The introduction of the acid-etch technique by Dr. Buonocore in 1955 was the genesis of adhesive dentistry. Currently, bonding to dental substrates may be accomplished through two adhesion strategies: (1) etch-and-rinse and (2) self-etch, which includes glass-ionomer based materials. More recently, a new family of dentin adhesives has been introduced (universal or multi-mode adhesives), which may be used either as etch-and-rinse or as self-etch adhesives.
In this paper the basic bonding mechanisms to enamel and dentin will be discussed to give the reader an overall understanding of the main differences among them.
The learning objectives are the understanding of the evolution of adhesive systems and which adhesion strategy might be more useful to clinical practice.
A introdução do conceito de condicionamento ácido do esmalte pelo Dr. Buonocore em 1955 iniciou a era da dentisteria adesiva. Atualmente, as resinas compostas podem ser aderidas ao esmalte e à dentina segundo duas filosofias adesivas: (1) os adesivos de condicionamento ácido total e (2) os adesivos de auto-condicionamento, que incluem os materiais derivados de ionómeros de vidro. Mais recentemente foram introduzidos adesivos universais que podem ser usados com condicionamento ácido total ou como adesivos auto-condicionantes.
Neste artigo pretende-se dar a conhecer os mecanismos que estão subjacentes a cada estratégia de adesão.
No final, os leitores conseguirão entender a evolução dos sistemas adesivos e, desta forma, qual a estratégia mais indicada para a sua prática clínica.
The paramount goal of bonding restorations is to achieve an intimate contact between restorative materials and dental hard tissues.1 For durable adhesion to occur in the mouth, the liquid adhesive must wet the solid adherent to allow structural interaction; the stress concentration at the interface must be reduced; and the interface must be protected from degradation in the oral environment.2
Dental adhesives are solutions of resin monomers that make the resin–dental substrate interaction achievable.3 Adhesive systems are composed of monomers with both hydrophilic groups and hydrophobic groups. The former enhance wettability to the dental hard tissues, while the latter allow the interaction and co-polymerization with the restorative material.4 The chemical composition of adhesives also includes curing initiators, inhibitors or stabilizers, solvents and, in some cases, inorganic fillers.4
The mineralized part of the tooth is a complex structure made of different hard tissues, which have a quite distinct ultra-morphology and composition. Enamel is composed of a hard solid crystalline structure – hydroxyapatite (HAp) (96% by weight) – with strong intermolecular forces, a high-energy surface,1 besides water and organic material (4% by weight) (Fig. 1).5 Dentin is a biological composite of HAp (50% by volume) that envelops collagen (30% by volume, mainly type I) (Fig. 2).6 Dentin is intrinsically humid (20% by volume of water),6 and less hard than enamel, with low intermolecular forces and low-energy surfaces.1 Dentin is also a substrate that undergoes change with age in an asymmetrical physiological aging process, leading to an increase of dentin thickness and decrease in dentin permeability.7 Furthermore, sclerotic and carious dentin suffer structural changes that result in an higher mineralization and a consequently reduced permeability.7,8 Unlike dentin, enamel can be dried easily, making the bonding process to enamel different from that of dentin.8
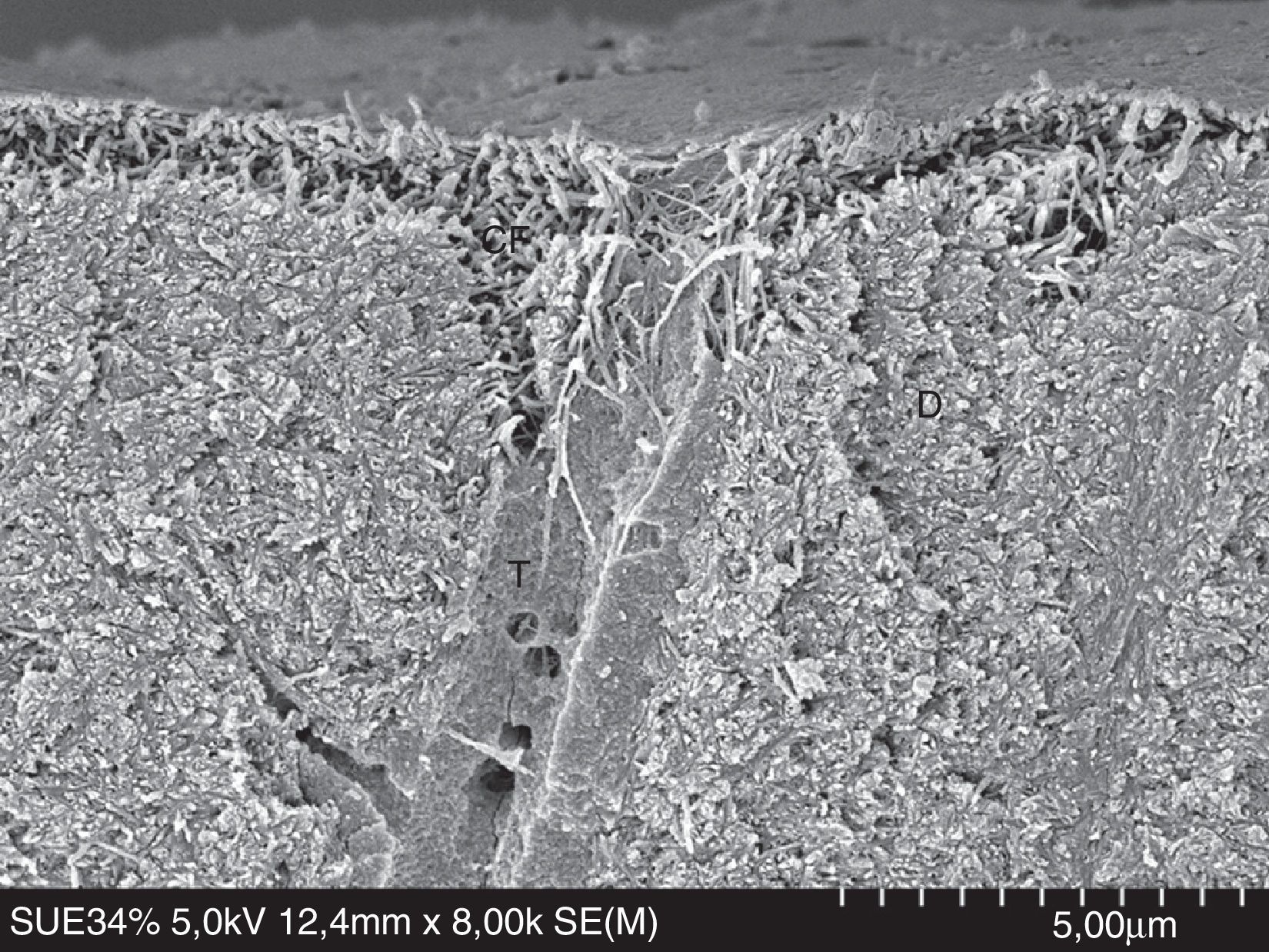
FESEM micrograph of human dentin etched with 32% phosphoric acid (3M ESPE) for 15s. Original magnification=×8000. CF=collagen fibrils exposed by the acid; D=dentin; T=dentinal tubule.
The new era of adhesive dentistry began with Dr. Buonocore in 1955.9 As a visionary, Dr. Buonocore proposed etching enamel with 85% phosphoric acid to improve the retention of acrylic resin to pit-and-fissures. This was the pioneering research of Minimally Invasive Dentistry.10 Enamel conditioning with phosphoric acid results in the formation of microporosities where resin penetrates to form “prism-like” resin tags.11 This yields an enamel bonding predominantly micromechanical.12 Recommendations for simultaneous etching of enamel and dentin were published in the 1970's.13 It was the beginning of the total-etch concept.
When a tooth is instrumented with a cutting instrument the surface becomes covered with an adherent layer of debris,14 forming a low-energy smeared layer.15 Salivary films and composite resins also have a low-energy surface.15 The smear layer (Fig. 3) is mainly formed of HAp and denatured collagen,16 plugging the dentinal tubules with smear plugs (Fig. 3). Smear layer behaves as a true physical barrier, reducing dentinal permeability by 86%.17 However, smear layer is permeable due to submicron channels that allow the flux of dentinal fluid.18
FESEM micrograph of smear layer and a smear plug. Original magnification=×10,000. SL=smear layer; SP=smear plug; Int=intertubular dentin; P=peritubular dentin; T=dentinal tubule.
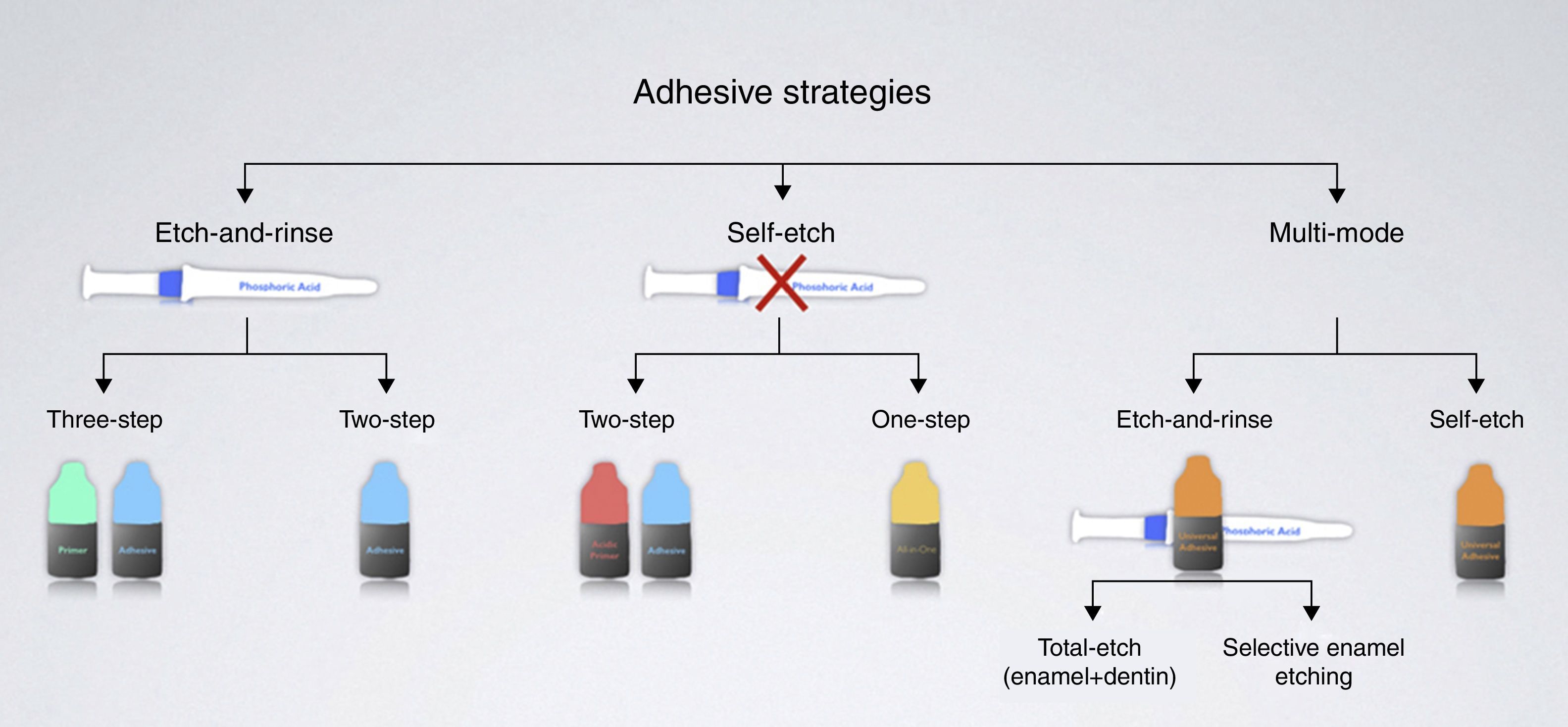
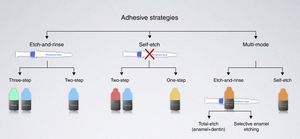
Contemporary adhesive strategies depend on how adhesive systems interact with the smear layer – dissolving it or making it permeable (Fig. 4). The classification of adhesive systems in generations is obsolete19 and may serve the purpose of making current adhesives sound as more advanced (6th generation, 7th generation, and so on) for marketing purposes.
Etch-and-rinse adhesive strategy (formerly known as total-etch)The etch-and-rinse strategy includes two types of adhesives according to the number of steps involved:
- 1.
Three-step etch-and-rinse adhesives (see Table 1 for examples): after phosphoric acid etching and rinsing off with water, a solvent-rich primer is applied (hydrophilic functional monomer) and air-dried, followed by a bonding resin (hydrophobic cross-linker resin), which must be polymerized.
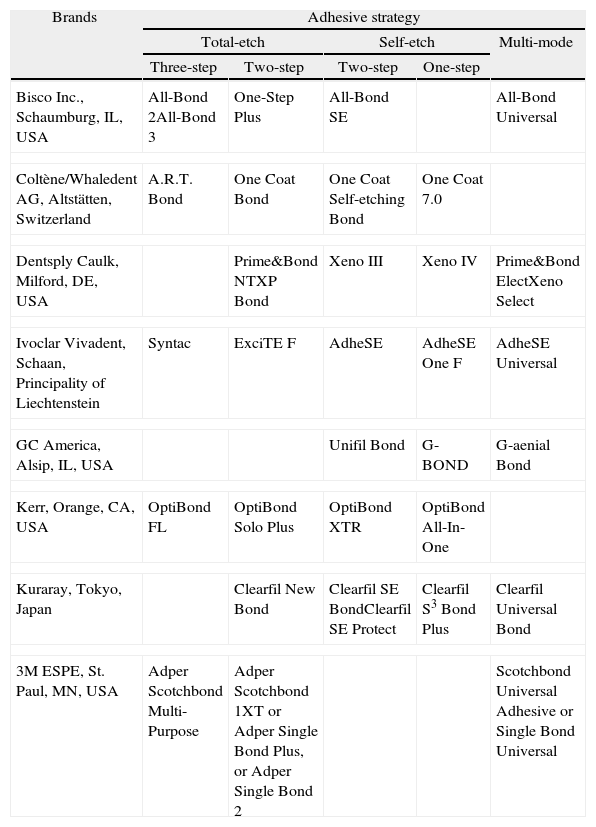
Table 1.Adhesive strategies: adhesive systems currently availablea by brand (in alphabetic order).
Brands Adhesive strategy Total-etch Self-etch Multi-mode Three-step Two-step Two-step One-step Bisco Inc., Schaumburg, IL, USA All-Bond 2All-Bond 3 One-Step Plus All-Bond SE All-Bond Universal Coltène/Whaledent AG, Altstätten, Switzerland A.R.T. Bond One Coat Bond One Coat Self-etching Bond One Coat 7.0 Dentsply Caulk, Milford, DE, USA Prime&Bond NTXP Bond Xeno III Xeno IV Prime&Bond ElectXeno Select Ivoclar Vivadent, Schaan, Principality of Liechtenstein Syntac ExciTE F AdheSE AdheSE One F AdheSE Universal GC America, Alsip, IL, USA Unifil Bond G-BOND G-aenial Bond Kerr, Orange, CA, USA OptiBond FL OptiBond Solo Plus OptiBond XTR OptiBond All-In-One Kuraray, Tokyo, Japan Clearfil New Bond Clearfil SE BondClearfil SE Protect Clearfil S3 Bond Plus Clearfil Universal Bond 3M ESPE, St. Paul, MN, USA Adper Scotchbond Multi-Purpose Adper Scotchbond 1XT or Adper Single Bond Plus, or Adper Single Bond 2 Scotchbond Universal Adhesive or Single Bond Universal - 2.
Two-step etch-and-rinse adhesives (see Table 1 for examples): after phosphoric acid etching and rinsing off with water, dentin and enamel are simultaneously primed and bonded (the hydrophilic primer and the hydrophobic resin are blended in one solution19), followed by air-drying and polymerization.
Phosphoric acid is generally used in a gel form with a concentration between 30% and 40% (pH=0.1–0.4). The low pH also kills most residual bacteria.20,21
EnamelWhen phosphoric acid is applied over an unground or ground enamel surface, HAp is selective dissolved creating macro- and micro-porosities. After the resin monomers infiltrate the enamel porosities through capillary attraction, polymerization makes the resin interlock within the porosities. This mechanism is responsible for the formation of micro and macro ‘prism-like’ resin tags (Fig. 5).11 Enamel adhesion has been considered the ‘golden standard’.22 Resin bonded to enamel protects the resin–dentin interface against degradation in vitro23,24 and clinically.25,26
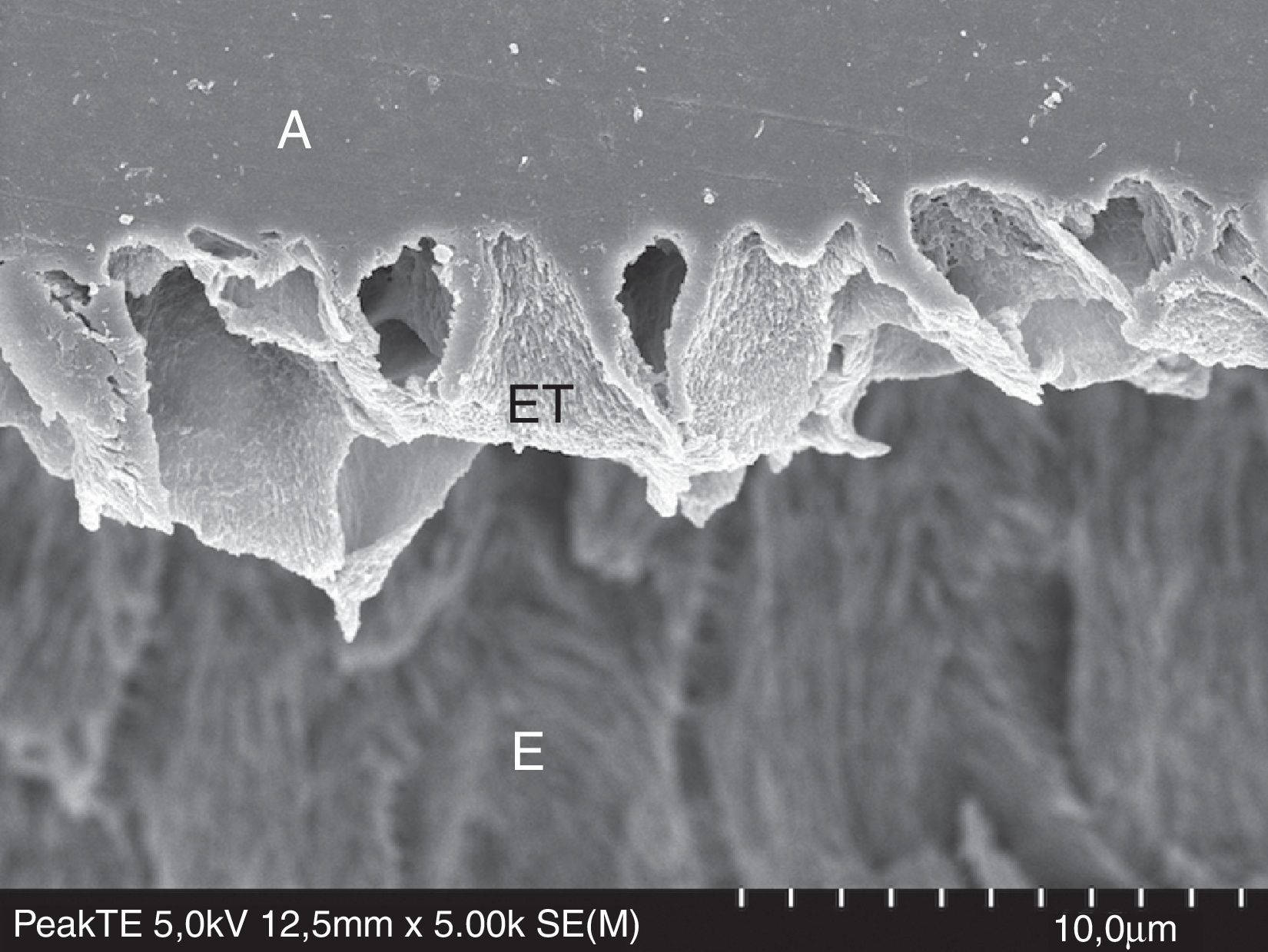
FESEM micrograph of resin–enamel interface formed with Peak Universal Bond (Ultradent) under the etch-and-rinse mode, after treatment with 6N HCL. Original magnification=×5000. A=adhesive; ET=enamel tags formed by enamel crystallites that were wrapped by the adhesive; E=enamel.
Dentin adhesion is more challenging than enamel adhesion due to dentin composition, rendering the etch-and-rinse strategy a highly sensitive technique.8,27
With the acid-etching step 50vol.% of the mineral is solubilized (smear layer and superficial HAp), extracted, and replaced by the rising water. Without the HAp backbone, the superficial collagen network becomes exposed. The rising water combined with the existent 20vol.% content of water from dentin results in 70vol.% of water surrounding the collagen fibrils anchored to the nondemineralized dentin.28 The water prevents the collagen fibers from collapsing.19
After the water is rinsed off, dentin cannot be air-dried to the same extent as enamel, because the dentin collagen network would collapse, blocking primer and bond infiltration.29
In vitro, etch-and-rinse adhesives must be applied following the ‘wet bonding technique’,30,31 especially when an acetone-based adhesive is used.31,32 However, it may be difficult to dry enamel without drying dentin. Clinically it is not easy to visualize how moist is moist or how dry is dry.33,34 Rewetting the dried dentin with water in vitro allows the collagen network to re-expand to a level similar to the moist technique, restoring bond strengths.35–37 De Goes and co-workers recommended to brush out the excess water with a cotton pellet, a disposable brush, or a tissue paper.38 In the same fashion, overwet conditions also result in lower bond strengths due to dilution of the adhesive.39
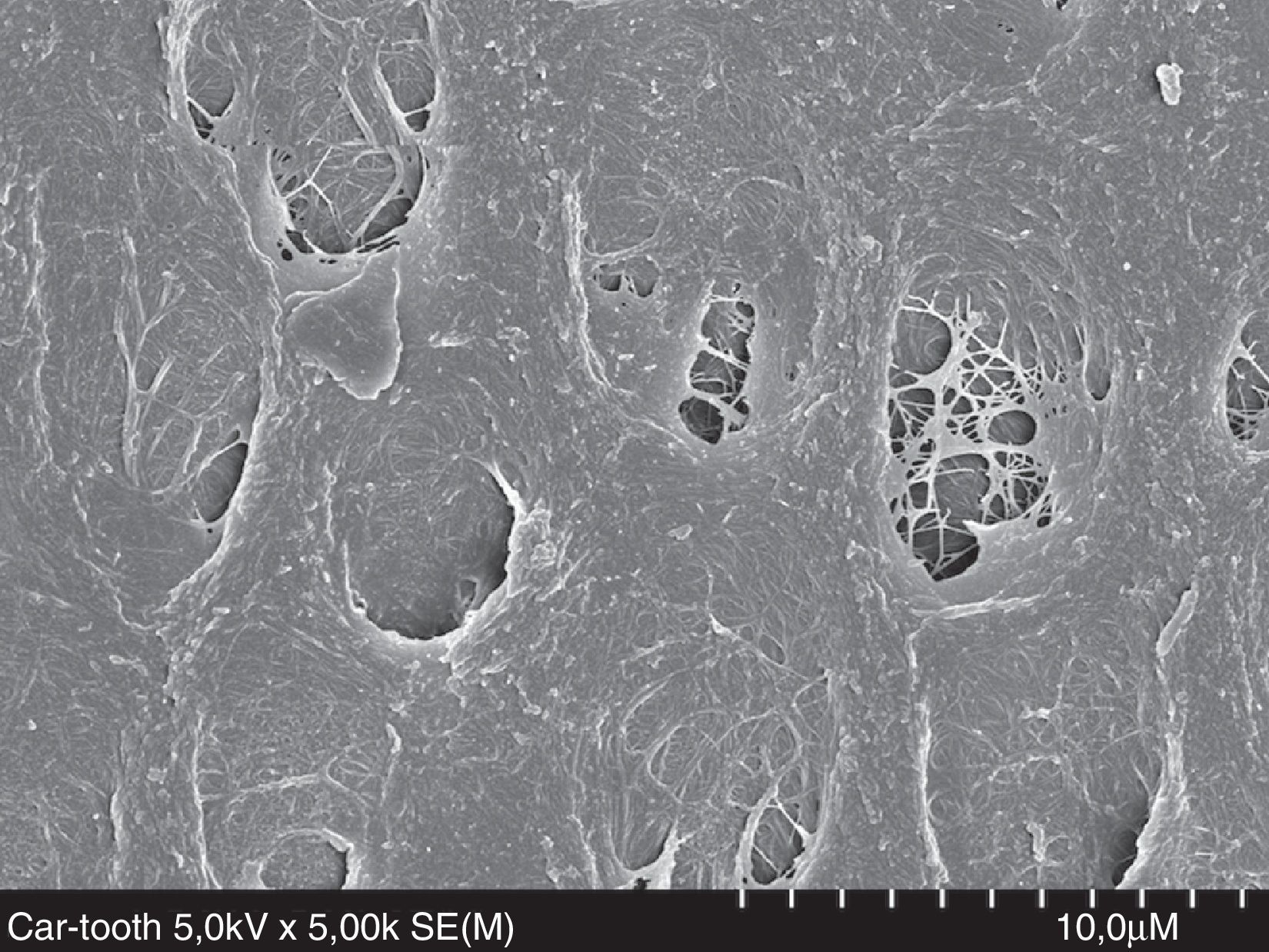
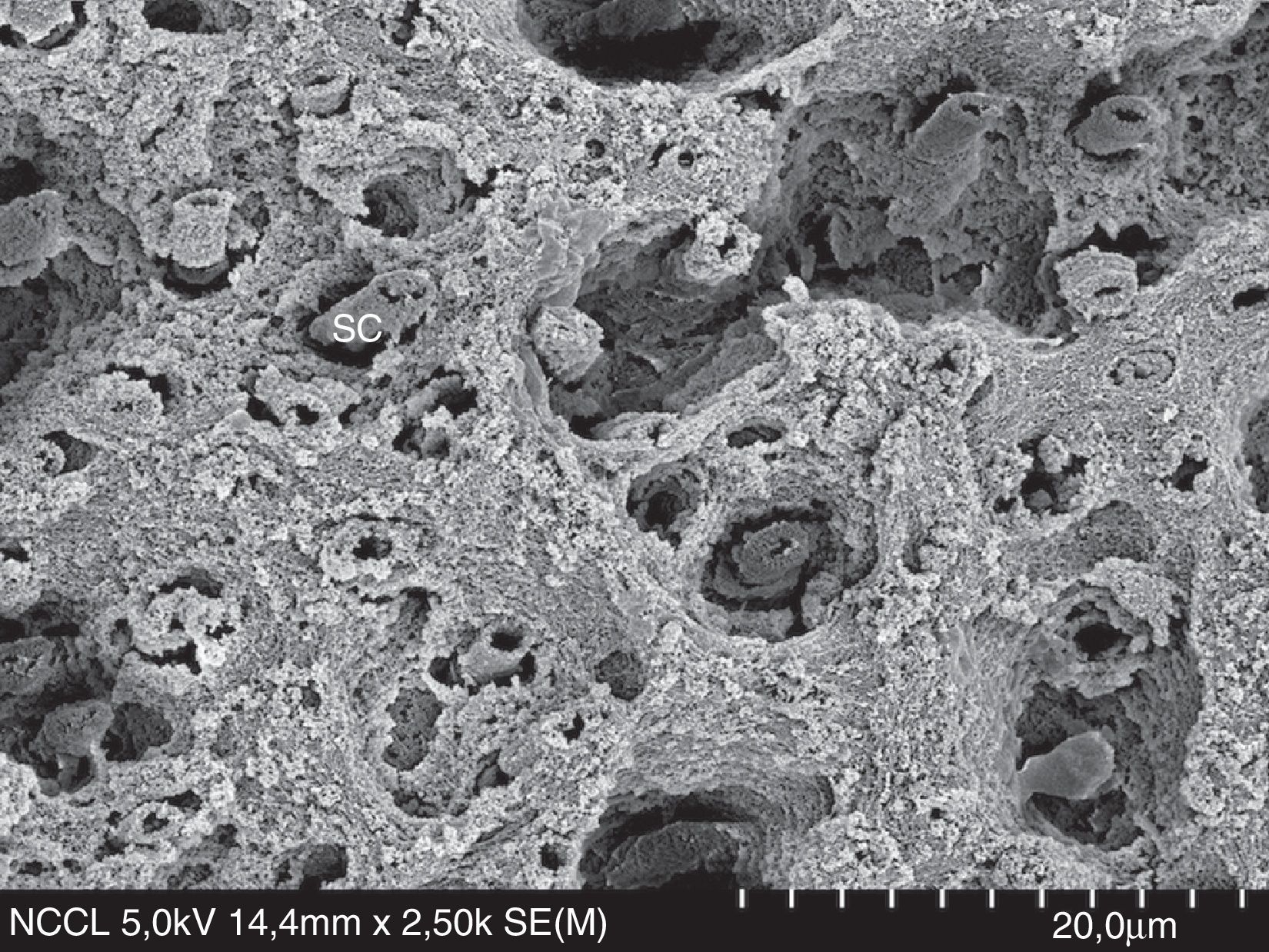
Recent studies have suggested that dentin moisture and solvent-content may not be so important if the etch-and-rinse adhesive is vigorously rubbed onto the dentin surface.40–43 Most of the in vitro studies about moist/dry dentin have been carried out in ideal laboratory dentin (intact dentin from extracted sound molars or bovine teeth). However, caries-affected dentin (Fig. 6) and hypermineralized dentin in non-carious cervical lesions (NCCL, Fig. 7) are the most usual clinical subtracts. An 18-month clinical study in NCCL resulted in similar retention rate for dried and moist dentin, regardless of the solvent based-adhesive (ethanol or acetone) used.44
Ideally, the organic primer (acetone or ethanol) should be able to completely displace the residual water and allow the adhesive resin to fully infiltrate and hybridize collagen after polymerization.37 Water replacement by resin is far from ideal due to the presence of residual solvent and dentin transudation during solvent-evaporation step, and before and after polymerization of the adhesive resin.45,46
The monomer polymerization within the collagen network spaces creates a hybrid layer of collagen and resin (Figs. 8 and 9)6 providing mechanical retention for resin-based restorative materials. Theoretically, for etch-and-rinse adhesives, the bond strengths obtained for hydrophilic adhesives is a sum of the strengths of the resin tags, hybrid layer and surface adhesion.47 However, the role of resin tags on bonding is debatable, as tags have to be firmly bonded to tubules wall to provide retention. For example, deep dentin is rich in tubules but bond strengths are generally lower due to an increase in permeability.48 Nevertheless, formation of resin tags can provide some information about the wettability of the adhesive.49
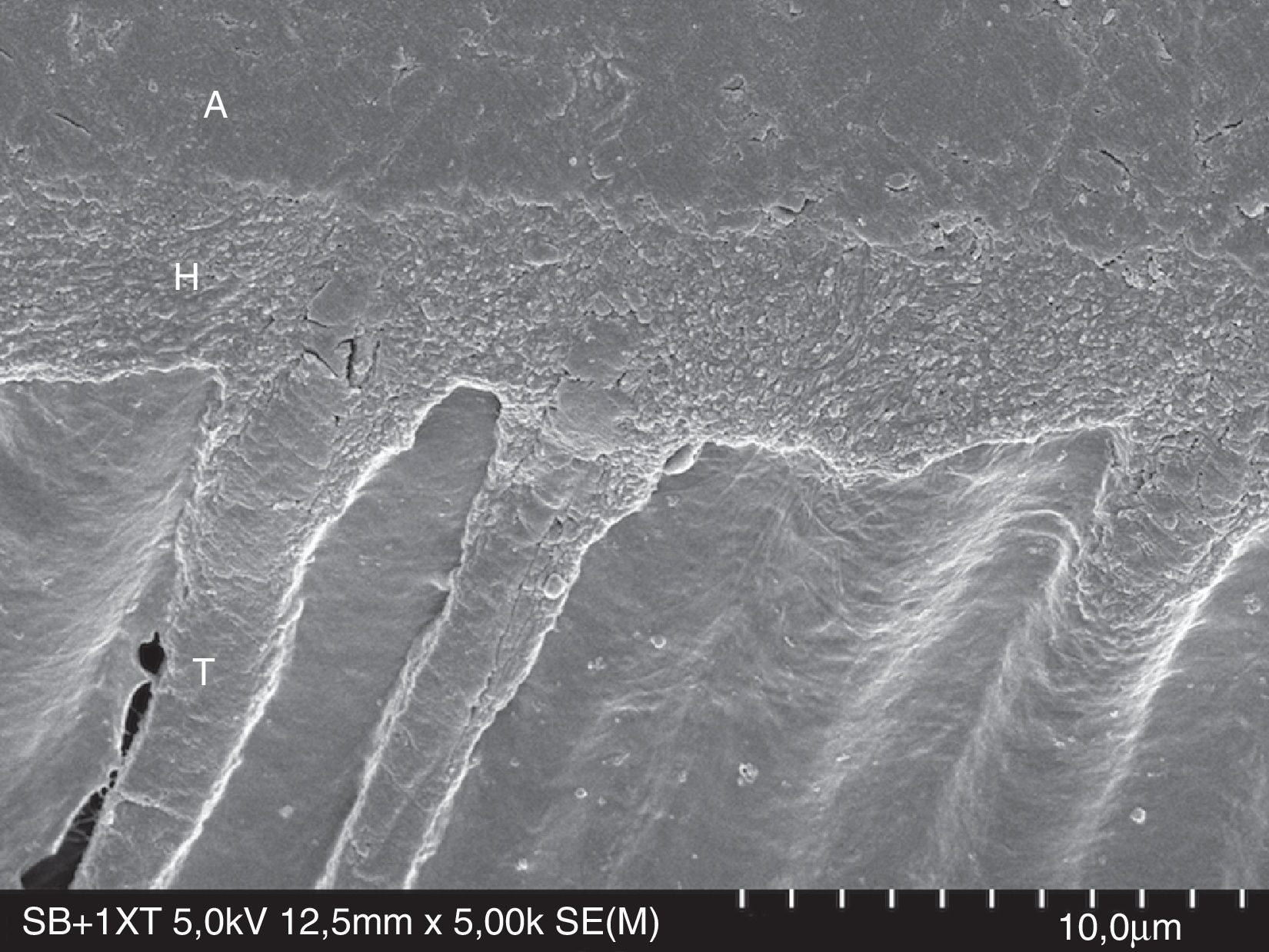
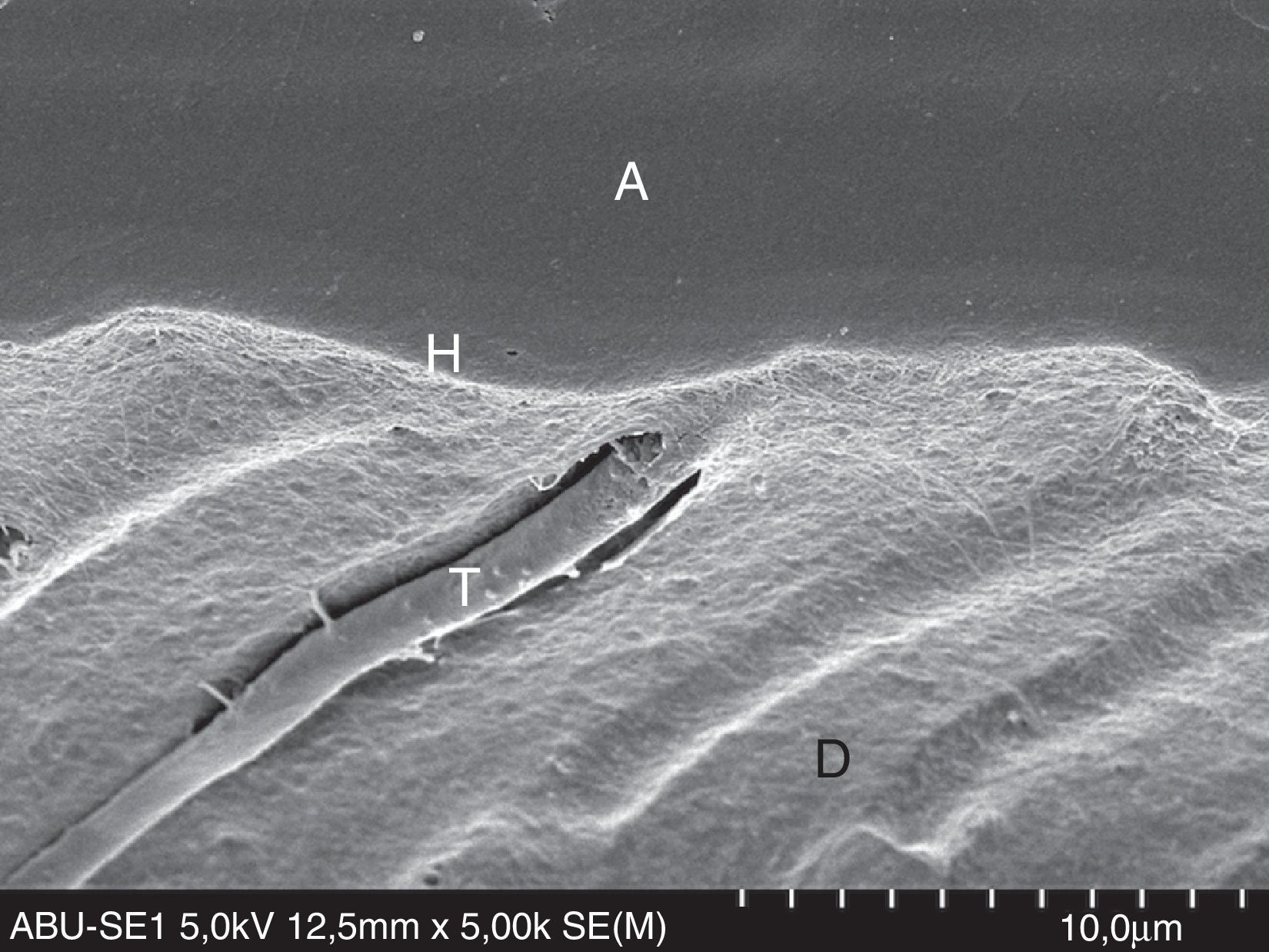
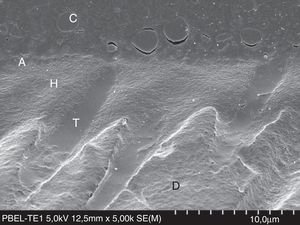
FESEM micrograph of resin–dentin interface formed with the ethanol/water-based etch-and-rinse adhesive Adper Scotchbond 1XT (3M ESPE) following the ‘wet-bonding technique’. Original magnification=×5000. A=adhesive; H=hybrid layer; T=resin tag.
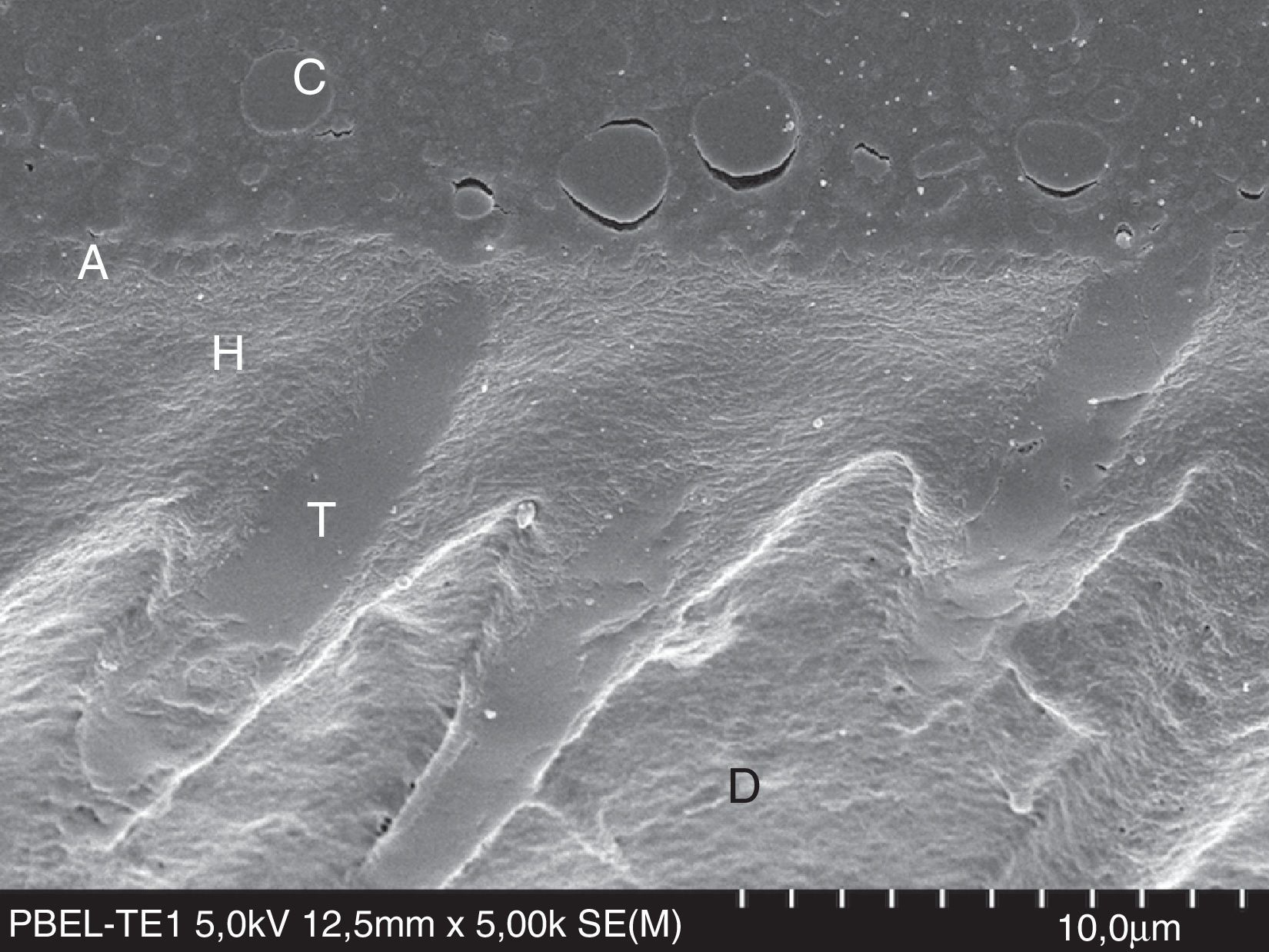
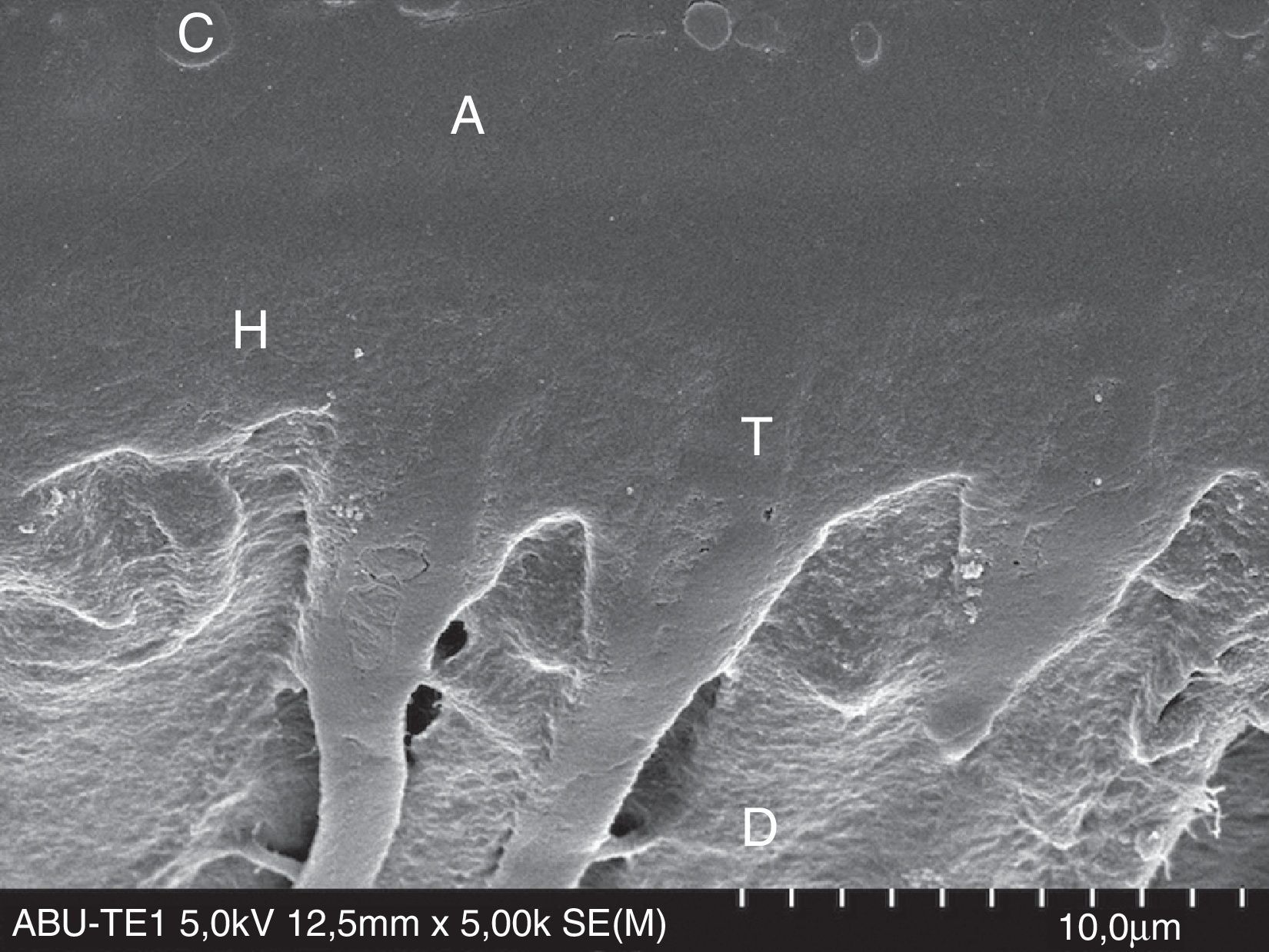
FESEM micrograph of a resin–dentin interface formed with the acetone-based Prime&Bond Elect Universal Adhesive (Dentsply Caulk) applied as an etch-and-rinse adhesive. Note the thin adhesive layer (A) than that of the Adper Scotchbond 1XT (3M ESPE) in Fig. 8. Original magnification=×5000. A=adhesive; C=composite; D=dentin; H=hybrid layer; T=resin tag.
Three-step etch-and-rinse adhesives resulted in better laboratory performance than two-step etch-and-rinse adhesives.22 A study comparing the dentin bond durability of the three-step etch-and-rinse adhesive Scotchbond Multi-Purpose (3M ESPE) with the two-step etch-and-rinse adhesive Single Bond (3M ESPE) resulted in a significant reduction in bond strengths for the latter after 6 months of water storage.50 De Munck and co-workers also observed that bond strengths obtained with two-step etch-and-rinse adhesives were affected after 4 years of water storage within specimens without the protective role of enamel margins.23
The primer/adhesive resin has hydrophilic and hydrophobic monomers. Due to the high hydrophilicity this mixture cannot provide a hermetic seal in deep dentin.51 Two-step etch-and-rinse adhesives behave as semi-permeable membranes after polymerization, allowing continuous transudation of the dentinal fluid.51 If the residual water is incompletely removed from the solvent, added to the diffused water from dentin, water filled channels or water trees form.52 Water-trees are usually visualized after the silver nitrate impregnation technique.53 Additionally, water reduces the degree of conversion of adhesives, resulting in sub-optimal polymerization of the polymer due to the residual water within the hybrid layer and adhesive layer. This water also results in worse mechanical properties of the polymer and, consequently, lower bond strengths.54 If the adhesive system is applied in extreme conditions (overdry/overwet), more voids will be formed at the base of the hybrid layer, which will not be fully infiltrated by resin, leaving a pathway for extrinsic and intrinsic water-flow over time.31
The nanoleakage pattern from etch-and-rinse adhesives is characterized by silver impregnation within the hybrid layer, the adhesive layer, and the fully or partially demineralized dentin at the base of the hybrid layer.51 The latter will be more prone to hydrolytic degradation.55 Even in adequate conditions of moisture and following manufacturers’ instructions, resin does not fully infiltrate the demineralized dentin.56–58
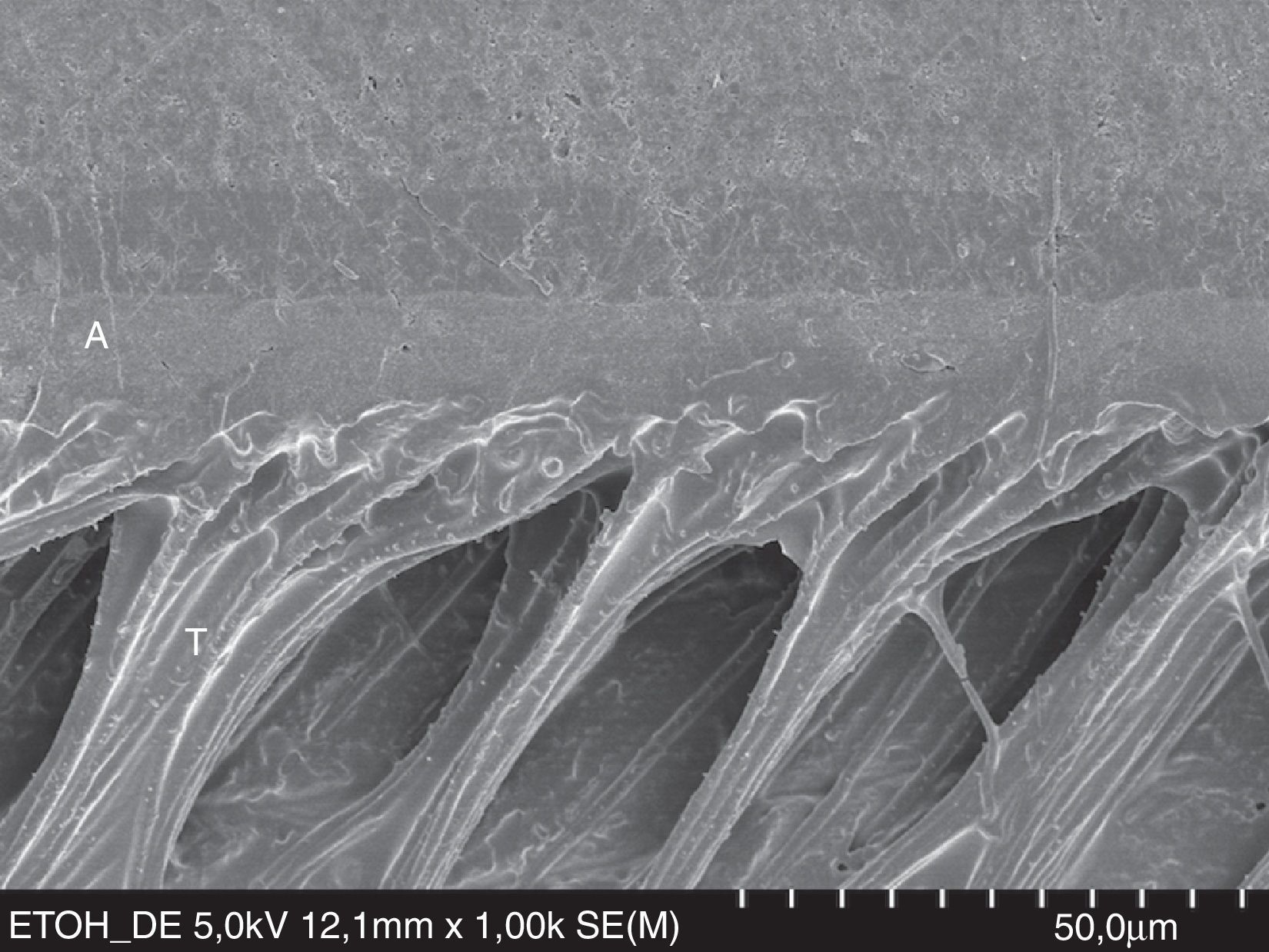
A new trend to overcome hydrolytic degradation of the dentin–resin interface has been developed – the ‘ethanol wet bonding’.59 This technique is very promising, as ‘ethanol wet bonding’ performed better than ‘water wet bonding’, in vitro.59 The concept is based on the exchange of the water in acid-etched dentin by ethanol to enhance resin monomer penetration into the collagen network (Fig. 10).
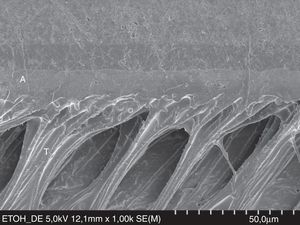
FESEM micrograph of a resin–dentin interface formed with the hydrophobic resin D/E Resin (Bisco Inc.) applied on etched dentin, following the ‘ethanol wet-bonding technique’. Note the area densely filled with resin tags (T). Dentin wettability was enhanced with ethanol saturation. Original magnification=×1000. A=adhesive; T= resin tag.
Ethanol wet bonding resulted in higher bond strength over time. It is believed that this technique results in higher resin uptake and better resin sealing of the collagen matrix, protecting it from endogenous collagenases.60 However, ethanol wet bonding is not achievable in clinical practice as the time required to fully saturate dentin with ethanol makes the clinical application unrealistic.
The bond strengths obtained with etch-and-rinse adhesives also depend on the respective solvent, mainly acetone or ethanol, as the ‘window of opportunity’ for optimal hybridization and tubular seal depends on the chemical composition of the adhesive system.33,46 Ideally, solvent should be totally evaporated before polymerization, to augment proximity of reactant molecules and prevent residual monomers from plasticizing the polymer. Acetone is not able to re-expand air-dried demineralized dentin.42 Under overdry conditions, the infiltration rate of acetone-based adhesives within the hybrid layer may be reduced to 50%.56 Acetone is more sensitive to dentin moisture than ethanol (Figs. 8 and 9).43 Clinical trials have shown a higher retention rate and better performance of the ethanol/water-based adhesive Adper Scotchbond 1XT (3M ESPE; see Table 1) in comparison to the acetone-based adhesive One-Step Plus (Bisco Inc.; see Table 1).40,61
In light of these limitations, several approaches have been suggested to improve the performance of etch-and-rinse adhesives:
- -
To use aqueous 2-hydoxyethyl-methacrylate (HEMA)-based rewetting agents prior to the application of an acetone-based adhesive to widen the window of opportunity.33
- -
Vigorous agitation of the primer solution of three-step etch-and-rinse adhesives might increase the ability for suspension or emulsion polymerization.62
- -
Extend the application/infiltration time of the primer/bonding solution in the two-step etch-and-rinse, even that may be clinical difficult to reach.63,64
- -
Double the number of coats or layers of the primer/bonding solution for two-step etch-and-rinse adhesives, mainly for acetone-based adhesives.65,66
A recent in vitro screening of three-step etch-and-rinse adhesives concluded that Optibond FL (Kerr) may be considered the golden standard of its class, as Optibond FL resulted in higher microtensile bond strengths, lower nanoleakage, and higher degree of conversion compared to Scotchbond Multi-Purpose (3M ESPE), All-Bond 3 (Bisco Inc.) and Fusion Duralink (Angelus).67
Self-etch adhesive strategyTo overcome the problems associated with acid-demineralized dentin depth and resin infiltration of etch-and-rinse adhesives, a user-friendlier and less-sensitive approach was introduced – self-etch adhesive systems. This strategy aimed at simplification and reduced application time.
The self-etch adhesive strategy includes two types of adhesives according to the number of steps involved:
- 1.
Two-step self-etch adhesives (see Table 1 for examples), in which enamel and dentin are simultaneously conditioned and primed with an acidic self-etching primer, followed by the application of an adhesive resin (hydrophobic resin), which must be polymerized.
- 2.
One-step self-etch adhesives (see Table 1 for examples), in which the acidic primer and the hydrophobic adhesive resin come all together in one self-etching solution. Supposedly, this unique solution conditions, primes and infiltrates the substrate prior to polymerization.
Independently of the numbers of steps, the acidic self-etch primer of the two-step self-etch adhesive and the one-step self-etch adhesive solution are complex aqueous solutions of functional monomers (phosphoric-acid and/or carboxylic-acid esters), cross-linkers monomers, monofunctional co-monomers and additives (e.g. fillers, photoinitiators, etc.).4,68 Water is an essential component for adhesive ionization, enabling the respective self-etching characteristic.4 In opposition to etch-and-rinse adhesives, the mandatory presence of water in their composition makes self-etch adhesives less susceptible to variations in the degree of substrate moisture.
Self-etch adhesives are classified according to their acidity:69
- a)
Strong (pH≤1), e.g. Xeno III (Dentsply DeTrey); allowing an interaction of some micrometers depth in dentin and enamel.
- b)
Intermediately strong or moderate (1<pH<2), e.g. AdheSE One F (Ivoclar Vivadent) allowing an interaction depth of 1–2μm.
- c)
Mild (pH≈2), Clearfil SE Bond (Kuraray); allowing an interaction depth of 1μm.
- d)
Ultra-mild (pH>2.5), e.g. Clearfil S3 Bond (Kuraray); nanometric interaction in depth, allowing a true nano-interaction zone,70 in opposition to the traditional and thicker hybrid layer.
The degree of enamel demineralization and interaction with the smear layer and underlying dentin is dependent on the adhesive aggressiveness, i.e. pH and chemical composition.69,71,72 In general, self-etch adhesives demineralize dentin more superficially than total-etch adhesives do, as self-etch are less acidic. The pH of self-etch adhesives is higher than that of phosphoric acid.4
EnamelThe etching pattern on unground enamel depends on the aggressiveness of the self-etch adhesive used,71,73 ranging from absent to moderate.74 Self-etch adhesives are unable to etch enamel to the same depth as phosphoric acid due to their lower pH.75–78 As a result, bond strengths to unground and ground enamel are lower than enamel bond strengths achieved with acid phosphoric etching.71,74
Perdigão and Geraldeli observed lower enamel–resin bond strengths in the absence of etching pattern.74 Self-etching adhesives performed better on prepared enamel than on unprepared enamel74; therefore, enamel roughening is recommended prior to the application of self-etch adhesives.71,77
A study, in which the sealing capacity of a self-adhesive Enamel Loc pit and fissure sealant (Premier Dental Products Co.) was tested, resulted in an higher silver nitrate infiltration at the enamel–sealant interface when Enamel Loc was directly applied to unprepared enamel, as per the respective manufacturer's instructions.79 Even the previous application of the strong self-etch adhesive Adper Prompt-L-Pop (3M ESPE) did not significantly reduce the silver nitrate infiltration. The sealing capacity was enhanced when enamel was etched with phosphoric acid previous to the application of the self-etch/self-adhesive sealant.79
Some manufacturers recommend pre-etching unground enamel with phosphoric acid prior to the application of self-etch adhesives to improve enamel–resin bond strength.71 Enamel selective etching is highly recommended when self-etch adhesives are used (Figs. 1 and 5).80
DentinThe concept of self-etching, self-priming adhesives is to simultaneously etch and prime dentin at the same extent, integrating the smear layer into the adhesive interface.69,81 Because the smear layer is left partially intact, this class of adhesives may cause less post-operative sensitivity than that of etch-and-rinse adhesives.82,83 However, this decrease in post-operative sensitivity associated to self-etch adhesives lack scientific validation, as several controlled clinical trials concluded that post-operative sensitivity might be more related to the operator technique than to the bonding strategy.84,85
The smear layer thickness and smear layer buffer effect may interfere with the demineralizing process of the self-etching adhesives.86 This interference is a controversial subject as the buffer capacity of the smear layer is low due to smear layer dissolution after self-etch adhesive application and also due to the presence of interconnecting channels at the superficial part of the smear layer.69 However, Kenshima and co-workers suggested that the smear layer could have regional differences in density that might influence the degree of adhesive infiltration, leading to interfacial gaps.87 For mild and moderate self-etch adhesives, if a thick smear layer is present, a narrow hybrid layer forms with low density of resin tags.87 Nonetheless, the depth of dentin infiltration and the thickness of the hybrid layer are not a predictor of bond strength.72 For enamel, on the other hand, the demineralization capacity is correlated to enamel bond strengths.
The question persists if resin tags contribute to long-term stability. It is believed that micromechanical interlocking can provide immediate resistance to adhesive debonding, but chemical interaction will improve the durability and overcome the adhesive-interface degradation.19,80
Mild- or ultra-mild self-etch adhesives have a two-fold interaction with dentin: (1) micromechanical interaction due to polymerization in situ of the infiltrated adhesive monomers; and (2) chemical interaction, due to ionic bonding between functional monomers of adhesive systems and the calcium in residual dentin HAp. Yoshioka and co-workers argued that specific functional monomers, such as phosphoric acid esters88 and carboxylic acids,89 could interact with HAp through an ionic reaction.90 The chemical interaction of self-etch adhesives with calcium in HAp is explained by the ‘adhesion–decalcification’ concept.91 According to this model, all acids bond ionically to calcium in HAp, through the exchange of phosphate ion from HAp into the solution.89 Polycarboxylic acids, regardless of their concentration/pH, can adhere or decalcify HAp, depending on the dissolution rate (solubility) and stability of the resulting calcium salts.91 In the first phase, carboxylic acids bond to calcium of HAp. In the second phase, depending on the diffusion rate of calcium–acid complex into solution, the acid will either remain attached to the HAp surface with only limited decalcification involved, or the calcium–acid complex will debond, resulting in a substantial decalcification effect.
Chemical adhesion is not a new finding in dental adhesion, as glass-ionomer-based materials have been used to bond chemically to dentin and enamel since 1971.92,93 Glass ionomer cements set through an acid–base reaction and form a strong ionic interaction between carboxylic groups (COO-) of the polyalkenoic acid (within the glass ionomer liquid), and calcium in HAp.93 The polyalkenoic acids interact with apatite substrates following the adhesion–decalcification concept.94 The strong and stable self-adhesiveness of glass ionomer-derived materials is responsible for the excellent long-term performance of these materials.95,96 A 13-year follow-up in NCCL, in which the three bonding strategies – etch-and-rinse, self-etch and glass-ionomer – were analyzed, resulted in a higher retention rate for the glass ionomer materials,95 despite the signs of degradation observed for all the adhesive systems over time.
Mitra and co-workers reported that the polyalkenoic acid first used in the Vitrabond (later Vitrebond, 3M ESPE) resin-modified glass-ionomer cement – Vitrebond co-polymer (VCP)97 – bonds chemically to calcium in HAp,96 being a significant factor for Vitrebond excellent long-term clinical performance.27,96,98 Fourier transformed infrared spectroscopy confirmed a chemical interaction between VCP and calcium in Hap (unpublished results). VCP is a component of several commercial dental adhesives made by 3M ESPE, namely Scotchbond Multi-Purpose primer and Adper Scotchbond 1XT adhesive (also known as Adper Single Bond Plus or Adper Single Bond 2), the self-etch adhesive Adper Easy Bond and the newest Scotchbond Universal Adhesive (see next section Multi-mode or Universal adhesive). The chemical interaction may be responsible for excellent long-term in vitro and clinical results of VCP-based adhesives.25,27,99–105
Clearfil SE Bond (CSE, Kuraray) is a mild (pH≈2) two-step self-etch adhesive known as the golden standard for self-etch adhesives (Fig. 11).4,27,102 A recent 8-year follow-up in NCCL recorded excellent clinical performance in dentin for CSE, with minor leakage in enamel without selective etching.83 The higher clinical and in vitro performance and durability are related to three main points: (1) being a mild self-etch, CSE slightly demineralizes dentin, allowing some residual hydroxyapatite to evolve and protect collagen fibrils; (2) presence of the functional monomer 10-methacryloyldecyl dihydrogen phosphate (10-MDP) component of the CSE primer; and (3) the presence of an hydrophobic coat, as a 2-step self-etch, in the second bottle of the CSE instructions for use. The application of a filled hydrophobic layer as second step for CSE improved the conversion rate of the adhesive as well its mechanical properties.106
FESEM micrograph of an interface formed with Clearfil SE Bond primer (Kuraray) applied on dentin following the manufacturer's instructions. A zone of mild dentin decalcification can be observed in the monomers enveloping HAp and collagen fibers. Original magnification=×20,000.
The monomer 10-MDP can ionically interact with calcium in HAp and form hydrolytically stable 10-MDP-calcium salts,80,107 through a self-assembled nano-layered interaction.107,108 This complex bonding of two 10-MDP molecules joined by stable MDP-calcium salt formation becomes the adhesive interface more resistant to degradation over time.108
Others functional acidic monomers such as phenyl-P and 4-MET have been introduced.4,107 However, none of these monomers could provide higher bond strengths than 10-MDP. Furthermore, their complex calcium salts are more soluble and hydrolytically instable, providing a lower bond stability.27,69,109 The instability/hydrophilicity and shelf-life problems of self-etch adhesives have been a concern for researchers.3,68,69
When one-step self-etch adhesives are applied onto dentin, air-drying the solvent may not be able to remove all the water and solvent,110,111 compromising the monomer polymerization and bond strength. Blisters are formed in the adhesive layer due to a rapid monomer-phase separation.112 In fact, monomers separate from water upon evaporation of the respective solvent. However, the sensitivity to air-drying seems to be material dependent.110,111 Tay and co-workers reported that one-step self-etch adhesives behaved as permeable membranes after polymerization as two-step etch-and-rinse adhesives,113,114 allowing water from the hydrated dentin to crossover the adhesive layer, forming blisters at the adhesive–composite resin interface and water-trees.52 Likewise, hydrophilic monomers in the oxygen inhibition layer enhance water sorption through osmosis.114 One-step self-etch adhesives are more prone to degradation of the resin–dentin interface by hydrolysis than two-step self-etch adhesives.45,52,115
In enamel, one-step self-etch adhesives also result in water blistering, which may compromise enamel bonding.116 To improve the immediate and long-term quality of the interaction between one-step self-etch adhesives and dentin, a few suggestions have been proposed:
- -
Apply more coats than those recommended by the respective manufacturer.117
- -
Actively apply the one-step self-etch adhesive scrubbing vigorously onto the dentin surface.118,119
- -
Apply an extra hydrophobic resin coating to improve in vitro and clinical performance of one-step self-etch adhesives, transforming them in two-step self-etch adhesives.120,121
With the expiration of the patent for the 10-MDP molecule from Kuraray, a new family of adhesive systems, with chemical adhesion potential, have been launched. They are called multi-mode or universal adhesives due to their versatile instructions for use (see Table 1 for examples).
According to the respective manufacturers, universal adhesives can be used under the etch-and-rinse mode (Fig. 12), the self-etch mode (Fig. 13), or with enamel selective etching.122 An exception is G-Bond Plus (GC), for which etching dentin is not recommended.123
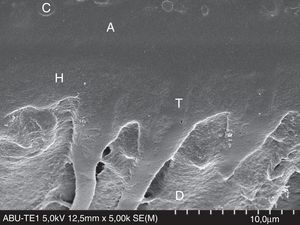
FESEM micrograph of a resin–dentin interface formed with All-Bond Universal Adhesive (Bisco Inc.), applied in etch-and-rinse mode. Original magnification=×5000. A=adhesive; C=composite; D=dentin; H=hybrid layer; T=resin tag.
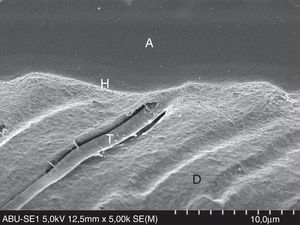
FESEM micrograph of a resin–dentin interface formed with All-Bond Universal Adhesive (Bisco Inc.), applied in self-etch mode. Note the thinner hybrid layer (H) and narrow resin tags (T). Original magnification=×5000. A=adhesive; D=dentin; H=hybrid layer; T=resin tag.
Scotchbond Universal Adhesive (SBU, 3M ESPE) was the first commercial universal adhesive124 and, consequently, often cited in the peer-reviewed literature.105,122–128 When SBU was applied on dentin, mean microtensile bond strengths did not vary with the variations in dentin moisture or the adhesive strategy used.124,125 However, nanoleakage was significantly lower when SBU was applied in self-etch mode.129 This means that SBU is not sensitive to the degree of dentin moisture, but residual HAp is needed to achieve optimal dentin–resin hybridization. The insensitivity of SBU to air-dried dentin may be explained by the water content of the adhesive (10–15% by wt) that allows the expansion of the collagen network. The dependence on HAp may be a result of the composition of SBU, which includes both 10-MDP and the polyalkenoic co-polymer, molecules capable of ionic bonding with calcium in HAp.124 Apart from the bond strengths, a low variability may be an indicative of a low technique sensitivity of SBU.115 The in vitro results were clinically confirmed through an 18-month follow-up in which Perdigão and co-workers only observed differences in the marginal adaptation among the adhesive strategies (etch-and-rinse; self-etch; self-etch with selective enamel etching).105 In fact, enamel selective etching is a pre-requisite as SBU is ‘ultra-mild’ self-etch adhesive (pH=3) unable to etch enamel at the same depth of phosphoric acid.124
The chemical interaction is a crucial characteristic of universal adhesives to enhance durability of dentin–resin interfaces. Bond strengths are usually lower than those of two- and three-step etch-and-rinse adhesives, but in line with results reported for self-etch adhesives.102,126
The in vitro performance of universal adhesives has been reported as material-dependent due to the complexity of their chemical composition.125,126,129 As explained elsewhere, all simplified adhesives behave as permeable membranes (either two-step etch-and-rinse or one-step self-etch adhesives). As universal adhesives are one-step self-etch adhesives, they behave in the same fashion.126,128 If the exposed collagen is not fully encapsulated by the polymerized adhesive monomers, demineralized collagen fibrils will be vulnerable to time-dependent hydrolytic degradation by water, leaving voids within the hybrid layer or demineralized nano-channels.53,130
As for one-step self-etch adhesives, coating universal adhesives with an extra layer of a hydrophobic resin improves their immediate123,128 and long-term bond strengths and degree of conversion, consequently lowering nanoleakage.
Furthermore, universal adhesive infiltration is enhanced if active application is used.128
ConclusionAccording to the current dental adhesion literature, adhesive systems which include an hydrophobic resin coating are able to better prevent water degradation in comparison to adhesive systems without the hydrophobic resin coating, regardless of the bonding strategy.23,128,131 However, laboratory and clinical performance of adhesive systems seem to be dependent on their specific chemical composition.80,131
One must be aware that, although simplification of the adhesive systems have been associated with loss of effectiveness,27 advances have been made to improve the chemistry of the newest adhesives. Two-step self-etch adhesives have shown that they can outperform the ‘golden standard’ etch-and-rinse adhesives.131
Conflicts of interestThe authors have no conflicts of interest to declare.
Special thanks to Professor Jorge Perdigão (Department of Restorative Sciences, University of Minnesota, Minnesota, USA) for providing the scanning electron micrographs.