The continuous subcutaneous insulin infusion (CSII) is an alternative to multiple daily injections therapy in type 1 diabetes and its use is increasingly common due to the beneficial effects on glucose control.
AimsTo analyze clinical features and biochemical parameters of patients on CSII therapy.
Type of studyLongitudinal retrospective.
SettingOutpatient clinic.
Population: Type 1 diabetic patients using insulin infusion pump in our department.
MethodsWe evaluated outcomes regarding the following set points: immediately before initiation of CSII therapy, 12 months after inclusion and in the last appointmentHH. For statistical analysis, we used non-parametric tests and linear regression analysis. We considered significant a value of p≤0.05.
ResultsWe studied 63 patients (24 men; 39 women) with a mean pre-CSII HbA1c of 8.2%±1.43; mean age at the time of placement of 32.7±10.94 years; and mean follow up time after placement of 2.1±1.92 years. There was a statistically significant reduction of HbA1c during follow-up (HbA1c 12 months: 7.2% [6.6–7.8] p=0.001; HbA1c at the end of follow-up: 7.4% [6.6–7.9] p=0,001). There was no significant variation of weight or total daily insulin dose. We registered a negative correlation between the last HbA1c before CSII and the reduction in HbA1c until the end of the follow-up period (ρ=−0.644 p=0.000). The median reduction in HbA1c was higher in women (W: –1.10 [−2.20––0.40] vs M: −0.10 [−0.80–0.40]; p=0.002). Female gender was a predictive factor of better results with CSII, even after adjustment to the last HbA1c before the initiation of therapy.
ConclusionsIn our sample, the last HbA1c before the beginning of CSII was the most powerful predictive factor of the reduction of HbA1c during follow up. Women had better results than men. There was no significant variation of weight and total daily insulin dose during follow up.
A terapêutica com infusão subcutânea contínua de insulina (Tisi) é uma opção ao uso de múltiplas injeções de insulina na diabete tipo 1 e o seu uso é cada vez mais frequente dados os benefícios no controle glicêmico.
ObjetivosAnalisar as características clínicas e os parâmetros bioquímicos dos doentes tratados com Tisi e procurar fatores preditores da resposta à terapêutica.
Tipo de estudoLongitudinal retrospectivo.
LocalConsulta externa.
PopulaçãoDiabéticos tipo 1 usuários de Tisi seguidos no nosso serviço.
MétodosForam registrados os resultados nos seguintes períodos: imediatamente antes da colocação da Tisi, 12 meses após colocação e à data da última consulta. Os resultados foram apresentados como média±desvio padrão ou mediana [quartis]. Foram usados testes não paramétricos para a análise estatística e foi efetuada regressão linear. Foram considerados significativos os valores de p<0,05.
ResultadosA amostra era constituída por 63 diabéticos tipo 1 (24 homens - H; 39 mulheres - M) com os seguintes valores antes da colocação: idade 32,7±10,94 anos; HbA1c 8,2%±1,43. O tempo médio de catamnese foi de 2,1±1,92 anos. Ocorreu uma redução estatisticamente significativa da HbA1c ao longo do tempo de seguimento (HbA1c aos 12 meses: 7,2% [6,6-8] p=0,001; HbA1c ao fim do tempo de seguimento: 7,4% [6,6,7,9] p=0,001). Não se verificou variação do peso e da dose diária total de insulina. Verificou-se uma correlação negativa entre a HbA1c prévia e a redução da HbA1c até ao fim do tempo de seguimento (ρ= −0,644 p=0,000). A mediana da redução da HbA1c até a data da última consulta foi maior no grupo das mulheres (M: −1,10 [−2,20 - −0,40] vs. H: −0,10 [−0,80 - −0,40]; p=0,002). O gênero feminino foi um fator preditivo de melhor resposta à Tisi mesmo após ajuste para a HbA1c prévia.
Conclusões: Na amostra estudada, a última HbA1c anterior ao início da Tisi foi o fator com maior capacidade de predição da resposta à terapêutica. As mulheres obtiveram melhores resultados da terapêutica com Tisi do que os homens. Não ocorreram variações estatisticamente significativas do peso ou da dose diária total de insulina.
After the publication of the DCCT1, the goal of the treatment of type 1 diabetes mellitus is to obtain glucose values as near as possible to normal, as long as the patient's hypoglycaemia risk isn’t unacceptably high. The optimum glucose control would prevent the occurrence of chronic complications of diabetes2 and result in improvement of the quality of life of the person with diabetes3. The review of target glucose values in type 1 diabetes mellitus has led to the increased use of intensive insulin therapy that was suggested by the same study as the most effective method to obtain the proposed goals.
Continuous subcutaneous insulin infusion (CSII) therapy is an alternative to the use of multiple daily injections (MDI) for the treatment of type 1 diabetes mellitus and its use is increasingly common due to the beneficial effects on glucose control. Pickup4 used CSII as a research tool to elucidate the relation between glucose control and diabetic complications. Later on, other authors described the improvement of glucose control in selected and motivated patients that previously had not attained the treatment goals with MDI. 5–11. Although several studies showed significant reductions in HbA1c with CSII, a metanalysis of randomized clinical trials comparing with MDI showed, by a random effects model, a non-significant trend towards mean reductions in HbA1c of −0.26 (IC 95% –0.57-0.05) after 6 months follow up and −0.61 (IC 95% −1.29–0.07) after 12 months follow up7. Other authors12 detected significant reductions in HbA1c (9.36±0.22 vs 8.96±0.11, p=0.039). Significant differences between therapeutic modalities are only apparent with follow up periods longer than 1 year.
Regarding hypoglycaemia risk, several observational studies suggest that CSII is associated with a significant risk reduction, particularly in terms of severe episodes7. The same metanalysis did not find data supporting sustained reductions in total daily insulin dose (TDID) in patients using CSII, nor significant reductions in weight. Other authors12,13 suggest that CSII might be associated with as much as 16% reductions in TDID comparing with multiple daily injections, as well as a significant increase in weight (68.2±0.7 Kg vs 71.2±0.3 Kg; p<0.001).
Greater patient satisfaction has been documented in patients using CSII therapy, although there is discordance between studies in terms of impact on quality of life3,6,7,14. However, it is of note that CSII therapy might be associated with other complications, namely an increase in risk of diabetic ketoacidosis, pump malfunction and infusion site infections12. Few studies have analyzed the variables associated with improved metabolic control after initiation of CSII therapy. Two studies have shown greater benefit in individuals with higher HbA1c before CSII15,16. The selection of patients with higher HbA1c (HbA1c>8.0%) was associated with superior benefit than described in two metanalysis12,13.
There is no significant data regarding the effect of age in CSII but several authors have suggested good results in pediatric patients in terms of HbA1c reduction as well as in risk of hypoglycaemia and quality of life17. One study on female adolescents showed a good response to CSII therapy in this population, which included individuals with eating disorders18.
AimsTo analyze the clinical characteristics and biochemical parameters in diabetic patients treated with CSII therapy and identify the predictive factors for a good response to this regimen.
MethodsWe included all individuals with type 1 diabetes that were users of CSII, under follow up in the Endocrinology consultation of our hospital. We recorded variables in the following time points: immediately before initiation of CSII therapy, 12 months after initiating use of the insulin pump and at the time of the last appointment. As the TDID is not recorded in the patient's clinical file, it was inferred from the insulin sensitivity factor, which is calculated using the 1800 rule, universally used in our consultation. Results were presented as mean±standard deviation or median [quartiles]. Changes in weight, TDID and TDID/Weight ratio were calculated as fractions. For the statistical analysis, we used SPSS Statistics 20.0. To compare differences in the variables and their correlations we used the Wilcoxon test, Mann-Whitney test, Spearman correlation and a linear regression model. We considered significant a value of p≤0.05. In a separate analysis, the sample was divided in two groups (better and worse response) according to the median of variation in HbA1c at the end of follow up (−0.8%), and we used the Mann-Whitney and Fischer exact tests to compare groups.
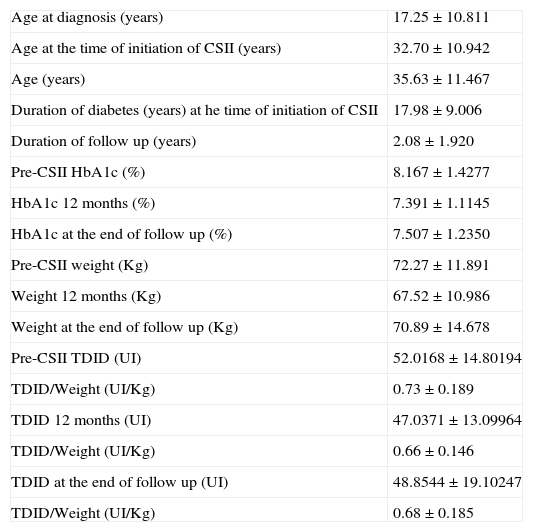
ResultsThe sample included 63 patients with type 1 diabetes (24 men; 39 women) whose clinical characteristics are presented in Table 1. The mean follow up was 2.08±1.920 years.
Characterization of the sample (n 63: 24♂ 39♀).
| Age at diagnosis (years) | 17.25±10.811 |
| Age at the time of initiation of CSII (years) | 32.70±10.942 |
| Age (years) | 35.63±11.467 |
| Duration of diabetes (years) at he time of initiation of CSII | 17.98±9.006 |
| Duration of follow up (years) | 2.08±1.920 |
| Pre-CSII HbA1c (%) | 8.167±1.4277 |
| HbA1c 12 months (%) | 7.391±1.1145 |
| HbA1c at the end of follow up (%) | 7.507±1.2350 |
| Pre-CSII weight (Kg) | 72.27±11.891 |
| Weight 12 months (Kg) | 67.52±10.986 |
| Weight at the end of follow up (Kg) | 70.89±14.678 |
| Pre-CSII TDID (UI) | 52.0168±14.80194 |
| TDID/Weight (UI/Kg) | 0.73±0.189 |
| TDID 12 months (UI) | 47.0371±13.09964 |
| TDID/Weight (UI/Kg) | 0.66±0.146 |
| TDID at the end of follow up (UI) | 48.8544±19.10247 |
| TDID/Weight (UI/Kg) | 0.68±0.185 |
TDID–total daily insulin dose
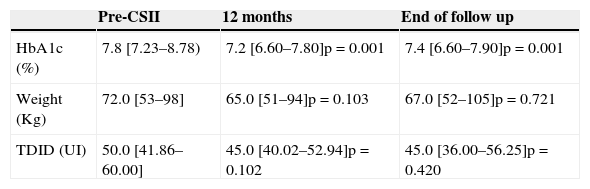
General outcomes. After CSII initiation, there was a significant mean HbA1c reduction at 12 months and at the end of the follow up period (Table 2). There was no significant variation in weight, TDID or TDID/weight ratio.
Clinical and biochemical outcomes after initiation of CSII.
| Pre-CSII | 12 months | End of follow up | |
|---|---|---|---|
| HbA1c (%) | 7.8 [7.23–8.78) | 7.2 [6.60–7.80]p=0.001 | 7.4 [6.60–7.90]p=0.001 |
| Weight (Kg) | 72.0 [53–98] | 65.0 [51–94]p=0.103 | 67.0 [52–105]p=0.721 |
| TDID (UI) | 50.0 [41.86–60.00] | 45.0 [40.02–52.94]p=0.102 | 45.0 [36.00–56.25]p=0.420 |
Results of HbA1c and TDID are presented as median [1st quartile–3rd quartile] and weight is presented as median [minimum–maximum]. p <0,05 was considered significant.
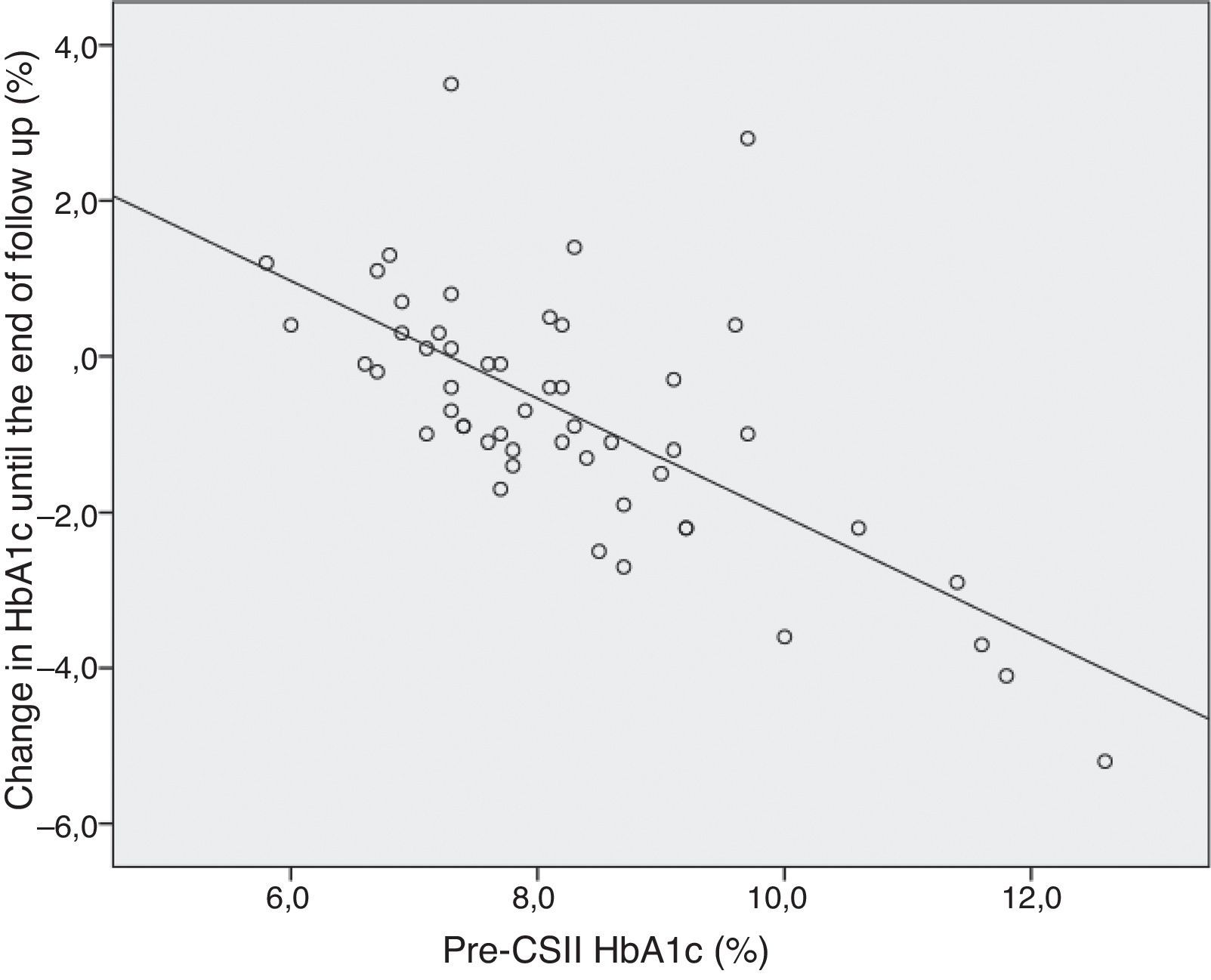
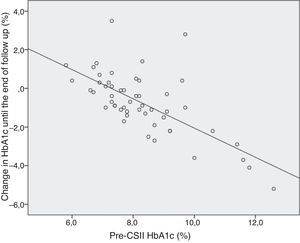
HbA1c. We found a significant correlation between pre-CSII HbA1c and its variation at 12 months and at the end of follow up (ρ=−0.643 p=0.000 and ρ=−0.644 p=0.000, respectively)–figure 1. The linear regression model showed that for each 1.0% increase in pre-CSII HbA1c there is a 0.76% reduction in post-CSII HbA1c (R=0,682, t=−6,593, p=0,000). We found a significant correlation between HbA1c variation at 12 months and age at the time of initiation of CSII therapy (ρ=0.312 p=0.033) and between HbA1c variation at the end of follow up and age at diagnosis (ρ=0.390 p=0.005) and pre-CSII TDID (ρ=−0.402 p=0.025). The total time of follow up had a significant correlation with pre-CSII HbA1c (ρ=0.260 p=0.049) and with the variation of HbA1c at 12 months and end of follow up (ρ=−0.448 p=0.002 e ρ=−0.304 p=0.028). The linear regression model showed that the total time of follow up is not a predictive factor of HbA1c variation at 12 month or at the end of follow up, after adjustment to the pre-CSII (p=0.403 e p=0.106, respectively).
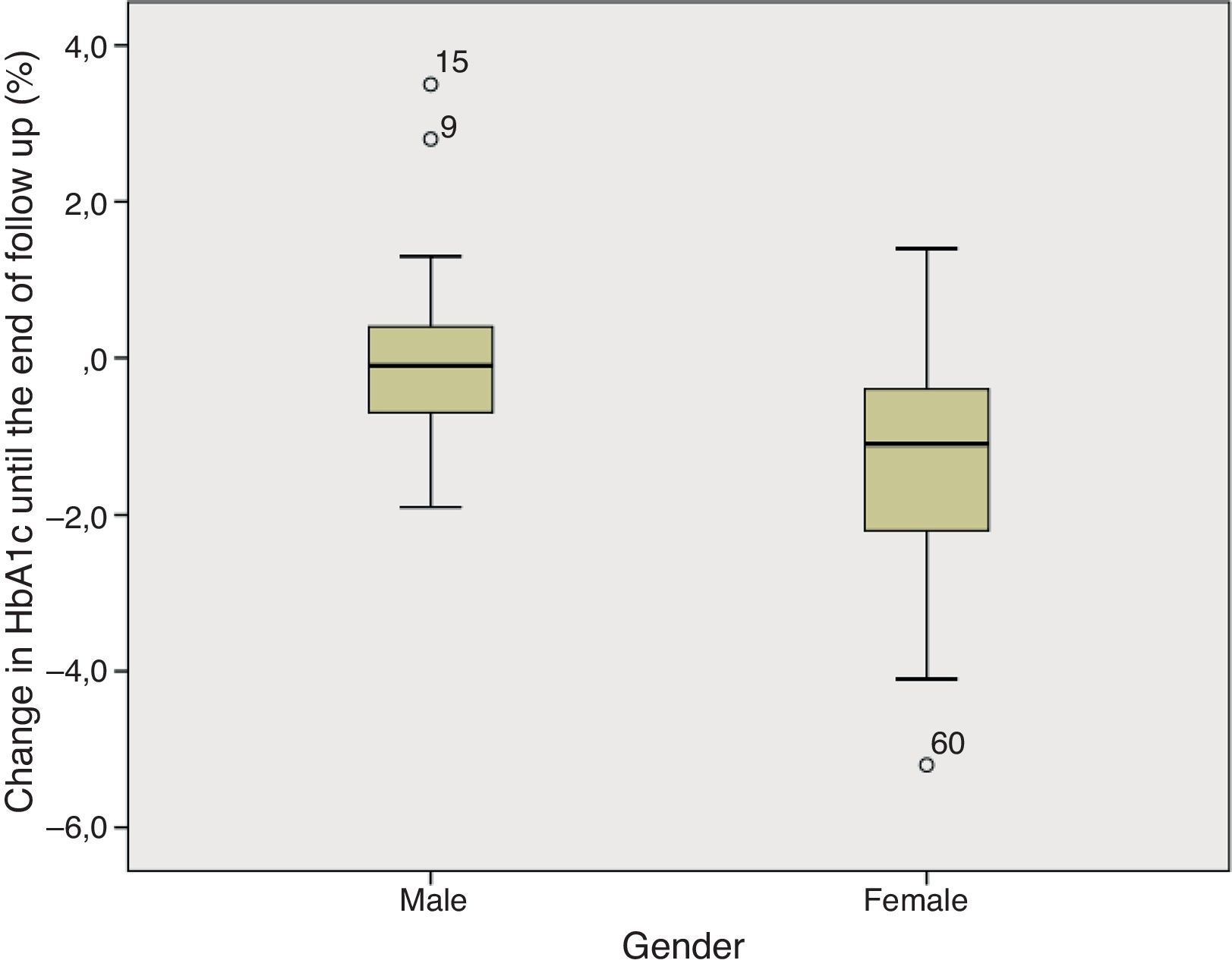
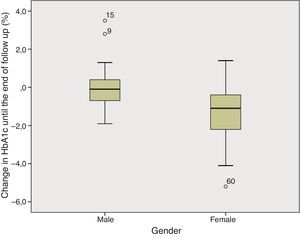
Gender. In the comparative analysis, we found a significant difference in HbA1c variation until the end of follow up, with a more pronounced reduction in women comparing to men (median [quartile]: −1.100 [−2.200–−0.400] vs −0.100 [−0.800–0.400]; p=0.002)–figure 2. It is of note that the two groups were not significantly different in terms of pre-CSII HbA1c (Men: 7.7 [7.2–8.2] vs Women: 8.2 [7.3–9.2]; p=0.119).
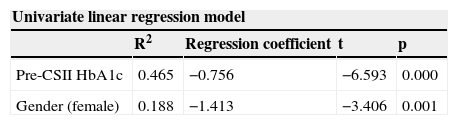
We evaluated the interaction between pre-CSII HbA1c and gender as determinants of variation of HbA1c until the end of the follow up period. According to this model, these two variables predict 52.3% of the variance of HbA1c. Female gender is associated with a 0.824 reduction in HbA1c (t=−2.449; p=0.018) and each 1.0% increase of the pre-CSII HbA1c is associated with a 0.67% reduction in HbA1c (t=-5.869; p=0.000)–table 3.
Univariate linear regression models for pre-CSII HbA1c and gender. Multivariate linear regression model including pre-CSII HbA1c and gender as independent covariates and a dependent variable - change in HbA1c until the end of the follow up period.
| Univariate linear regression model | ||||
|---|---|---|---|---|
| R2 | Regression coefficient | t | p | |
| Pre-CSII HbA1c | 0.465 | −0.756 | −6.593 | 0.000 |
| Gender (female) | 0.188 | −1.413 | −3.406 | 0.001 |
| Multivariate linear regression model (R2=0.523) | |||
|---|---|---|---|
| Regression coefficient | t | p | |
| Gender | −0.824 | −2.449 | 0.018 |
| Pre-CSII HbA1c | −0.672 | −5.869 | 0.000 |
Weight and TDID. We found a significant correlation between weight and TDID, and between the weight variation and TDID variation until the end of the follow up period (ρ=0.557 p=0.031). There is a significant correlation between pre-CSII TDID and its variations at 12 months and end of follow up (ρ=−0.561 p=0.005 and ρ=−0.619 p=0.001, respectively), as well as with pre-CSII HbA1c and HbA1c variation until the end of follow up (ρ=0.381 p=0.024 and ρ=−0.402 p=0.025, respectively). The pre-CSII TDID/weight ratio has a significant correlation with the variation in TDID/weight ratio until the end of follow up (ρ=−0.771 p=0.001).
Characterization of groups according to the quality of response. The sample was divided in two groups according to the median HbA1c variation until the end of follow up: a reduction in HbA1c>0.8% was considered a «better response» (BR) and a reduction in HbA1c inferior or equal to 0.8%, a stable HbA1c or an increase in HbA1c were considered a «worse response» (WR). When the two groups were compared, we found a significant difference between pre-CSII HbA1c (BR 8.65% [7.775-9.775] vs WR 7.30% [6.900–8.100]; p=0.000), age at diagnosis (BR 10.0 years [5.5–18.0] vs WR 20.0 years [12.25–26.0]; p=0.003) and age at the time of CSII initiation (BR 28.5 years [22.75–34.25] vs WR 33.0 years [27.5–41.5]; p=0.028). We found a significantly higher number of women in the group with a BR to CSII (p=0.017). There were no other significant differences between groups.
DiscussionIn concordance with previous literature, we found a significant reduction in HbA1c after initiation of CSII, representing a median variation in HbA1c of −0.50% [−1.20–0.1] at 12 months follow up and −0.80% [−1.475–0.3] at the end of follow up. These results suggest that there is a more marked reduction in HbA1c with longer periods of follow up, as reported by other authors12. In our sample we did not find a significant change in weight, TDID or TDID/weight ratio.
The association between the improvement in glucose control with CSII and pre-CSII HbA1c was previously reported by Bode15 et al., as well as DeVries16. In concordance with those findings, we also report a negative correlation between pre-CSII HbA1c and its variation at 12 months and at the end of follow up.
Women had better outcomes with CSII than men, and this difference persisted even when adjusted to pre-CSII HbA1c. In a recent study19, this gender difference was reported, but the authors did not find a causal explanation for the results.
The fact that women presented with higher pre-CSII HbA1c values could be related to the existence of a patient subset with brittle diabetes20. This classification is very controversial and little understood. The results of CSII therapy in brittle diabetes are very discordant between studies, some of which report benefit20–24. However, it has already been reported improvement in metabolic control in female adolescents and young adults with CSII therapy18. Battaglia and colleagues considered that the studied population has a high prevalence of insulin injection omission, particularly in individuals with body image disorders, and showed that CSII was associated with a significant improvement in metabolic control, adherence to the prescribed regimen and better self-efficacy. Occasionally, this type of behavior is not identified, and these patients’ diabetes is wrongly classified as brittle.
The response to the intervention in the group of women might also be related to other variables, which we did not evaluate, perhaps associated with the receptivity and adherence to new technologies, including the appropriate use of an insulin pump. It is also possible that there are other variables related to glucose control that we cannot infer from pre-CSII HbA1c.
The finding of a significant correlation between pre-CSII TDID and its variation at 12 months and at the end of follow up, and with HbA1c variation can hypothetically be related to: 1) the individuals that most benefited from CSII therapy were already highly motivated and, beyond the rigorous compliance with their insulin regimen, they also increasingly adhered to their meal plan, with subsequent weight loss; 2) individuals that most benefited from CSII therapy were the ones that had more variability of their needs of insulin throughout the day and hence, were using a higher TDID under a MDI regimen; 3) individuals that most benefited from CSII therapy are the ones that tend to occasionally neglect insulin injections and are reported has keeping elevated HbA1c values despite using higher TDID.
The correlation between age at diagnosis and HbA1c variation at the end of follow up, and between age at the time of initiation of CSII and HbA1c variation at 12 months follow up can be related to the presence of a subset of older individuals in our sample, with the same duration of diabetes, and less capable of using electronic devices.
The characterization of two groups in terms of response to CSII confirmed our previous results, including the ones relative to a probable gender effect–higher prevalence of women in the BR group; the influence of pre-CSII HbA1c–individuals with higher HbA1c tend to obtain more benefit from CSII; age at diagnosis and at the time of initiation of the intervention–younger individuals have more marked reductions in HbA1c.
LimitationsOur study did not include other variables that might influence the success of CSII, such as patients’ socioeconomic data (ie education level, employment pattern or characterization of the family aggregate), as this information was not originally included in our database. As ours is a convenience sample, we need to consider selection bias. On the other hand, we did not study the benefits of CSII in terms of incidence and severity of hypoglycaemia episodes and incidence of ketoacidosis. We considered information on hypoglycaemia unreliable because this was a retrospective study, and the registry is frequently based on subjective qualitative measures of incidence, as determined by several clinicians. On the other hand, ketoacidosis was extremely rare. We were also not able to extensively characterize the subgroup of women with greater improvement in metabolic control as our registry did not include more data on these patients, namely psychometric measures.
ConclusionsWomen with type 1 diabetes mellitus seem to have greater benefit with CSII therapy, presumably in association with the existence of a subset of individuals with unsatisfactory glucose control with multiple daily injections. However, it is possible that other variables related with the female gender might contribute to this difference. Our findings should not interfere with priority criteria for the use of insulin pumps in type 1 diabetics.
Presented at the ICE/ECE 2012 meeting with a grant provided by the Sociedade Portuguesa de Endocrinologia, Diabetes e Metabolismo.