To evaluate the effects of external tooth whitening on the surface of two types of resin composites, by scanning electron microscopy (SEM).
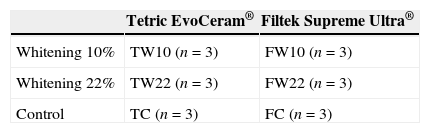
MethodsEighteen (N=18) specimens were assigned into six groups using two composites: a nanohybrid (Tetric EvoCeram®) and a nanofill (Filtek Supreme XT®); and three protocols: 10% carbamide peroxide (CP) whitening (TW10 and FW10); 22% CP whitening (TW22 and FW22); control group (TC and FC). For each group, the resin composite was introduced into a silicon mold and light-cured according to manufacturer's instructions. Specimens were stored in distilled water (37°C, 24h), polished, and then randomly divided into the mentioned groups. Specimens from the whitening groups were exposed to the CP gels (White Dental Beauty®) for 6h per day. TC and FC specimens were exposed to distilled water. All specimens were stored in an incubator (37°C, 14 days) and observed by SEM. It was measured the hydrogenionic potential (pH) of the CP gels.
ResultsSEM analysis of both composites exposed to the CP gels showed surface changes, namely superficial cracks and numerous porous, highlighting a real separation between the resin matrix and the composite fillers, regardless of the gel concentration. These changes were more notorious in the nanohybrid composite. 10% and 22% CP gels have a pH value of 6.71 and 7.42, respectively.
ConclusionThe 10% and 22% CP led to changes on both surface composites. These alterations are pronounced in the nanohybrid composite and are independent of the concentrations used.
Avaliar os efeitos do branqueamento dentário externo na superfície de duas resinas compostas, através da sua observação ao microscópio electrónico de varrimento (SEM).
MétodosDezoito (N=18) espécimes foram distribuídos em 6 grupos, tendo-se utilizado duas resinas compostas: uma nanohíbrida (Tetric EvoCeram®) e uma nanoparticulada (Filtek Supreme XT®); e três protocolos: branqueamento com peróxido de carbamida (CP) a 10% (TW10 e FW10); branqueamento com CP a 22% (TW22 and FW22); grupo controlo (TC e FC). Para cada grupo a resina composta foi introduzida num molde de silicone e fotopolimerizada de acordo com as instruções do fabricante. Os espécimes foram armazenados em água destilada (37°, 24h), polidos, e depois aleatoriamente divididos nos grupos mencionados. Os espécimes dos grupos de branqueamento foram expostos ao gel de CP (White Dental Beauty®) por 6h diárias. Os espécimes dos grupos TC e FC foram expostos a água destilada. Todos foram armazenados numa incubadora (37°C, 14d) e observados ao SEM. Foi medido o potencial hidrogeniónico (pH) dos géis de CP.
ResultadosA análise ao SEM das resinas compostas expostas aos géis de CP revelou alterações de superfície, nomeadamente poros e fendas superficiais, demonstrando a separação entre a matriz orgânica e o conteúdo inorgânico, independentemente da concentração do gel. Estas alterações foram mais marcadas na resina composta nanohíbrida. Os géis de CP a 10% e 22% têm um valor de pH de 6,71 e 7,42, respectivamente.
ConclusãoO CP a 10% e 22% provoca alterações na superfície de ambas as resinas compostas. Estas modificações são mais pronunciadas na resina composta nanohíbrida e independentes da concentração utilizada.
At home external tooth whitening, popularized by Haywood and Heymann,1 is one of the most common dental esthetic treatments. Hydrogen peroxide (HP), in the form of carbamide peroxide (CB), an HP releasing agent, is widely used as whitening agent.2 Regardless of its success, the safety and side effects of tooth whitening cannot be neglected. Whitening techniques and products have influence not only on enamel, dentin and pulp tissues, but also on restorative materials exposed to them, like resin composites. The polymer network of composites consists of carbon–carbon bonds, which may be degraded by HP, through oxidative reactions, influencing the physical properties of the restorations.3 Among other properties, surface alterations in resin composites after whitening have been widely studied3–16 and several authors found an increase in topographic changes after whitening.3–13 The increase of surface irregularities in composite restorations, beyond esthetic issues, facilitates bacterial accumulation,4,5,8,17,18 making the resin composite more susceptible to external pigmentation.8,18 These modifications seem to be unequal in different composite types: if the polymeric chains are characterized by strong bonds between polymers of high molecular height the whitening agent will not be easily spread.9
Currently, innumerous resin composite and different whitening agents concentrations are available for clinicians. Treatment plans using both composite restorations and whitening treatment are very common in oral rehabilitation.19,20 It is important to understand the impact of whitening on these restorations, namely on their quality and longevity.
The purpose of this investigation was to evaluate, through an in vitro study, the effects of external tooth whitening on the surface of two resin composites, a nanohybrid and a nanofill. The following null hypotheses were tested:
- 1.
External tooth whitening with a 10% PC causes no changes on the surface of nanohybrid and nanofill composite samples, compared to samples of these materials exposed to distilled water;
- 2.
External tooth whitening with a 22% PC causes no changes on the surface of nanohybrid and nanofill composite samples, compared to samples of these materials exposed to distilled water;
- 3.
There are no differences on the surfaces of nanohybrid composite samples exposed to tooth whitening with a 10% or 22% PC gel, compared to nanofill composite samples exposed to the same protocol;
- 4.
There are no differences on the surfaces of nanohybrid or nanofill composite samples exposed to tooth whitening with a 10% PC gel compared to samples of the same composite type exposed to a 22% PC gel.
Two resin composites were used: one nanohybrid (Tetric EvoCeram® – Ivoclar Vivadent AG, Schaan, Liechtenstein) and one nanofill (Filtek Supreme XT® – 3M ESPE, St. Paul, MN, USA), all A3 (Vita Classical shade guide, Vita Zahnfabrik, Bad Sackingen, Germany). Eighteen (N=18) composite disks were prepared with 10mm×2mm (diameter/thickness), using silicon casts (Putty Prestige®, Vannini Dental Industry, Grassina, Italy), to obtain a total useful area of 78.5mm2 per specimen. The resin composite was inserted on the silicon cast in only one increment with an insertion spatula. Each surface was cured on both sides, through a glass plate with a curing light (Coltolux® Led, Coltène Whaledent, Cuyahoga Falls, OH, USA), according to manufacturer's instructions. The tip of the curing light unit was in contact with the glass plate during polymerization and the curing light intensity (460nm) was verified with a radiometer (Demetron L.E.D. Radiometer, Kerr, Danbury USA). All specimens were stored in distilled water in a dry incubator (37°C, 24h).
The composite disks were polished with medium, fine and extra-fine polishing disks (OptiDisc®, Kerr, Orange, MN, USA) and then, with a polishing rubber point (Dimanto®, Voco, Cuxhaven, Germany). For each specimen, a new polishing disk was used. Each polishing step was performed with a slight uniform pressure, with a circular pattern for 10s. After polishing, the specimens were stored in distilled water in a dry incubator (37°C, 24h). The same operator performed all specimens’ preparation. Each specimen was randomly assigned to one of the 6 experimental groups (n=3), as explained in Table 1.
Specimens from the groups TW10/FW10 and TW22/FW22 were respectively exposed to 10% CP (White Dental Beauty 10%®, Optident, West Yorkshire, United Kingdom) and 22% CP (White Dental Beauty 22%®, Optident, West Yorkshire, United Kingdom) for 14 continuous days. The gel application was repeated at the same time every day and was performed by spreading the gel, in order to cover the entire surface of the polished specimens with a uniform thickness (0.5mm). During the period of contact with the gel (6h, according to the manufacturer's instructions), the specimens remained in a dry incubator at 37°C. After this period of time, each specimen was washed with distilled water for 1min and placed in 10mL of distilled water in the incubator.
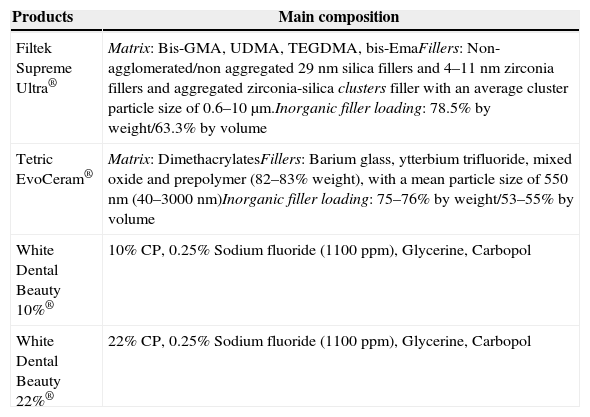
The specimens of Groups TC and FC were exposed to distilled water for 14 days. Distilled water was changed every 24h, and then remained in the dry incubator at 37°C. All specimens remained 24h in distilled water in the incubator after the 14 days protocol. All products used in laboratory testing are described in Table 2.
Main composition of the products used (according to manufacturer's information).
| Products | Main composition |
|---|---|
| Filtek Supreme Ultra® | Matrix: Bis-GMA, UDMA, TEGDMA, bis-EmaFillers: Non-agglomerated/non aggregated 29nm silica fillers and 4–11nm zirconia fillers and aggregated zirconia-silica clusters filler with an average cluster particle size of 0.6–10μm.Inorganic filler loading: 78.5% by weight/63.3% by volume |
| Tetric EvoCeram® | Matrix: DimethacrylatesFillers: Barium glass, ytterbium trifluoride, mixed oxide and prepolymer (82–83% weight), with a mean particle size of 550nm (40–3000nm)Inorganic filler loading: 75–76% by weight/53–55% by volume |
| White Dental Beauty 10%® | 10% CP, 0.25% Sodium fluoride (1100ppm), Glycerine, Carbopol |
| White Dental Beauty 22%® | 22% CP, 0.25% Sodium fluoride (1100ppm), Glycerine, Carbopol |
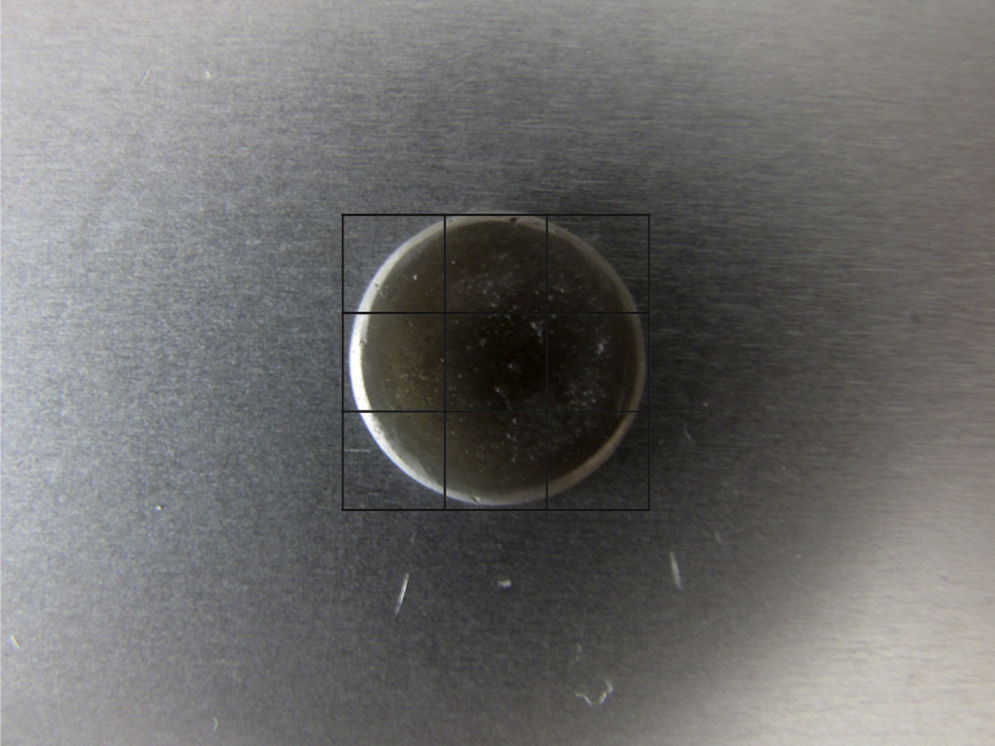
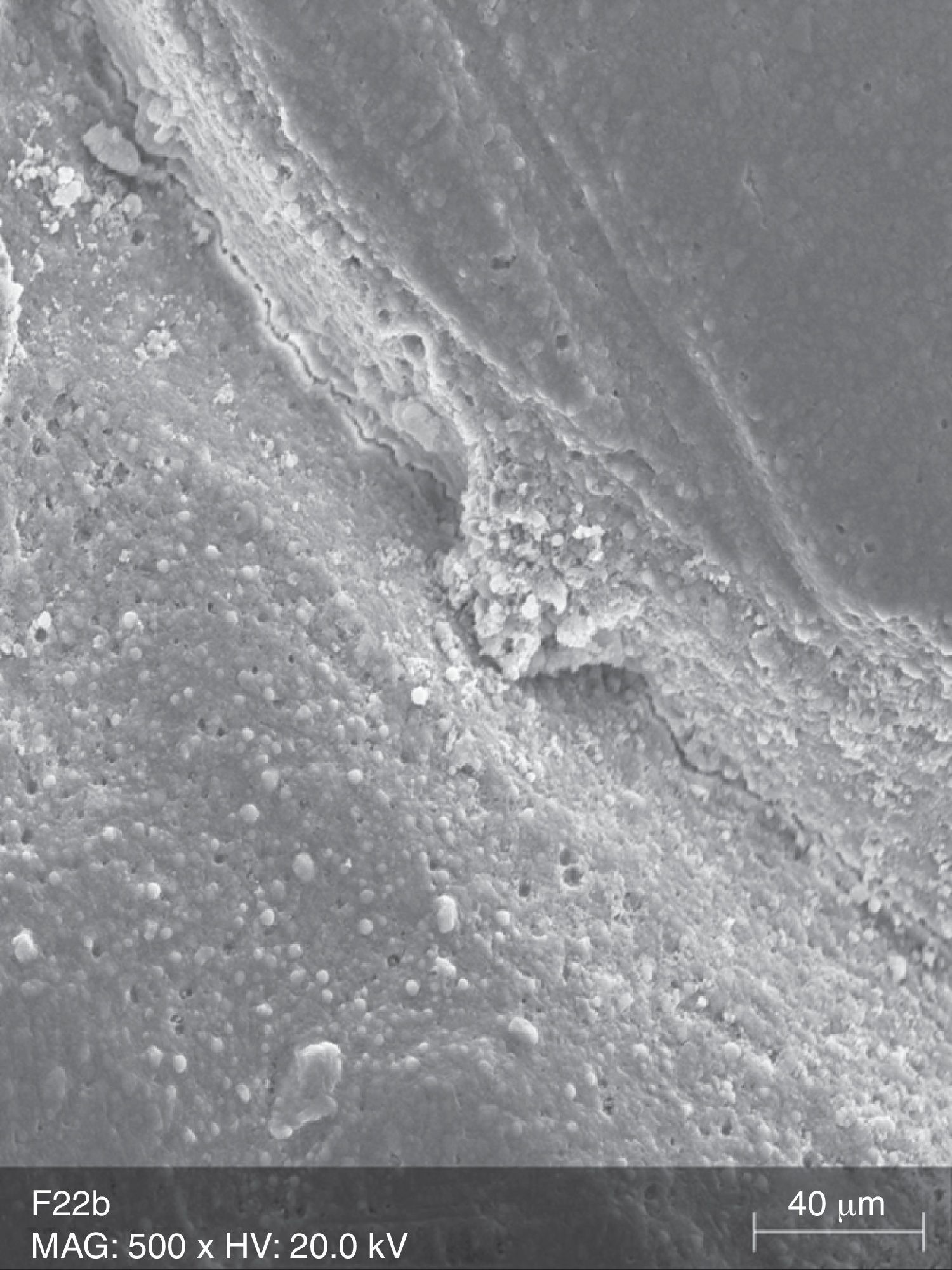
The polished surface of each specimen was coated by sputtering with gold (Jeol JFC-1100 E®, TakeOff Corporation, Tokyo, Japan) and then was examined by SEM (Hitachi S-450®, Faculdade de Medicina Dentária da Universidade de Lisboa). An imaginary grid that divided each specimen surface into 9 parts was created (Fig. 1), to facilitate and systematize the qualitative analysis of surface changes. In each specimen, all these parts were observed and microphotographs were taken at magnifications of 33×, 250×, 500× and 1000×. The image that best characterized what occurred on the specimen surface was chosen as a representative.
The hydrogenic potential (pH) of the two whitening products used was measured using a pH potentiometer (pH Meter Basic 20®, Crison Instruments SA, Barcelona, Spain), after calibration with buffer solutions.
ResultsRepresentative SEM microphotographs are illustrated in Figs. 2–7.
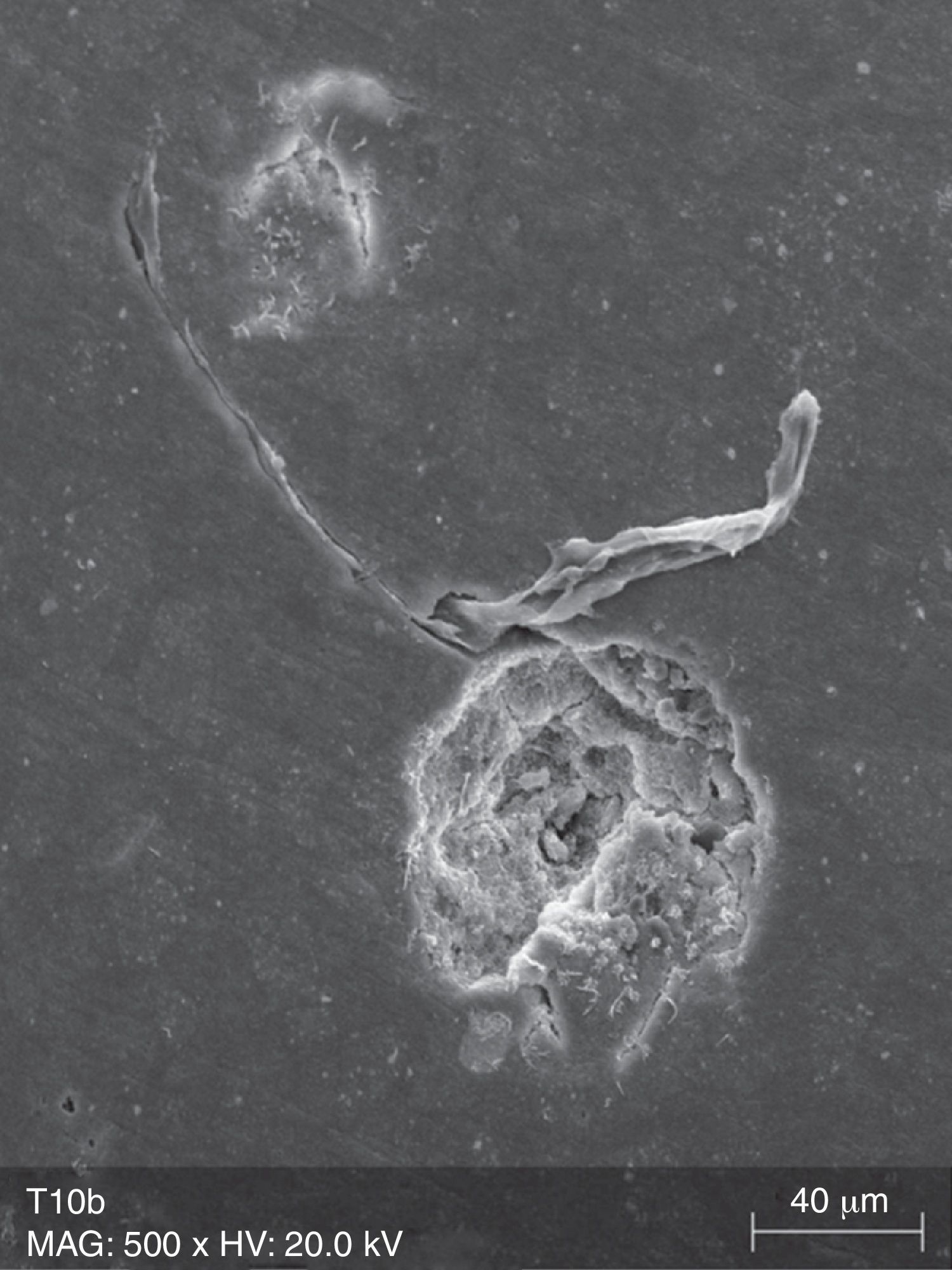
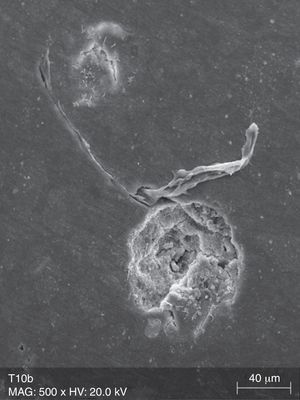
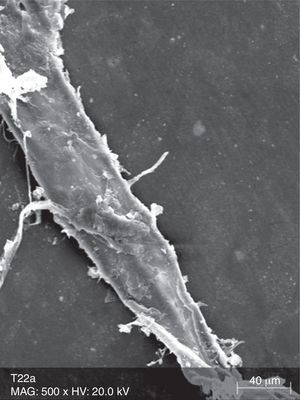
The surface analysis of the specimens of group TW10 (Fig. 2) and TW22 (Fig. 3) revealed the existence of multiple surface pores and cracks, around which we can observe the breakdown of the resin composite components, resulting in an irregular surface with loss of homogeneity. On the other hand, the TC specimens (Fig. 4) showed no loss of substance, and only a few pores were noticeable.
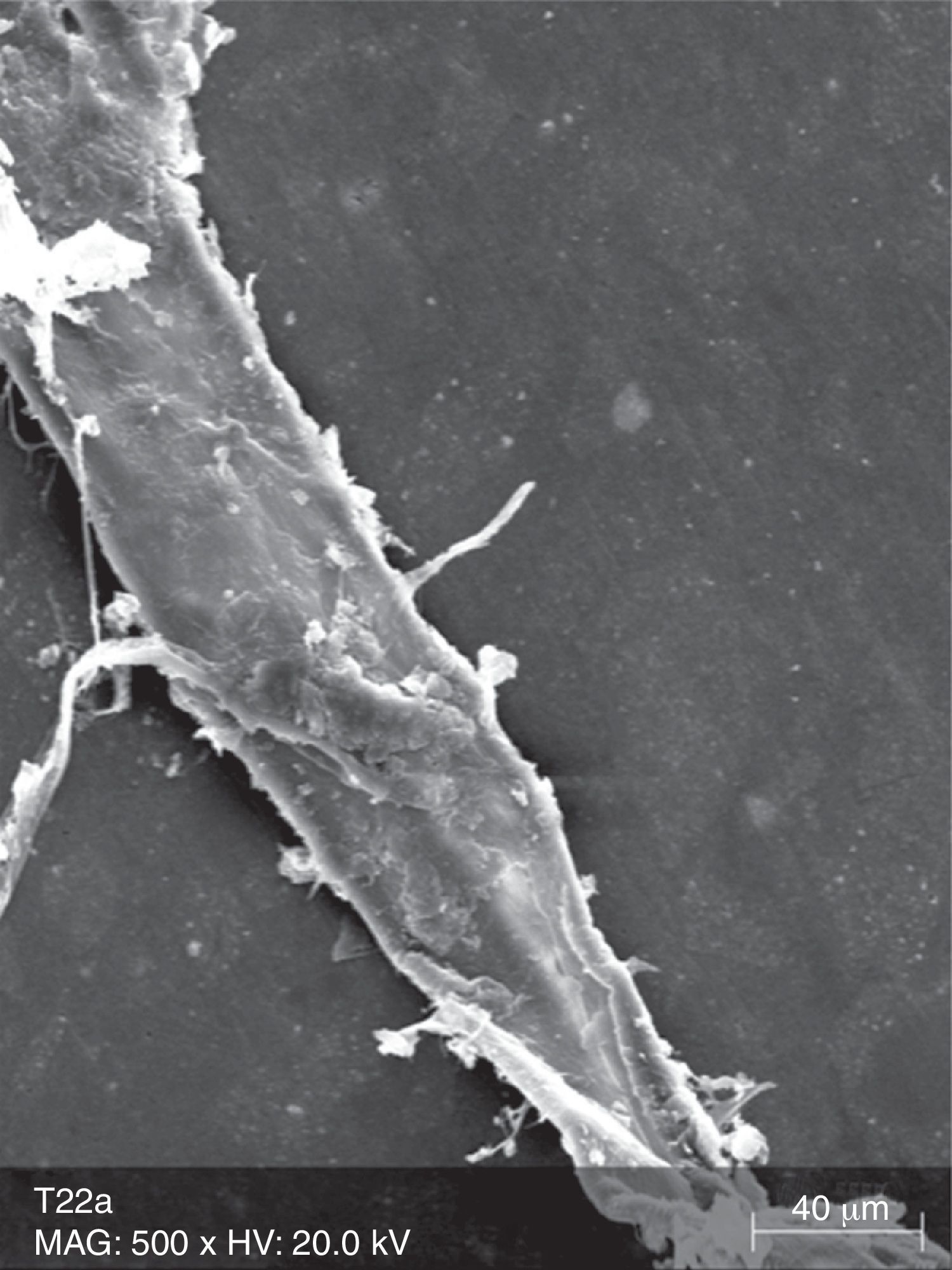
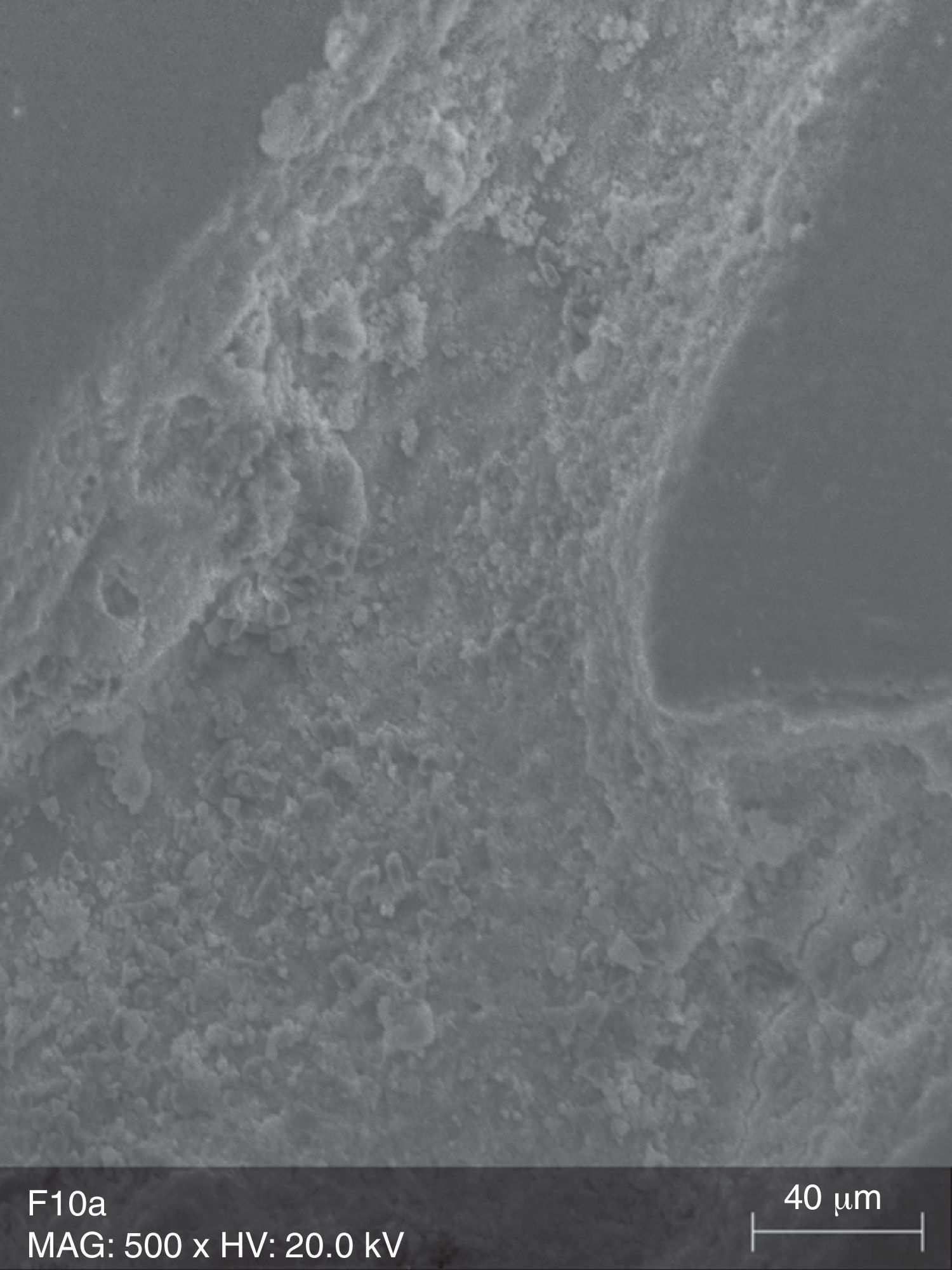
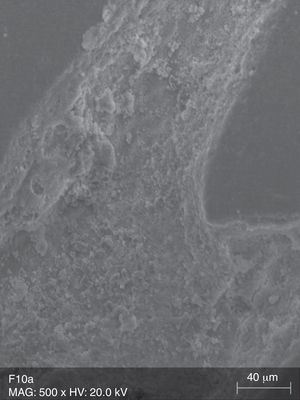
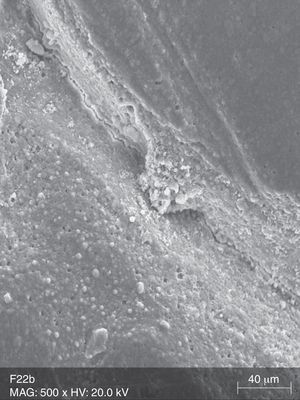
The surface analysis of the specimens of FW10 (Fig. 5) and FW22 (Fig. 6) revealed the existence of numerous cracks. In these specimens it was possible to distinguish some superficial uniformity loss, namely the separation between various nanoclusters and the composite matrix. There was also the presence of pores. In the FC group specimens some marks were mainly observed (Fig. 7).
The pH measurement of the whitening agents resulted in pH values of 6.71 for White Dental Beauty 10%® and 7.42 for White Dental Beauty 22%®.
DiscussionVery often in clinical practice, there are resin composite restorations on teeth subjected to whitening procedures.21 The prognosis and longevity of these restorations will depend, not only on their mechanical properties, but also on the physical and biological properties of the materials used.22 Attin et al. demonstrated that whitening agents may have a negative effect on restorative materials, including changes in surface morphology.23 As this issue remains under debate in the scientific community, the present work intended to evaluate the effects of dental whitening on the surface of two resin composites, one nanohybrid (Tetric EvoCeram®) and other nanofill (Filtek Supreme XT®), using two CP concentrations: 10% and 22%.
In this study, SEM analysis revealed no significant differences between the two PC gels concentrations, for the same composite type. Therefore, the null hypothesis corresponding to this objective was accepted. However, all experimental groups exposed to a whitening agent (10% or 22%) revealed superficial morphologic and topographic changes, when compared to control groups. So, the null hypotheses corresponding to these objectives were rejected.
It is known that surface texture and morphology have not only a great influence on the esthetic properties of the restorations but, in addition, also are associated with the accumulation of plaque,4,5,8,17,18 the development of caries and gingivitis and eventually will promote long-term failure of the restoration.10,15 Several authors found significant superficial alterations in resin composites after whitening.3–13
Due to their organic matrix, the resin composites are more susceptible to chemical changes, relatively to metallic or ceramic restorations.24 Indeed, the whitening agents affect mainly the organic matrix of the resin composites, while the fillers are probably unaffected, even in an extreme acidic environment.20 The Bis-GMA matrix of the resin composites can be softened by chemical substances with identical solubility parameters.24 The whitening agents contain various solvents, which may contribute, alone or in combination, to decrease the resin matrix solubility.25 During a surface spectral analysis of microfill resin composites after whitening, a decrease in silica and silicon content was detected, suggesting erosion of the matrix.26 In this study, it was possible to detect surface changes consistent with post-whitening composite matrix degradation, which was reflected as a significant increase of surface porosity in the case of the Tetric EvoCeram® specimens, and in the propagation of cracks and increase in surface roughness in both tested resin composites. The control specimens (TC and FC) only showed a few pores and marks, without loss of substance, which probably resulted from the polishing procedure and the proper resin composite insertion.
Some aspects of the chemical dissociation of CP and HP can even accelerate the hydrolytic degradation process of the resin composites, reported by Soderholm et al. which chemically occur in vivo, contributing to the wear of resin composites in areas of greatest stress.27 Again, it is expected that any difference on surface morphology to occur on resin composites with higher resin content. The results of this study are in agreement with those findings, since it was found that Tetric EvoCeram® (53–55% of inorganic content in volume) shows more pronounced surface alterations than Filtek Supreme XT® (63.3% of inorganic content by volume). Some authors concluded that Filtek Supreme XT® was more resistant to surface alterations comparing with Tetric EvoCeram®.7
For other authors,3,22 the free radicals produced by peroxides can negatively affect the resin-filler interface, causing breaks in the connections of these components, contributing to an increase in roughness and crack propagation. In recent studies eluted products (silane) in composites after whitening were detected which indicates that it can lead to a breakdown of the chemical bonding between the inorganic filler particles and the organic matrix.3 The amount of filler content of a resin composite is directly related to the surface area that is occupied by filler particles, versus that occupied by the matrix, the esthetic properties (surface uniformity) being dependent on the largest fillers of the restoration surface. Since the increase in surface roughness results in erosion of the matrix, the resulting breakdown of the matrix-fillers connections leads to the displacement and elution of these particles. Thus, the higher the volume and size of these particles, the more irregular and porous the surface will be.6,11,12,20,22 Some authors suggested that spherical fillers can reduce the mechanical stress and the risk of fracture propagation, which would make the microfill resin composites less prone to surface changes, even considering their high content of matrix.27 This study also corroborates the findings of those authors,27 since the nanofill (Filtek Supreme XT®) presents a greater uniformity in the average fillers size compared with the nanohybrid (Tetric EvoCeram®). In 2002, other authors found that the surface of microfill resin composites is noticeably more uniform than the surface of hybrid, so it is natural that micro- and nanofill composites would exhibit less variation, even after whitening.25
For other researchers,9,22,28 the causes for surface changes seem to be the whitening agents pH,28 exposure time, PH concentration, and other components of the whitening products.9,22,28 Among these are, usually, carbomer and glycerin, which are present in the products tested in this study. These compounds act as thickeners, increasing the adherence of the whitening agent to the tooth surface and extending the time of the whitening agent release.29 Their damaging action on enamel has been showed.28 Nonetheless, there is no sufficient supporting data about the effects of these substances on restorative materials. Regarding the concentration of whitening agents, there were no significant differences among the specimens subjected to 10% and 22% CP. Regarding the pH of various whitening products commercially available for at home whitening, it ranges from 5.66 to 7.35.30 The whitening gels used in this study have a neutral pH, which is less acidic than those found in some foods or drinks.30–32 Therefore, the pH will not, most likely, contribute as a confounding variable.
On the other hand, some studies did not suggest any surface change in composite after whitening.14–16,22 Wattanapayungkul and Yap found no significant differences on the surface of microfill and hybrid resin composites after whitening with 10% and 15% CP.22 However, these authors did not polish the restorations. Since that, clinically, any restoration always requires some degree of finishing and polishing, in this study the specimens polishing was performed as an attempt to better replicate a clinical scenario. Furthermore, the different results among the various studies published to date4,6–12,15,16,18,20–22,26 are likely the results of the use of different agents, whitening products, restorative materials and experimental protocols, namely, differences in the frequency of the whitening agents application. In the future, it would be important to clarify whether these changes yield a real decline in the clinical quality and in the longevity of resin composite restorations.
ConclusionsWith the limitations of this study, it can be concluded that:
At home bleaching with a 10% or 22% CP gel causes surface changes, observed by SEM, on the surfaces of nanofill and nanohybride composites;
These changes are most marked on the surface of the nanohybrid composite, regardless of the CP concentration (10 or 22%), compared to the surface changes in the nanofill resin composite, which suggests that this type of resin composite is more susceptible to the action of whitening agents;
The same type of composite has similar surface changes whether exposed to 10% or 22% CP. In this study, the concentration of the whitening agent was not significant for the effects on the surface of composite restorations.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.