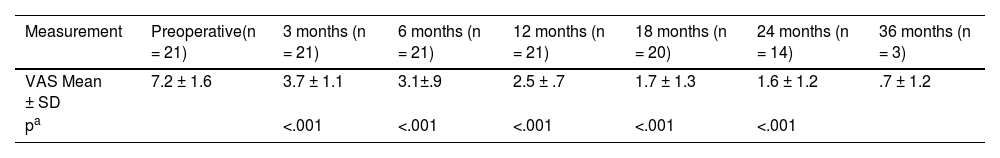to assess clinical safety and postoperative audiological outcomes in postlingual deafness Spanish speaking patients, who underwent surgery with Nurotron™ cochlear implant.
Material and methodsRetrospective descriptive case series study. We performed follow-up of complications and audiological measurements before and after cochlear implantation. Patients with bilateral severe to profound sensorineural hearing loss or patients with unilateral deafness with/without tinnitus were included. Repeated-measures within-subjects for assess pure tone thresholds and speech performance (bilingual test) with a detailed monitoring to establish security or adverse effects were performed. Analysis of variance tests, repetitive measures, were used for statistical analysis.
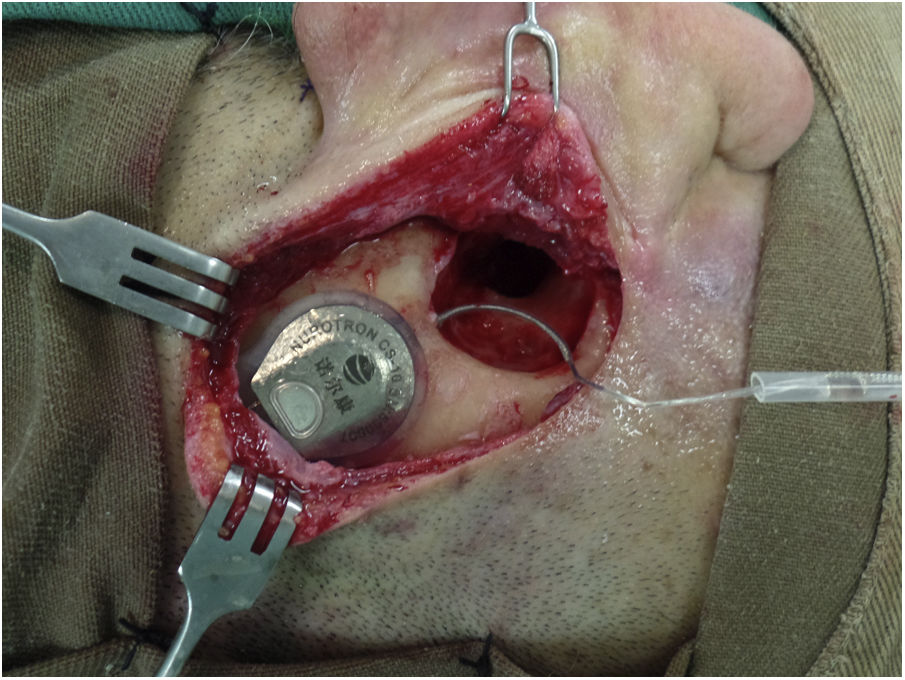
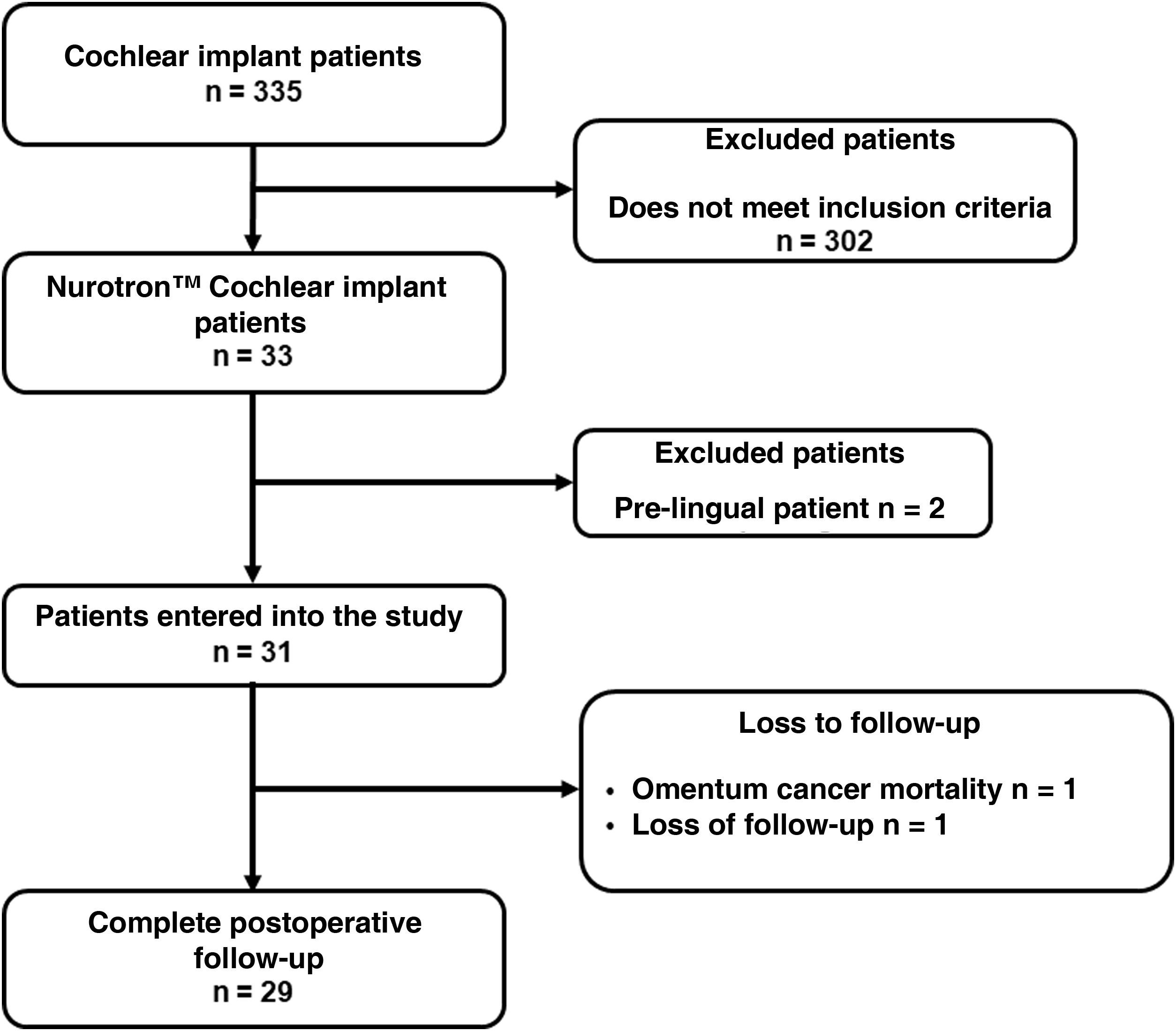
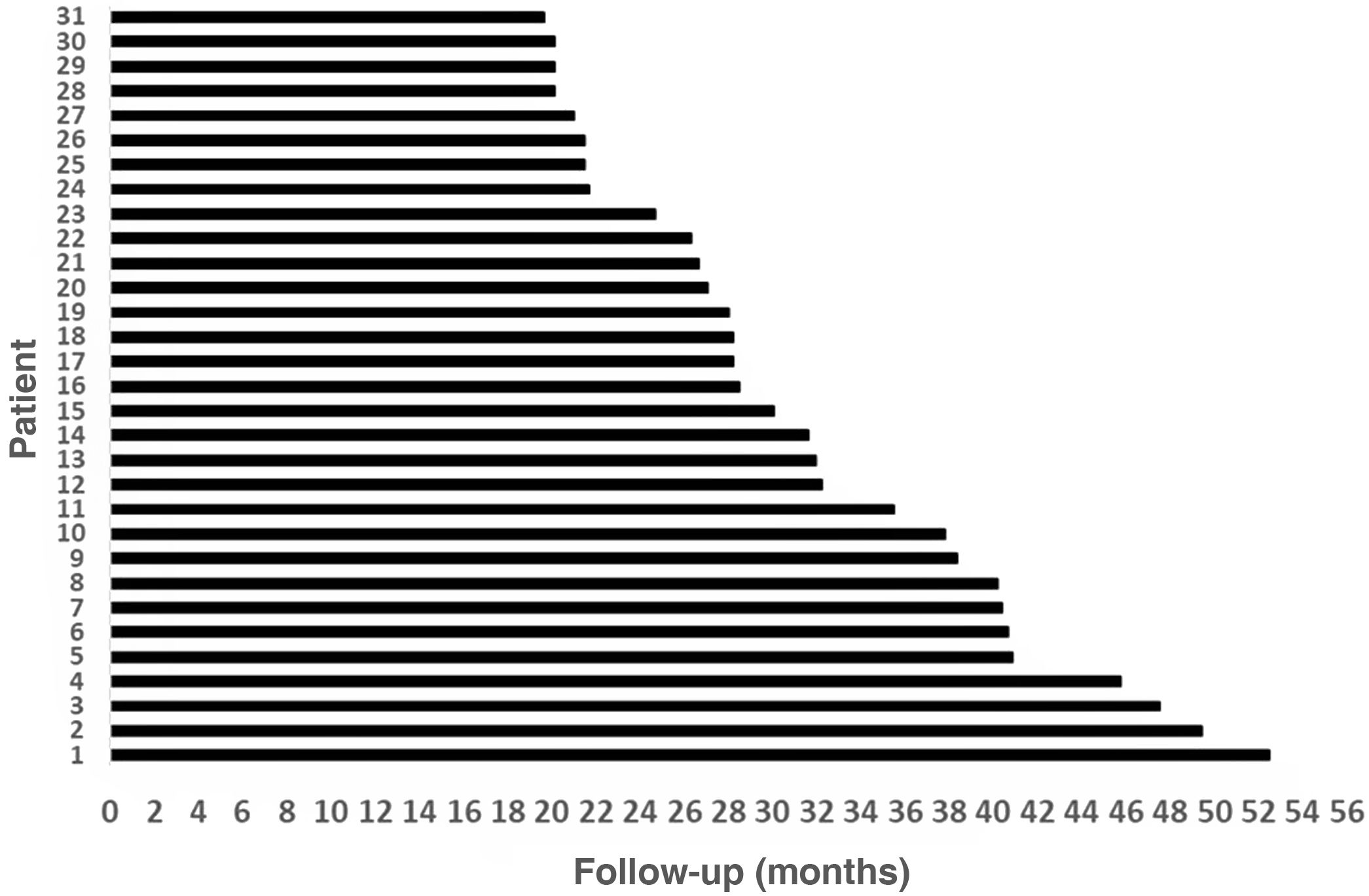
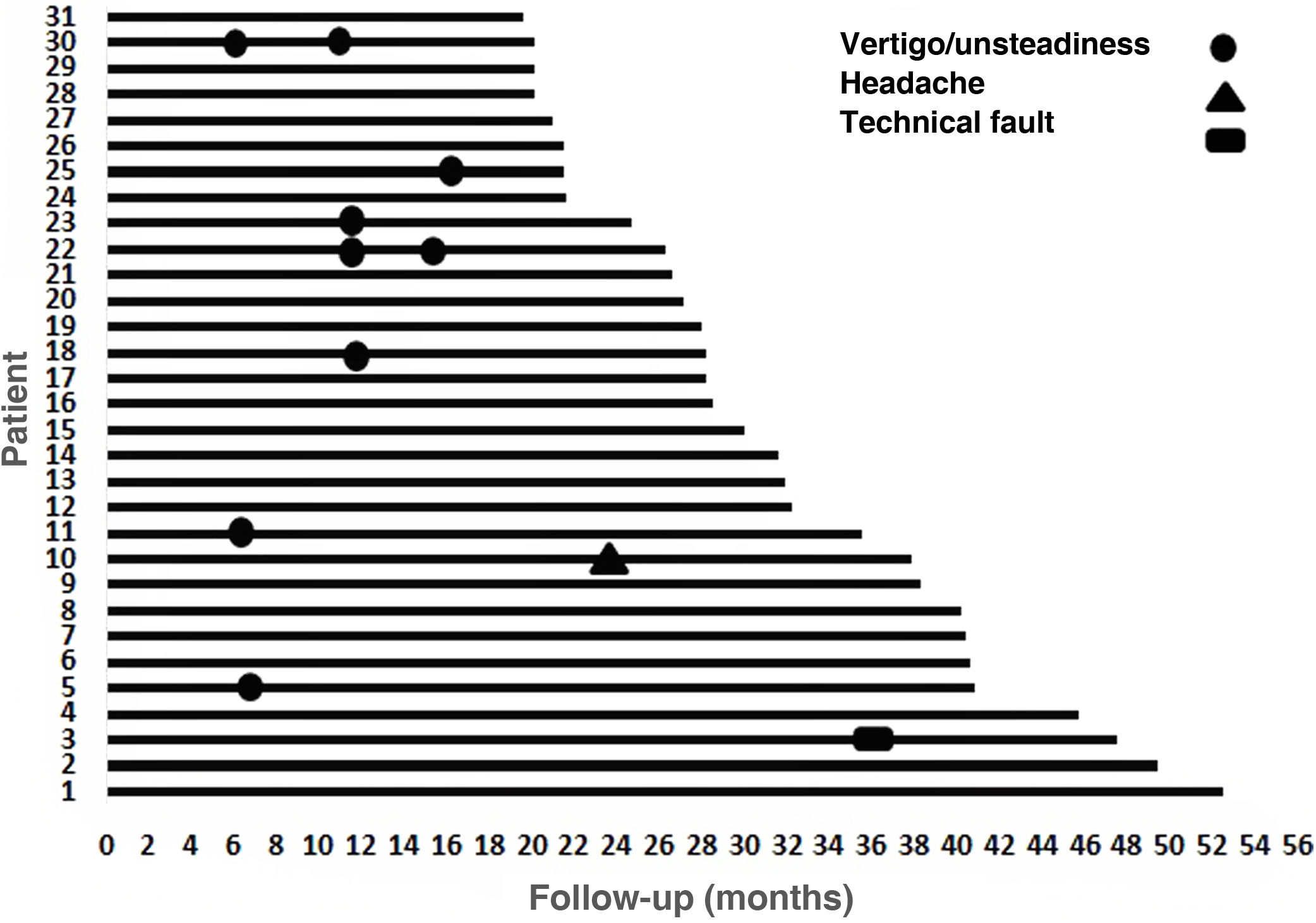
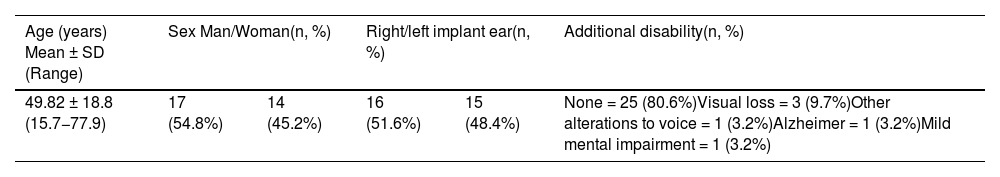
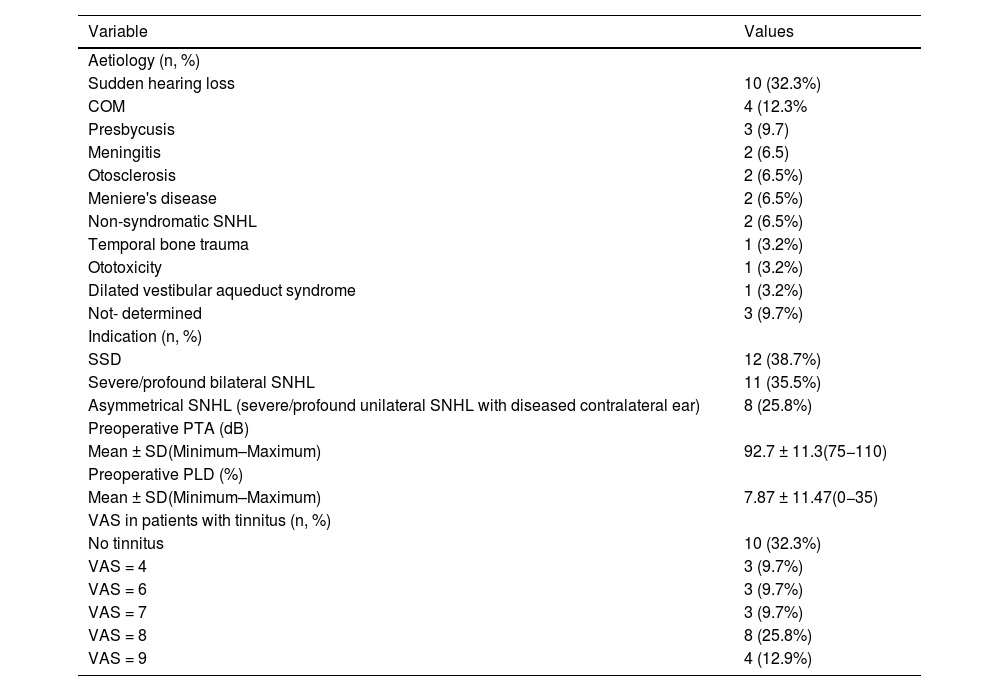
Results31 patients were included, 17 (54.8%) men and 14 (45.2%) women. Mean age at the time of surgery was 49.82 ± 18.8 years. The mean follow-up of the group was 31.56 ± 9.57 months (minimum = 19.6 months and maximum = 52.50 months). As major complication one patient (3.23%) had a hard failure that required removal and re-implantation. 25.8% of the patients presented minor complications, the most frequent being vertigo/unsteadiness in 22.6%.
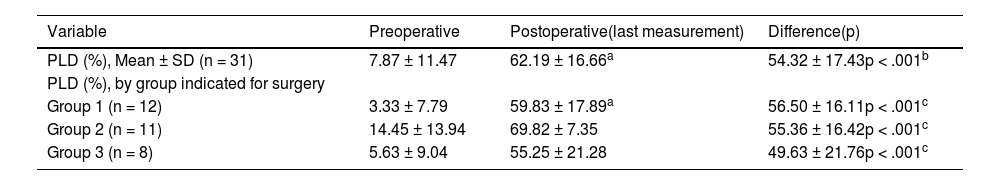
The mean of language discrimination (free field at 65 dB SPL) was 62.19% ± 16.66; being 69.82% ± 7.35 in the group of severe to profound bilateral sensorineural hearing loss. A statistically significant reduction was observed in patients with tinnitus, assessed using the visual analogue scale, preoperative = 7.2 ± 1,6 vs postoperative (18months postoperative) = 1.7 ± 1.3 (p < .001).
ConclusionsThe Nurotron™ cochlear implant shows satisfactory audiological results, in accordance with what has been reported in the literature. Minor complications were similar to previous studies, but the percentage of hard failure should continue to be observed, which was higher than other reports with comparable follow-up.
evaluar la seguridad clínica y resultados audiológicos postoperatorios en pacientes de habla hispana con sordera postlingual, que recibieron implante coclear Nurotron™.
Materiales y métodosEstudio descriptivo retrospectivo tipo serie de casos. Se hizo seguimiento de complicaciones y mediciones audiológicas antes y después del implante coclear. Se incluyeron pacientes con pérdida auditiva neurosensorial bilateral severa a profunda o pacientes con sordera unilateral con/sin tinnitus. Se realizaron medidas repetidas dentro de los sujetos para evaluar los umbrales de tonos puros y el rendimiento del habla (listados de bisílabas), así como seguimiento clínico para establecer la seguridad del dispositivo. En el análisis estadístico se utilizaron análisis de la varianza y pruebas de medidas repetitivas.
Resultadosse incluyeron 31 pacientes, 17(54,8%) hombres y 14(45,2%) mujeres. La edad media en el momento de la cirugía fue de 49,82 ± 18,8años. El seguimiento medio fue de 31,56 ± 9,57meses (mínimo = 19,6meses y máximo = 52,50meses). Como complicación mayor un paciente (3,23%) tuvo un fallo técnico que requirió remoción y reimplantación. El 25,8% de los pacientes presentaron complicaciones menores, siendo la más frecuente el vértigo/inestabilidad en el 22,6%.
La media de discriminación del habla (campo libre a 65 dB SPL) fue de 62,19% ± 16,66; siendo del 69,82% ± 7,35 en el grupo de hipoacusia neurosensorial bilateral severa/profunda. Se observó una reducción estadísticamente significativa en los pacientes con tinnitus, evaluados mediante la escala analógica visual, preoperatorio = 7,2 ± 1,6 vs postoperatorio (18 meses postoperatorio) = 1,7 ± 1,3 (p < 0,001).
ConclusionesEl implante coclear Nurotron™ muestra resultados audiológicos satisfactorios, acordes con lo reportado en la literatura. Las complicaciones menores fueron similares a estudios previos, pero se debe continuar observando el porcentaje de fallo técnico que fue superior a otros reportes con seguimiento equiparable.