To evaluate the clinical behavior of reccurent aphtous stomatitis (RAS) in a Cuban population.
MethodsIn the present study adult patients from the Vedado's University Polyclinic (Cuba) diagnosed with RAS, from September 2009 to May 2010, were examined. Clinical classification, site, associated predisposing factors and clinical evolution of aphthae were evaluated. All patients were reevaluated at 10 and 14 days after the diagnosis to analyze the healing process.
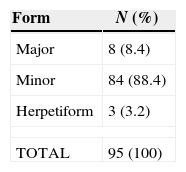
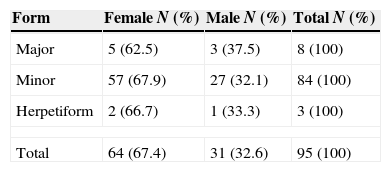
ResultsMinor aphthae was the more frequent clinical variant of RAS (88.4%) followed by Major (8.4%) and Herpetiform (3.2%) aphthae. Females were more affected (67.4%) than males (22,6%). Major aphthae was more common in patients aged between 35 and 59 years old, however Minor and Herpetiform aphthae were more common in individuals aged between 19 and 34 years old. Stress was a possible predisposing factor for the development of RAS in all patients. The most frequent site affected was the lip (35.7%) and the least was the palate (7.8%). Aphthae healing period was 7–10 days in 90.5% of the studied population.
ConclusionThe most common clinical variant of RAS in the population studied was the Minor form, affecting mainly the female gender and the younger age groups, with a healing period time between 7 and 10 days. The labial mucosa was the most common site.
Avaliar o comportamento clínico da Estomatite Aftosa Recorrente (EAR) numa população cubana.
MétodosOs pacientes adultos que compareceram na consulta da Policlínica da Universidade de Vedado (Cuba) e foram diagnosticados com EAR, de setembro de 2009 a maio de 2010, foram examinados tendo em conta as seguintes características das aftas: forma clínica, localização, fatores de risco associados e evolução clínica. Todos os pacientes foram reavaliados 10 a 14 dias após o diagnóstico para acompanhar o processo de cicatrização.
ResultadosA afta pequena foi a forma clínica mais frequente de EAR (88,4%), seguida pela afta grande (8,4%) e afta herpetiforme (3,2%). As mulheres foram mais afetadas (67,4%) do que os homens (22,6%). A afta grande foi mais frequente em pacientes com idades compreendidas entre os 35 e os 59 anos, enquanto que a aftas pequena e herpetiforme foram mais frequentes entre os 19 e 34 anos. O stress foi um possível fator de risco para o desenvolvimento da EAR em todos os pacientes. A mucosa labial foi a localização mais frequente (35,7%), enquanto que o palato foi a menos frequente (7,8%). O tempo decorrido entre o diagnóstico e a cicatrização da EAR foi de 7 a 10 dias em 90,5% da população estudada.
ConclusãoA afta pequena foi a forma clínica mais comum de EAR na população estudada, afetou principalmente o sexo feminino e as faixas etárias mais jovens e cicatrizava ao fim de 7 a 10 dias. A mucosa labial foi a localização mais frequente da EAR.
The recurrent aphthous stomatitis (RAS) is a common benign clinical entity already described by Hippocrates in 460–370 BC.1,2 It is characterized by the sudden appearance of painful, recurrent ulcers, located in the oral mucosa which, usually, heal spontaneously.3 Its precise etiology is unknown with various supporting factors being pointed out that seem to facilitate its occurrence.4 Three forms are clinically distinguished: minor (MiRAS), major (MaRAS) and herpetiform (HU). The first one covers about 80% of the cases. It can be unique or multiple, and it is characterized by the appearance of round or oval-shaped ulcers with a diameter of less than 0.5cm. They most frequently appear on the non-keratinized oral mucosa such as lip, bottom of the vestibule, floor of the mouth and lips of the tongue not being, however, excluded the remaining sites such as the gingiva, the dorsum of the tongue and the hard palate. Usually, they heal spontaneously within 10–14 days, with the possibility, however, to relapse within 3–4 months.3,5,6 Major aphthae, also called Sutton's disease or recurrent necrotic mucosa periadenitis, cover about 10% of the cases. They can be several, up to a maximum of 10, with a diameter exceeding 1cm. The interior is deeper than the one observed in minor aphthae, it is characterized by intense pain and the sites of predilection are the labial mucosa, the soft palate and the isthmus of the fauces. They sometimes are associated with dysphonia and/or dysphagia. The duration varies between 4 and 6 weeks and can leave scars.1,3,5,7,8 The herpetiform aphtha covers the remain 10% of the RAS. Many ulcers, between 10 and 100, occur with diameters between 1 and 3mm, they are very painful with no preferential location site and they have the tendency to coalesce. While the first two clinical forms predominate during childhood and youth the latter tends to appear in adult life with a scarring period between 7 and 10 days, being more frequent in women.1,3,5,7,8
Of unknown pathogenesis some factors are usually mentioned in the literature as probable risk factors.9 Among them the following are highlighted: gastrointestinal alterations, such as gastroenteritis, malabsorption syndrome, ulcerative colitis and giardiasis,10 food or respiratory allergies,11–13 endocrine changes related to the estrogen content since the aphthae often precede the beginning of the menstrual period and are absent during pregnancy5 and, lastly, vitamin deficiencies, namely folic acid, vitamins C, B-12 and B-1.1,5,14
The aim of this study was to determine the behavior of the RAS in patients attended at Vedado's University Polyclinic located in the municipality Plaza de la Revolución (Cuba).
Materials and methodsPatients above the age of 19 who sought the Dental Emergency service of the Vedado's University Polyclinic (Havana-Cuba) to treat aphthae located in the oral mucosa, from September 2009 to May 2010, were examined. Individuals with confirmed RAS diagnosis and with a history of similar chart were included in the study. Patients with physical or mental disabilities were excluded.
In order to accomplish this study, the topic and goal of the assignment as well as the steps to achieve it were all explained to the patients. The study is in line with what is established in the Helsinki Declaration on research in humans for therapeutic purposes.
On the basis of these criteria, the final sample consisted of a total of 95 patients with RAS in any given clinical presentations according to the classification of Scully and Porter.3,5
Data was collected concerning the following parameters: clinical classification, gender and age group, site, associated predisposing factors and clinical evolution according to the clinical classification.
The exam took place on a dental chair with good light and using a mouth mirror size No 5. The data were collected using a survey prepared for the purpose (Annex 1). The survey consisted of multiple choice and open-ended questions. Participants answered to demographic questions about their age and gender. They were also questioned about 42 stress symptoms and their frequency of occurrence (0=never, 1=rarely, 2=sometimes, 3=often, 4=very often, 5=always) for the last 12 months, using Lipp's inventory of stress symptoms for adults.15–17 The answers were accounted between a minimum of zero and a maximum score of 210. All subjects with scores equal to or greater than 50 were considered in stress.
The exam took place on a dental chair with good light and using a mouth mirror size No 5. The data were collected using a survey prepared for the purpose (Annex 1). The survey consisted of multiple choice and open-ended questions. Participants answered to demographic questions about their age and gender. They were also questioned about 42 stress symptoms and their frequency of occurrence (0=never, 1=rarely, 2=sometimes, 3=often, 4=very often, 5=always) for the last 12 months, using Lipp's inventory of stress symptoms for adults.15–17 The answers were accounted between a minimum of zero and a maximum score of 210. All subjects with scores equal to or greater than 50 were considered in stress.
Patients diagnosed with RAS were reevaluated at 10 and 14 days after the beginning of the lesions to evaluate the healing process. Those who did not provide full healing of the lesions repeated the appointment at 6 weeks.
Data processing was performed immediately after the collection and a database was created on a Microsoft Office Excel 2010 spreadsheet. The percentage was used as a summary measure.
Data is presented in tables. For the final information Microsoft Office Word 2010 was used.
ResultsThe most frequent clinical form was the minor Aphthae (88.4%) followed by the major one (8.4%) being the Herpetiform the less common one with only 3.2% of the population sample (Table 1).
According to gender distribution RAS was more frequent among females (67.4%) being this predominance evident in any of the clinical forms (Table 2).
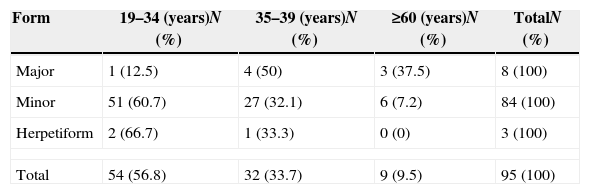
The distribution of the different clinical forms of aphthae covered all age groups. The major aphtha form is more common in individuals aged between 35 and 59 years old. The minor one, just as the Herpetiform one, are more common in individuals aged between 19 and 34 years old (Table 3).
Recurrent aphthous stomatitis distribution according to age groups.
| Form | 19–34 (years)N (%) | 35–39 (years)N (%) | ≥60 (years)N (%) | TotalN (%) |
|---|---|---|---|---|
| Major | 1 (12.5) | 4 (50) | 3 (37.5) | 8 (100) |
| Minor | 51 (60.7) | 27 (32.1) | 6 (7.2) | 84 (100) |
| Herpetiform | 2 (66.7) | 1 (33.3) | 0 (0) | 3 (100) |
| Total | 54 (56.8) | 32 (33.7) | 9 (9.5) | 95 (100) |
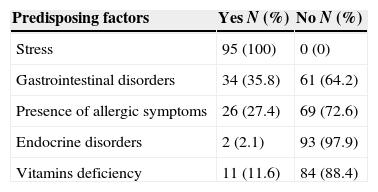
In most of the studied cases more than one associated risk factor is present. The entire sample examined mentioned stress as a possible risk factor for the development of RAS (Table 4).
Population distribution according to predisposing factors.
| Predisposing factors | Yes N (%) | No N (%) |
|---|---|---|
| Stress | 95 (100) | 0 (0) |
| Gastrointestinal disorders | 34 (35.8) | 61 (64.2) |
| Presence of allergic symptoms | 26 (27.4) | 69 (72.6) |
| Endocrine disorders | 2 (2.1) | 93 (97.9) |
| Vitamins deficiency | 11 (11.6) | 84 (88.4) |
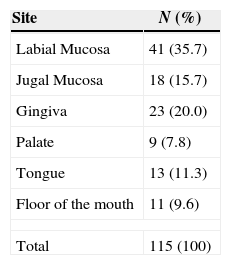
The most frequent site for the oral aphthae to be located was the lips with 35.7% and the least represented were area of the palate with 7.8% (Table 5).
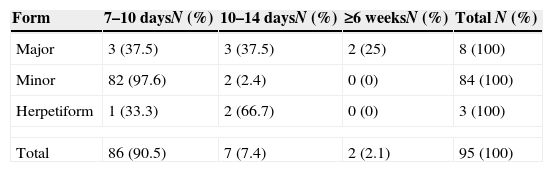
In 90.5% of the represented population aphthae evolution period till their healing was 7–10 days. Only in 2.1% of the cases was the evolution time equal to or greater than 6 weeks (Table 6).
DiscussionThis study has shown that the MiRAS (minor recurrent aphthous stomatitis) was the most frequent clinical variant of RAS (recurrent aphthous stomatitis) followed by the MaRAS (major recurrent aphthous stomatitis) whereas the HU (herpetiform ulcer) was the least common one. These results are consistent with previous studies, namely, in a Cuban population,18 in a Brazilian population19 and in an English population.20
In our studied sample MiRAS was more frequently found in the female gender. In a systematic review, points out that there is a slightly higher susceptibility for females.21 However other studies indicate there is no gender differences.22
The minor form of RAS was more frequent in the younger age group of the population studied (19–34) and its frequency decreases with age. Schulman in a comparative study made by crossing two American data bases (National Health and Nutrition Examination Survey III Oral Examination Component, Oral Health of United States Children: Public Use Data File Documentation and Survey Methodology) demonstrated that in children between the ages of 4 and 17 the most common average age for the diagnosis of aphthous lesions would be between 13 and 17 years old.22 Our study was done in older patients therefore there is no direct comparison. Nevertheless, we have noticed that in our adult population the incidence was higher in young adults which is consistent with the general consensus.
Although several studies have been made, investigators are still not able to determine the exact etiology and pathogenesis of this disease. Heredity, hematologic deficiencies, immunological disorders, diet, medication, stress, trauma, hormonal imbalances, infections, poor oral hygiene, among others, have been described as possible supporting factors in the emergence of these oral ulcers.23
Among the predisposing factors studied in our population, stress, intestinal alterations and food allergies stood out as the most frequent. However, in most cases, more than one factor coexisted.
Emotional tension, in susceptible people, can trigger or exacerbate the RAS.24–29 According to some authors, stress has a higher influence on triggering an episode than on its duration.30,31
The evaluation of stress in the context of a dental appointment, using inventories of stress symptoms, allows a stress diagnosis. In the present study Lipp's Inventory of Stress Symptoms was used and there was evidence that all patients that developed RAS showed stress symptoms.
The most common sites affected by RAS are the non-keratinized surfaces, although it can emerge in any region of the oral mucosa.32 In the present study the labial mucosa was the most frequently affected site followed by the gingiva, the jugal mucosa, the ongue, the floor of the mouth and the palate.
Characteristically RAS heals spontaneously. However, depending on its clinical variant, it may present different healing time as well as leaving a scar or not.32 In the population studied, 90.5% of the cases presented a clinical healing time between 7 and 10 days with 86.3% of the cases corresponding to the MiRAS form. The two cases with longer healing period, 6 or more weeks, corresponded to the MaRAS form.
ConclusionThe most frequent clinical variant of RAS in the population studied was the Minor form, affecting mainly the female gender and the younger age groups, with a healing period time between 7 and 10 days. The labial mucosa was the preferred site.
Conflicts of interestThe authors have no conflicts of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.