Clozapine is the first choice in drug-resistant schizophrenia but also causes important weight changes. This might discourage clinicians who are concerned about the risk of developing health problems. To assess this issue we measured change in body mass index (cBMI) induced by clozapine at 18 and 56 weeks.
MethodsBaseline body weight and height were measured and weight weekly thereafter during the first 18 weeks of treatment. After that, measurements were made monthly. Steady clozapine dose, clozapine and norclozapine blood concentrations, concomitant medication, gender and age were recorded.
ResultsAt 18 weeks (n=76) mean cBMI was 1.83kg/m2. Baseline BMI was inversely correlated with cBMI. At 56 weeks (n=57) cBMI was 2.67kg/m2 and was inversely correlated with basal BMI. Multiple regression analysis replicated the results. When split with BMI categories, obese patients had lesser risk for further weight gain.
ConclusionsObesity should not discourage clinicians from starting clozapine in drug-resistant patients.
Clozapina es el fármaco de elección en el tratamiento de la esquizofrenia resistente, pero genera importantes cambios ponderales que pueden disuadir al clínico de utilizarlo, preocupado por los posibles riesgos para la salud del paciente. Para valorar estos aspectos evaluamos los cambios producidos en el índice de masa corporal (IMC) a las 18 y 56 semanas de tratamiento con clozapina.
MétodoEn una muestra de pacientes diagnosticados de esquizofrenia, el peso y la estatura fueron medidos a nivel basal y posteriormente de forma semanal durante las primeras 18 semanas de tratamiento con clozapina. Posteriormente la evaluación fue mensual. Se registraron así mismo las dosis regulares de clozapina, los niveles plasmáticos de clozapina y norclozapina, la medicación concomitante, el género y la edad.
ResultadosA las 18 semanas (n=76) el incremento medio en IMC era de 1,83kg/m2. El IMC basal se correlacionaba de forma inversa con el incremento en IMC. A las 56 semanas (n=57) el incremento medio en IMC era 2,67kg/m2 y se correlacionaba de forma inversa con el IMC basal. Análisis de regresión múltiple replicaron estos resultados. Considerando categorías diferentes según el IMC basal, los pacientes que partían de un sobrepeso tenían menor riesgo de incremento ponderal continuado.
ConclusionesLa presencia de sobrepeso no debiera disuadir al clínico de considerar el tratamiento con clozapina en pacientes con esquizofrenia resistente.
Weight gain is a common side effect of antipsychotics, and it is mostly associated with newer antipsychotics.1 Obesity is common among people suffering with schizophrenia, affecting more than 40% of subjects.2 Central obesity is one of the components of the metabolic syndrome,3 a cluster of cardiovascular risk factors linked to increased mortality. This is particularly relevant among people with schizophrenia who have a 20 year reduced lifespan, mostly attributed to cardiovascular events.4 Among all antipsychotics, clozapine seems to cause the greatest weight gain1 along with associated effects but is the treatment of choice in drug-resistant schizophrenia, which affects nearly 20–30%.5 Patients started on clozapine have already been prescribed other antipsychotic drugs; which may have induced weight gain, thus discouraging the prescription of clozapine for fear of further weight gain. In different studies, basal BMI has been shown to be a predictor for increasing BMI in clozapine treatment, at 16 weeks,6 1-year7 and at 8-year follow up,8 suggesting that lower basal BMI may be correlated with greater BMI increase. Clozapine dose and cessation of smoking have also been associated with weight gain.
In this study we analyze the predictors for body mass index (BMI) change induced by clozapine at 18 weeks and 56 weeks, in a sample of drug-resistant schizophrenic patients and, specifically, the role of basal BMI.
MethodsDesignRetrospective analysis of weight and BMI change in a cohort of patients with a diagnosis of schizophrenia.
SubjectsInclusion of subjects started in January 1999 and finished on December 2003. Schizophrenic patients were recruited consecutively at the time of their first contact with the Clozapine Clinic in a general academic hospital (Hospital Clinic of Barcelona). The Clozapine Clinic is a clinical program focused in the treatment of psychotic patients living in the urban area of Barcelona and considered eligible for treatment with clozapine. All patients fulfilled criteria for drug-resistant psychosis and were evaluated before starting clozapine, by one of us (AP). Previous prescribed medication was recorded. Exclusion criteria for this study were: (1) patients on any additional antipsychotic medication, (2) failure to complete at least 90% of the assessment. Written informed consent was obtained from each patient, and the protocol was approved by the Ethics Committee of the institution.
AssessmentsBody weight and height were measured at baseline and weight weekly thereafter during the first 18 weeks of treatment, and monthly thereafter. Body Mass Index (defined as the weight in kilograms divided by the square of height in meters) was calculated at baseline, 5 weeks, 10 weeks, 18 weeks, 6 months, 10 months and 56 weeks. Basal BMI was divided into four categories: BMI less than 20 (underweight), BMI between 20 and 25 (normal weight), BMI between 25 and 30 (overweight) and BMI over 30 (obesity).
Steady clozapine dose, concomitant medication, gender and age at baseline were recorded. Blood samples for the determination of clozapine and norclozapine concentrations were obtained at the end of week 18 at 9a.m. approximately, 12h after the bedtime dose. Concentrations of clozapine and norclozapine were measured by using high performance liquid chromatography.
Statistical analysisThe main variable of the study was change in BMI (cBMI), measured as the arithmetic subtraction between each of the two time points and baseline: (1) at week 18 of treatment and; (2) at week 56. Weight was not the main variable as it could be influenced by height and by gender.
We conducted the same set of tests for the short and long term cBMI analyses. First, a correlation between basal BMI and cBMI using Pearson r coefficient was carried out. Secondly, a multiple regression analysis was carried out, using cBMI as dependent variable and basal BMI, mood stabilizer use (yes/no), norclozapine blood level, age and gender as independent variables.
As a secondary analysis, we compared cBMI according to the four baseline BMI categories (underweight, normal, overweight, and obese). As groups were relatively small and with different sample size, we tested the normality of distribution of the groups using the Shapiro–Wilk test and the variance homogeneity with the Levene's test. One-way ANOVA test was then performed, using BMI categories as independent factor. As BMI categories were ordinated categories, a linearity test was also carried out. As post hoc analyses, Tukey comparisons were carried out.
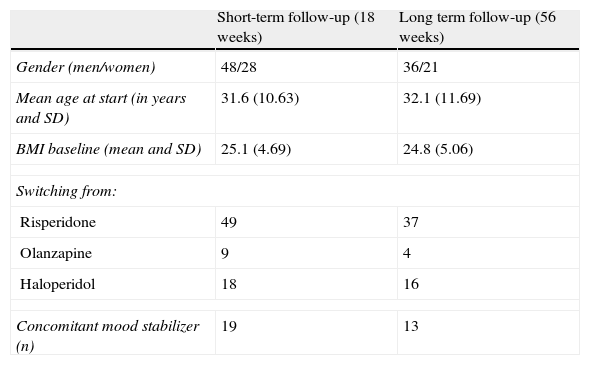
ResultsFinal sample was composed of 76 for the 18 week analysis and 57 for the 56 week analysis. Table 1 describes demographics and the clinical data of both samples. Actual dose of treatment was available for 50 patients at 18 weeks and 39 at 56 weeks. Clozapine and norclozapine blood levels were available in 60 and 45, respectively. No subject's medication was altered because of weight change.
Sociodemographic and clinical data of the two samples studied.
| Short-term follow-up (18 weeks) | Long term follow-up (56 weeks) | |
| Gender (men/women) | 48/28 | 36/21 |
| Mean age at start (in years and SD) | 31.6 (10.63) | 32.1 (11.69) |
| BMI baseline (mean and SD) | 25.1 (4.69) | 24.8 (5.06) |
| Switching from: | ||
| Risperidone | 49 | 37 |
| Olanzapine | 9 | 4 |
| Haloperidol | 18 | 16 |
| Concomitant mood stabilizer (n) | 19 | 13 |
SD: standard deviation; BMI: body mass index, expressed in kg/m2.
Mean cBMI at 18-week was 1.83kg/m2 (SD=2.42), ranging from −3.81 to 8.71kg/m2. 18 patients (19.7%) showed decreased BMI during follow-up. There was a statistically significant inverse correlation between baseline BMI and cBMI to 18 weeks, expressed by a Pearson r=−0.412 (p=0.0002). There were no differences in cBMI between women and men (p=0.803). cBMI was not correlated with the steady dose at week 6 (r=0.199; p=0.166, n=50) or clozapine or norclozapine blood levels (r=0.03, p=0.821 and r=0.037; p=0.779; n=60).
We further explored these results with a multiple regression, using cBMI to 18 weeks as a dependent variable and gender, age and BMI at baseline as independent variables. With this approach, only BMI at baseline was significant, showing again the inverse relationship (data not shown).
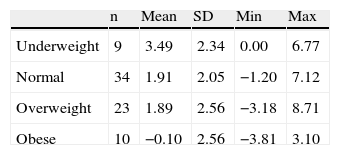
A further analysis of cBMI among the four categories of basal BMI was carried out. Table 2a shows cBMI among the groups. Shapiro–Wilk's and Levene's tests showed groups’ normal distribution and variance homogeneity. ANOVA analysis suggested difference between them (F=6.384, d.f. 3, p=0.001), and polynomial linear analysis suggested an inverse association (lower basal BMI, greater cBMI; p=0.001) that was replicated with the Tukey post hoc contrast, which showed statistical significant difference in cBMI between basal underweight and obese groups (p=0.006).
Changes in BMI (expressed as kg/m2) after 18 weeks of clozapine treatment, split by baseline BMI categories.
| n | Mean | SD | Min | Max | |
| Underweight | 9 | 3.49 | 2.34 | 0.00 | 6.77 |
| Normal | 34 | 1.91 | 2.05 | −1.20 | 7.12 |
| Overweight | 23 | 1.89 | 2.56 | −3.18 | 8.71 |
| Obese | 10 | −0.10 | 2.56 | −3.81 | 3.10 |
SD: standard deviation; BMI: body mass index, expressed in kg/m2.
Mean cBMI at 56 weeks was 2.67kg/m2 (SD=3.26), ranging from −4.16 to 10.51kg/m2. 10 patients (17.5%) showed decreased BMI during the follow-up. There was a statistically significant inverse correlation between basal BMI and cBMI at 56 weeks, expressed by a Pearson coefficient r=−0.422 (p=0.001). There were no differences in cBMI between women and men (p=0.560). cBMI was not correlated with the steady dose at week 6 (r=0.262; p=0.107, n=39) or clozapine or norclozapine blood levels (r=0.027, p=0.861 and r=0.064; p=0.677; n=45).
We further explored these results with a multiple regression, using cBMI to 1 year as a dependent variable and gender, age and BMI at baseline as independent variables. With this approach, baseline BMI and concomitant use of mood stabilizers were statistically significant, and basal BMI showed again the inverse relationship (data not shown).
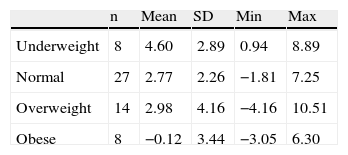
A further analysis of cBMI among the four categories of basal BMI was also carried out. Table 2b shows mean cBMI among the groups. Shapiro–Wilk's and Levene's tests showed groups’ normal distribution and variance homogeneity. ANOVA analysis suggested differences between them (F=3.309, d.f. 3, p=0.027), and polynomial linear analysis suggested an inverse association (lower basal BMI, greater cBMI; p=0.005) that was replicated with the Tukey post hoc contrast, which showed statistical significant difference in cBMI between basal underweight and obese groups (p=0.017).
Changes in BMI (expressed as kg/m2) after 56 weeks of clozapine treatment, split by baseline BMI categories.
| n | Mean | SD | Min | Max | |
| Underweight | 8 | 4.60 | 2.89 | 0.94 | 8.89 |
| Normal | 27 | 2.77 | 2.26 | −1.81 | 7.25 |
| Overweight | 14 | 2.98 | 4.16 | −4.16 | 10.51 |
| Obese | 8 | −0.12 | 3.44 | −3.05 | 6.30 |
SD: standard deviation; BMI: body mass index, expressed in kg/m2.
We also correlated cBMI at weeks 18 and 56, which showed a strong significant correlation (r=0.874, p<0.0001).
DiscussionOur results indicate that a change in BMI in both short and long term in patients treated with clozapine monotherapy is inversely correlated with the BMI at baseline. Age, gender and norclozapine blood levels at week 18 were not correlated with change in BMI. Concomitant mood stabilizer prescription was a significant predictor of greater BMI increase after one year.
Weight gain has been previously inversely correlated with BMI at baseline, with clozapine7–9 as well as with other newer antipsychotic drugs.10–13 Indeed, our results at 16 weeks mimic quite well the results showed by other researchers, suggesting that obese subjects will not experience, on an average, weight gain.6 For the first time, the same sample has been followed up for one-year. Our study supports all these studies; furthermore, stratification of the sample in four categories according to baseline BMI, stresses the study's conclusion, that is, that underweight and normal weight subjects are at higher risk of weight gain. It also provides the timeline of the weight gain. On the one hand, there is a strong correlation between the cBMI at both week 18 and week 56. On the other hand, a mere observation of the resulting data (mean cBMI from baseline to week 18 1.83kg/m2, compare to 2.67 at week 56) reflects a proportionally lower increase in BMI from week 18 up to 56. Both indicate that if weight gain occurs, it does so mainly in the first 18 weeks. Therefore, early remediation strategies should be implemented among people who experience significant weight gain after initiating clozapine. Our data indicates that there are no differences in cBMI between men and women, at either 18 or 56 weeks.
This study shows that clozapine can be prescribed without further risk of weight gain to overweight and obese drug-resistant schizophrenic patients. Indeed, these represent a large proportion of schizophrenic patients; a recent survey indicates that more than 50% present overweight or obesity,2 and nearly 25% meet criteria for drug-resistant schizophrenia.5 Moreover, these patients may benefit from an improvement in their mental state and, therefore, be able to attend better to structured therapies focused on well-being and weight loss strategies.
This study has some limitations. The data analysis was limited to the first year of follow-up. It could be argued that at longer term, weight gain would be induced. However, previous studies suggest9 that changes in BMI and weight after the first year of treatment with clozapine are minor8 and our correlation between week 18 and 56 suggests that weight gain is an early phenomenon. Moreover, our study includes both short and long term analyses, showing the same significant negative correlations, suggesting a trend of stability over time in weight gain. In our study, all patients received only three types of different antipsychotic before switching to clozapine, which could affect the generalization of our results. However, these drugs are or have been some of the most widely prescribed medications for schizophrenia (and psychosis). In any case, further studies with larger populations would be warranted.
FundingFunding for this study was provided partially by a Government of Catalonia, Comissionat per Universitats i Recerca del Department d’Innovació, Universitats i Empresa (DIUE) grant (2009SGR1295, 2009SGR1119), and by the Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de salud Mental, CIBERSAM; work was carried out in part at the Esther Koplowitz Center, Barcelona. These institutions had no further role in the study design; in the collection, analysis and interpretation of data; and in the decision to submit the paper for publication.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestDr. Goti, Dr. Undurraga, Dr Fernandez-Egea, Mrs A. Romero and Dr. Carne have no conflict of interest to disclose. Dr. Pons has provided consulting services to Janssen-Cilag, Johnson&Johnson and he is speaker/advisory board member for Janssen-Cilag. Prof. Dr. M. Bernardo has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory boards of Adamed, Almirall, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Mylan, Pfizer, Roche y Rovi.
Authors would like to thank Carmen Colmenar for the recruitment of volunteers.
Please cite this article as: Pons i Villanueva A, et al. ¿Debería considerarse la obesidad un factor limitante para el tratamiento con clozapina? Rev Psiquiatr Salud Ment (Barc.). 2013;6:75–9.