To evaluate the effects of tooth whitening with 10% carbamide peroxide on the surface ultramorphology of three dental restorative materials.
Materials and methodsThree materials were tested: (1) a reinforced zinc oxide-eugenol cement (IRM® Powder Zinc Oxide Eugenol, Dentsply, Konstanz, Germany), (2) a high copper amalgam with spherical particles (Ventura Finest®, Madespa SA, Toledo, Spain) and (3) a nanohybrid composite (EvoCeram Tetric®, Ivoclar Vivadent AG, Schaan, Liechtenstein). 6 specimens of each material (N=18) were inserted into silicone molds with circular cavities of 10mm×2mm. 3 specimens of each material were randomly assigned into the whitening or control groups. In the whitening group, the specimens were exposed to a 10% carbamide peroxide gel (Opalescence® PF 10%, Ultradent, South Jordan, UT, USA) for 14 consecutive days, 6h per day. In the control group, the specimens were exposed to distilled water. After 14 days stored at 37°C, all specimens were analyzed by scanning electron microscopy.
ResultsThe specimens exposed to carbamide peroxide revealed an irregular surface, with cracks and pores. Specimens from all control groups showed a smooth surface.
ConclusionThe 10% carbamide peroxide gel may cause changes in surface ultramorphology of the materials tested: reinforced zinc oxide-eugenol cement, amalgam and composite.
Avaliar os efeitos do branqueamento dentário com peróxido de carbamida a 10% na ultra-morfologia de superfície de materiais restauradores dentários.
Materiais e MétodosForam testados três materiais: 1) um cimento de óxido de zinco-eugenol reforçado (IRM® Powder Zinc Oxide Eugenol, Dentsply, Konstanz, Alemanha), 2) um amálgama de alto teor de cobre de partículas esféricas (Ventura Finest®, Madespa SA, Toledo, Espanha) e 3) um compósito nanohíbrido (Tetric EvoCeram®, Ivoclar Vivadent AG, Schaan, Liechtenstein). 6 espécimes de cada material (N=18) foram inseridos em moldes de silicone com cavidades circulares de 10mm x 2mm. 3 espécimes de cada material foram distribuídos aleatoriamente para os grupos de branqueamento ou de controlo. No grupo de branqueamento, os espécimes foram expostos a um gel de peróxido de carbamida a 10% (Opalescence PF 10%®, Ultradent, South Jordan, UT, EUA) durante 14 dias seguidos, 6 horas diárias. No grupo de controlo, os espécimes foram expostos a água destilada. Após 14 dias de armazenamento a 37°C, todos os espécimes foram analisados através de microscopia electrónica de varrimento.
ResultadosOs espécimes expostos ao peróxido de carbamida revelaram uma superfície irregular, com fendas e poros. Os dos grupos de controlo apresentaram uma superfície regular.
ConclusãoO gel de peróxido de carbamida a 10% pode causar alterações na ultra-morfologia de superfície dos materiais testados: cimento de óxido de zinco-eugenol reforçado, amálgama e compósito.
Tooth whitening is a popular technique used in esthetic dentistry,1 being widely accepted as an effective clinical procedure.2 Although considered relatively safe with regard to systemic effects, recently, some controversy has arisen related to its effects on restorative materials.1 The effect of whitening agents on restorative materials should be analyzed for their potential deleterious consequences on physical, mechanical and corrosive properties. The changes on materials properties may have important clinical implications, since the prognosis and the longevity of a dental restoration may depend upon them.3
Rotstein et al. have concluded that both 10% carbamide peroxide (CP) and 10% hydrogen peroxide (HP) altered the surface ultramorphology of reinforced zinc oxide-eugenol cement fillings, through scanning electron microscopy (SEM) analysis, with differences on the zinc oxide levels.4
Regarding amalgam, the greatest point of interest and research has been the mercury, with several authors detecting an increased concentration after whitening on the: (1) restorative surface, (2) immerging water, and (3) whitening product.1,5–8 Nevertheless, the mercury concentration was lower than the guidelines recommended by the World Health Organization (WHO) and National Academy of Sciences’ Food and Nutrition Board (NASFNB).1 The mercury release from fillings is dependent on the: (1) duration of the whitening treatment, (2) amalgam age, (3) surface polishing conditions, (4) composition and pH of the whitening agent,7 and (5) surface area of the restoration.7,9
Concerning the surface changes, Rotstein et al.5 and Gurgan et al.7 concluded that slight differences in amalgam surface regularity can be observed on SEM micrographs, after application of CP and HP. Nonetheless, other two experimental studies6,10 have not reported surface alterations after treatment with CP and HP.
Several studies have demonstrated an increase on the surface roughness of resin composites after whitening with 10% CP and/or 10% HP.11–13 Furthermore, some authors14 verified the existence of cracks visible to the naked eye with this material. Wattanapayungkul et al.3 concluded that the effect of whitening agents on surface roughness of composites is dependent on the specific material tested and time, with higher concentrations of HP causing higher roughness. In these cases, repolishing or replacing these restorations may be necessary after long periods of whitening treatment to allow the reestablishment of the esthetic properties and to prevent the colonization of cariogenic microorganisms.3,14,15
The objective of the present study was to assess the effects of tooth whitening on the surface ultramorphology of reinforced zinc oxide-eugenol cement, amalgam and resin composite analyzed by SEM. The null hypothesis tested on this study was that tooth whitening using a commercially available 10% CP gel does not cause any changes on the surface ultramorphology of reinforced zinc oxide-eugenol cement, amalgam and resin composite when compared with the surface ultramorphology of specimens of these materials exposed to distilled water.
Materials and methodsThree restorative materials were studied: a nanohybrid composite (EvoCeram Tetric®, Ivoclar Vivadent AG, Schaan, Liechtenstein) with A4 color, a high copper amalgam with spherical particles (Ventura Finest®, Madespa SA, Toledo, Spain) and a reinforced zinc oxide-eugenol cement (IRM® Powder Zinc Oxide Eugenol, Dentsply, Konstanz, Germany). 18 specimens were prepared, 6 specimens of each material, according to manufacturer's instructions and using silicon molds of 10mm diameter and 2mm thickness.
Amalgam capsules were vibrated on an amalgam vibrator for 5s and, with the aid of an amalgam carrier, the material was placed in the molds, being condensed with an amalgam condenser. For the reinforced zinc oxide-eugenol cement, powder and liquid were mixed with a spatula, in a glass plaque, and the material was inserted in the molds. After the material setting, the specimens were removed from the molds. The composite disks were obtained by the insertion of a single increment, with a spatula, into the molds. Each specimen surface was light-cured for 40s with a curing light (XL 3000®, 3M ESPE, St. Paul, MN, USA). The disks were then removed from the molds, placed inside sealed test tubes containing distilled water and stored at 37°C for 48h. Three specimens of each material were randomly assigned into the whitening or control groups.
All the specimens were polished with a different standardized method for each material. The amalgam disks were polished using, sequentially, a green stone (Komet®, Gebr. Brasseler, Lemgo, Germany), and three amalgam rubber points of increased rugosity (Identoflex®, Kerr-Hawe, Bioggio, Switzerland). Besides, the specimens were polished with pumice using a brush (Komet®, Gebr. Brasseler, Lemgo, Germany). The reinforced zinc oxide-eugenol cement specimens were polished with a green stone (Komet®, Gebr. Brasseler, Lemgo, Germany). The composite disks were polished with polishing disks (Sof-Lex®, 3M ESPE, St. Paul, MN, USA) and a rubber point (Kenda Composite Microfill®, Kenda, Vaduz, Liechtenstein). After the polishing procedures, the specimens were placed in distilled water and stored at 37°C for 24h.
For 14 consecutive days, the whitening groups specimens were exposed to 10% CP (Opalescence® PF 10%, Ultradent, South Jordan, UT, USA). The gel was applied every day at the same time. The application was made covering the entire polished surface and the specimens were left undisturbed for 6h in a dry incubator at 37°C. After this period of time, each specimen was washed with distilled water for 1min and placed in 10mL of distilled water and returned to the incubator. The specimens from the control groups were exposed to distilled water for 14 days and kept inside of an incubator at 37°C for all duration of the study. The water was changed every 24h. After the last application of whitening gel on the whitening group specimens, all specimens, from both groups, remained 24h in distilled water and then left to air dry for 7 days in a covered container.
The polished surface of each specimen was coated by sputtering with gold–palladium (Jeol JFC-1100 E®, TakeOff Corporation, Tokyo, Japan). The entire surface was examined using a scanning electron microscope (Hitachi S-450®, Tokyo, Japan). An imaginary grid that divided each sample surface into 9 parts was created. In each sample all these parts were observed and microphotographs were taken at magnifications of 33×, 250×, 500× and 1000×. The image that best represented what occurred in most of the specimen surface was chosen as representative.
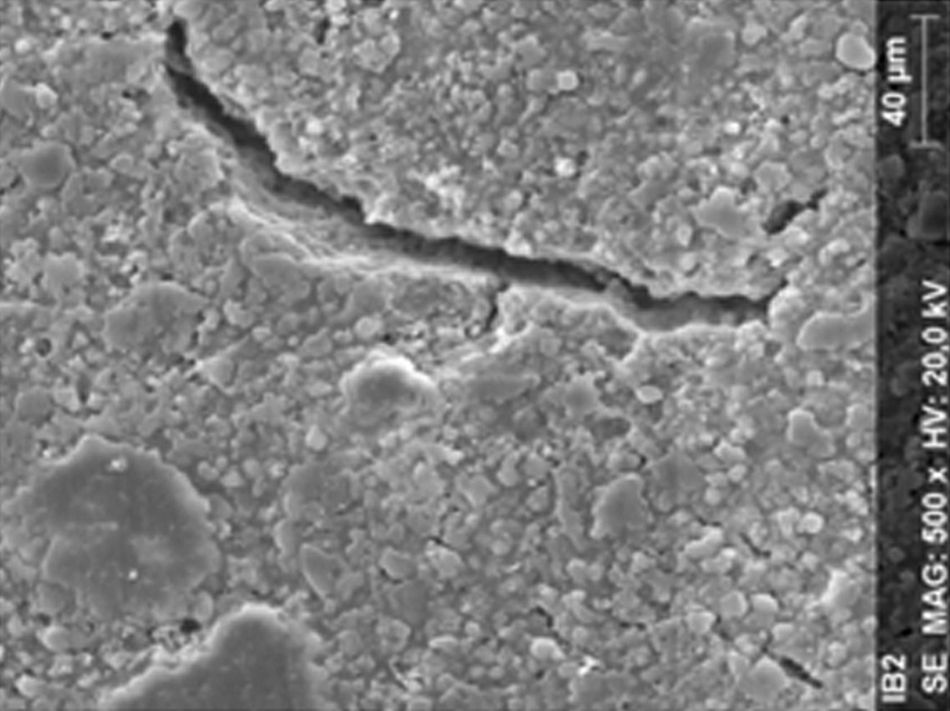
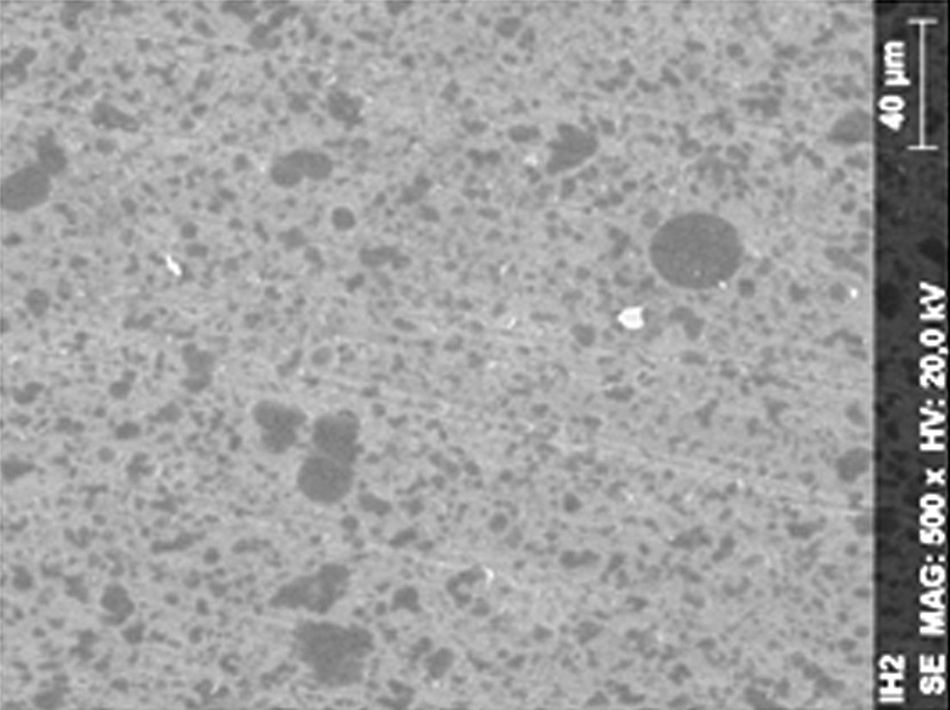
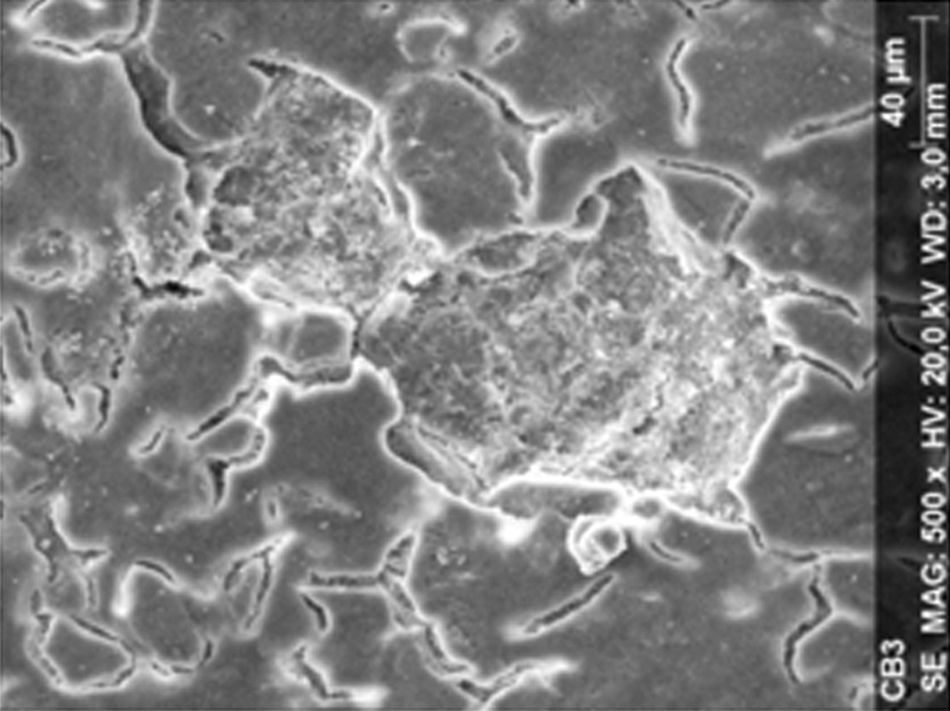
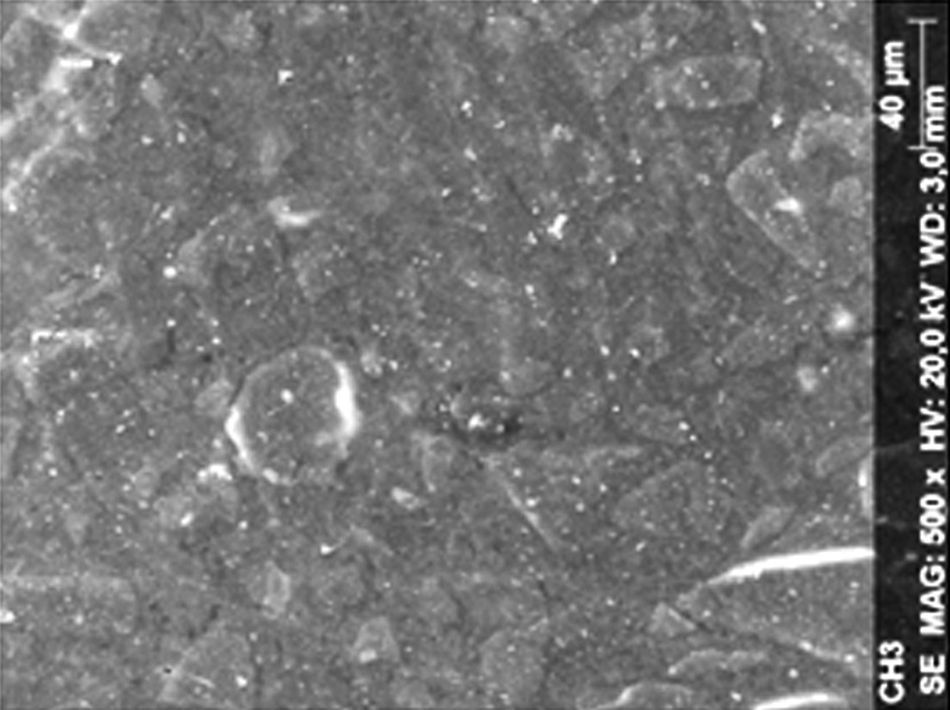
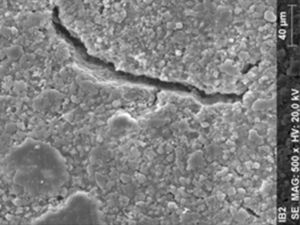
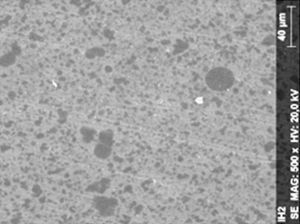
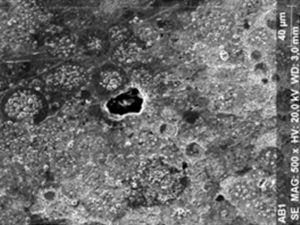
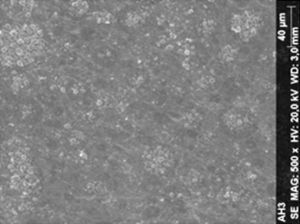
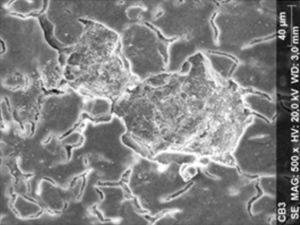
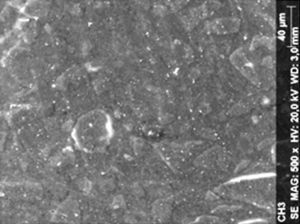
ResultsThe microscopic analysis of the reinforced zinc oxide-eugenol cement specimens, exposed to CP, revealed an irregular surface, with numerous and relatively large cracks and pores (Fig. 1). Specimens from the control group showed a smooth surface, and although some cracks and pores were visible, these were few and small in size (Fig. 2).
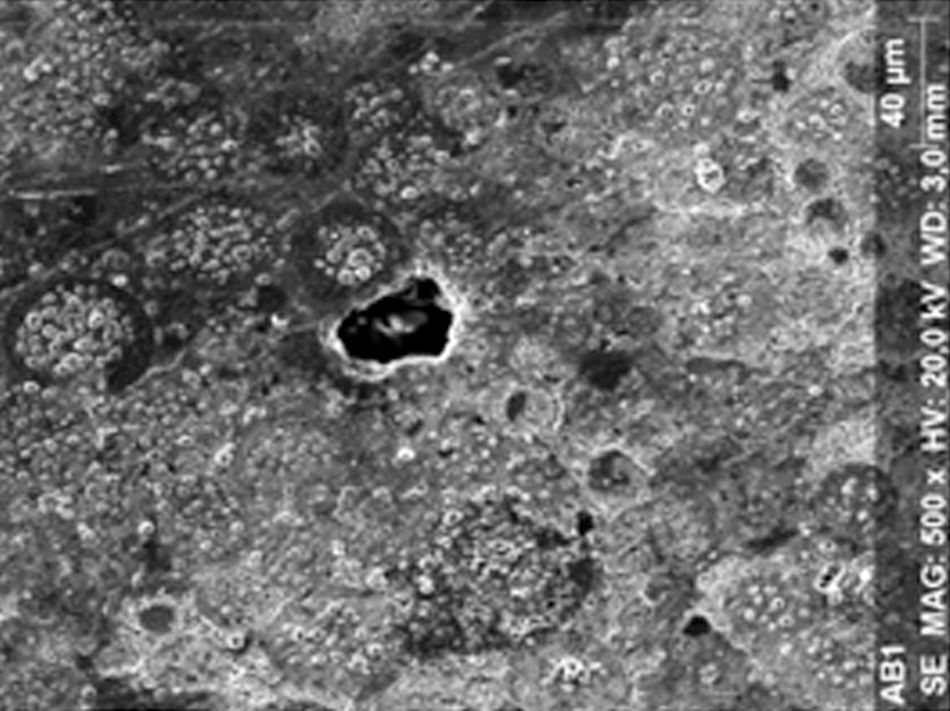
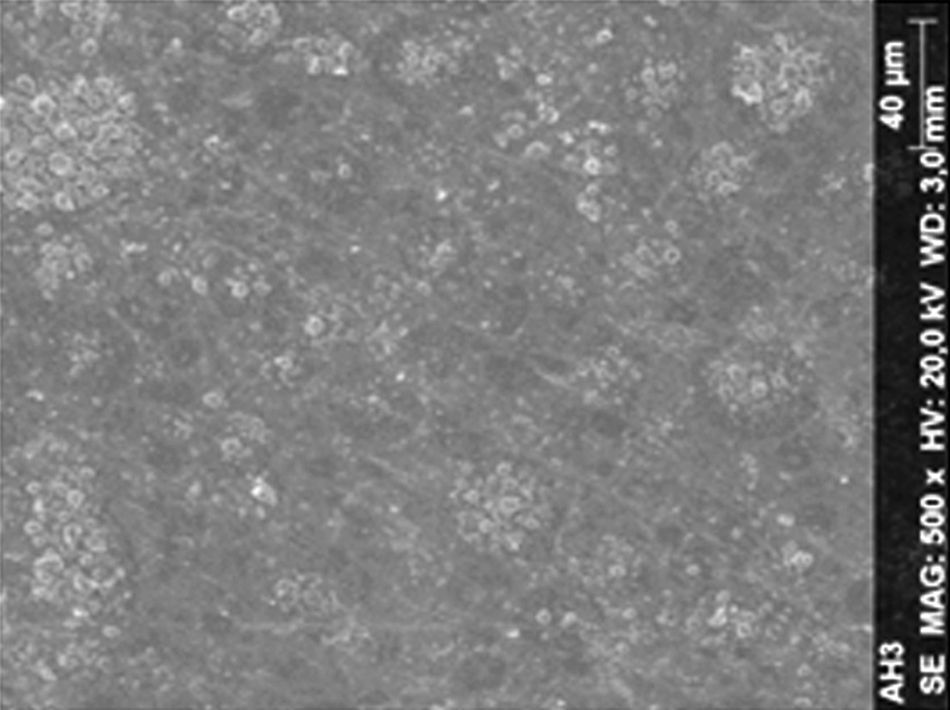
Regarding the amalgam groups, the specimens subjected to the whitening procedure had an uneven surface ultramorphology with pores and some scattered cracks (Fig. 3). In the control group, the micrographs revealed a regular surface, with evidence of the spherical nature of the tested amalgam (Fig. 4).
Composite specimens exposed to CP revealed on the microscopic analysis multiple cracks and an increase in surface porosity (Fig. 5). Specimens of the control protocol presented a regular surface morphology, without the existence of cracks (Fig. 6).
With all materials, a few notches were visible, that are thought to be the result of the polishing procedures.
DiscussionAccording to the results obtained, it was clear that there were differences between the surface ultramorphology of the whitening groups and the control groups, for all the materials tested through SEM observation. The null hypothesis was rejected, since the 10% CP gel may cause changes in surface ultramorphology of the materials tested.
The results obtained with the reinforced zinc oxide-eugenol cement specimens are consistent with those found by Rotstein et al., where the 10% CP and HP solutions were applied for 7 days. Until date, that was the only article published assessing the effect of whitening on this material, using SEM.4
Regarding amalgam, the differences observed between specimens of the whitening and the control groups are supported by the study of Rotstein et al.5 On that study, the authors established an association between the surface degradation induced by the whitening agent and, a decrease on the levels of tin and copper, or increased levels of mercury and silver. Usually with amalgam, the anticorrosion effect is related with the presence of tin and copper. The values found by those authors allowed the conclusion that the decrease in the level of these constituents was caused by the oxidative effect of the whitening agent. In the present study, the surface degradation observed may be due to the action of reactive oxygen molecules caused by the decomposition of CP. These molecules may have reduced the copper–tin phase, leaving the surface material unprotected, and leading to a dissolution of the mercury phase, the largest of any amalgam, which resulted on the existence of cracks and pores, in contrast to the specimens exposed only to distilled water.
Nevertheless, the results of this study contrast with other in vitro studies,6,10 which used SEM observation to analyze the amalgam surface. On those studies, no differences were found between the experimental and control specimens. The reasons for different results among studies are uncertain in this case. However, it can be speculated that the different protocols or whitening products used may explain some of the differences.
Regarding resin composite, the differences found between the whitening and the control specimens are supported by several studies.12,15–17
Composites are more prone to chemical changes, compared to metal or ceramic restorations, due to their organic matrix.17 HP has the ability to degrade the matrix polymers of the resin composites.17 It has been suggested that HP and highly energetic radicals may have an effect on the filler–resin interface and cause a separation of the matrix and the filler, which results in crack propagation and increased surface roughness.3
According to Polydorou et al.,15 the lower content of inorganic filler of some materials may be the cause of their greater susceptibility to changes during the whitening procedure, and differences in surface roughness are more likely to be expected to occur with composites with higher content of resin and less of inorganic filler. In the present study, the surface changes were displayed as an increased occurrence of porosity and cracks. This seems to be related to the relatively low content of inorganic filler of the nanohybrid composite used, which was reported by the manufacturer as being approximately 61%. These changes may have been triggered by complex interactions among the various components of whitening products, particularly products of decomposition of CP that caused the erosion of the composite matrix surface.12,16
Other studies,2,10,18,19 using different protocols did not obtain significant differences between control and experimental specimens.
It is important to acknowledge some limitations of this study. Firstly, only a limited number of materials and manufacturers were tested. This can lead to an extrapolation of results only occurring due to specific interactions between materials. Finally, the in vitro nature of this study does not permit an extrapolation to a clinical tooth-whitening situation. Nonetheless, an effort was made to include in the protocol measures trying to mimic the intra-oral conditions. One of those measures was the inclusion of the specimens in an incubator at a temperature of 37°C in distilled water. Besides, the whitening protocol chosen (6h daily for 14 days) was based on the average time for a 2 weeks-night application, which is considered to be a safe time period for the dental pulp.20
According to SEM protocol, in order to view a sample is necessary to treat the surface by coating it with gold by sputtering. This does not allow any subsequent changes in the specimen surface, which prevents us from seeing alterations in the same area/specimen before and after bleaching. In this way, a strict protocol has to be adopted to create the exact same conditions in specimens from whitening and control groups, before, during and after the treatment.
In future studies, materials from other manufacturers should be tested to better understand this subject. It would also be of interest to compare various concentrations of whitening products, both with CP and HP, to assess whether the surface changes observed are dependent on the concentration of these agents. It would be relevant to evaluate polished and not polished, to investigate the impact of polishing procedures on the decrease of possible side effects. Also, a quantitative analytical method, such as profilometry, should be used to complement the present data, since SEM only gives qualitative and not quantitative information.
ConclusionThe 10% CP gel may cause changes in surface ultramorphology of the materials tested: reinforced zinc oxide-eugenol cement, amalgam and composite.
Ethical responsibilitiesProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
This study was performed at UICOB, R&D unit no. 4062 of FCT. The authors would like to thank Prof. Doutor Manuela Lopes for her help in SEM observation and, Prof. Doutor Luis Pires Lopes and Prof. Doutor António Mata for the lab facilities.
Dentina, Lda kindly provided the whitening gel.