patients about prostate biopsy efficiency, and if percentages of infrastaging, subgrading and prediction of insignificant PCa are in accordance with the literature. At 3 years, we reproduced the results of the longest series of AS, so we have ascertained that our AS protocol can be implemented with increasingly more patients.
Conocer la información necesaria para reproducir los resultados de la literatura en vigilancia activa (VA) en cáncer de próstata (CaP) en nuestro propio centro, de tal forma que dicha información sea objetiva y se le pueda dar al paciente de forma fehaciente. Contemplamos estudiar el porcentaje de pacientes candidatos a VA y que la escogen en nuestro ambiente, los datos de infraestadificación, infragradación y predicción de CaP insignificante, depurar el poder predictivo de distintas variables clínicas para mejorar nuestros criterios de selección y analizar los resultados de nuestros pacientes en VA.
Material y métodosRevisión retro y prospectiva de nuestras bases de datos. Se analiza un periodo de un año natural seleccionando posibles candidatos a VA. Análisis de nuestras prostatectomías radicales para conocer las tasas de infraestadificación, infragradación y tasa de CaP insignificante (criterios de Epstein). Análisis uni/multivariado de variables clínicas en pacientes con tumor insignificante en pieza de prostatectomía radical. Valoración prospectiva de supervivencia global y libre de tratamiento activo (SLTA) en pacientes en VA.
ResultadosEntre octubre de 2010 y octubre de 2011, un 44,7% de los CaP cumplían criterios para ser incluidos en VA, y un 11,2% la escogieron. Nuestros porcentajes de infraestadificación, infragradación y tasa de CaP insignificante fueron 14%; 31,4%; y 55,7% respectivamente, pero solo 6 pacientes (6,97%) tuvieron CaP≥pT3a+Gleason≥7+volumen>0,5cc. En el estudio multivariado para predicción de tumor insignificante, la densidad de PSA y el número de cilindros afectos son factores independientes. Con un seguimiento medio de 36±39meses, de 232 incluidos en VA, 63 pacientes pasaron a tratamiento activo (27,1%), solo 13 por ansiedad sin progresión patológica. La mediana del tiempo de SLTA es de 72,7meses (IC 95%: 30,9–114,4). La SLTA a los 24 meses es del 76,4% (69,7–83,1%) y a 48 meses es del 58,1% (48,8–67,4%). Solo 10 pacientes (4,3%) fallecieron, 9 por causa diferente al CaP. La supervivencia global estimada a 5 años es del 92,8% (IC 95%: 86,7–98,9%).
ConclusionesEl conocimiento exacto de la casuística de cada centro debería ser obligatorio para informar a los pacientes verazmente de la rentabilidad de la biopsia y de si los porcentajes de infragradación, infraestadificación y de CaP insignificante se adecuan a los de la literatura. A 3 años reproducimos los resultados de las series más longevas de VA, por lo que el programa de VA puede seguir implementándose e incluyendo cada vez a más pacientes.