To review the literature about the prevalence of vitamin D deficiency and its consequences in children and adolescents with sickle-cell disease.
Data sourcesThe literature survey was performed through the bibliographic databases MEDLINE; U.S. National Library of Medicine and National Institutes of Health (PubMed); Literatura Latino-Americana e do Caribe em Ciências da Saúde (Lilacs), and the Cochrane Library. The keywords were selected using Medical Heading Terms (MeSH): “vitamin D” OR “vitamin D deficiency” AND “anemia, sickle cell” AND “child” AND “adolescent”. The search was limited to articles in English, Spanish and Portuguese, published until April 2014.
Data synthesisEleven articles were selected among the 18 found. In 6 of the 11 studies, serum levels of vitamin D in children and/or adolescents with sickle-cell anemia were low. The prevalence of vitamin D deficiency in patients with sickle-cell anemia exceeded that of the comparison group. The low intake of vitamin D, seasonality, exposure to sun, increased metabolism associated with the hemoglobinopathy, and age increase were factors associated with the deficiency. There was an association between a significant vitamin D deficiency and bone weakness and painful crises. There was a positive correlation between increased levels of vitamin D by supplementation and functional, physical capacity.
ConclusionsThe vitamin D deficiency in children and adolescents with sickle-cell disease is prevalent and requires further studies to demonstrate its association with comorbidities and possible benefits of vitamin D supplementation.
Revisar a literatura sobre a frequência da deficiência de vitamina D e suas consequências em crianças e adolescentes com anemia falciforme.
Fontes de dadosO levantamento bibliográfico foi feito nas bases bibliográficas MEDLINE, U.S. National Library of Medicine e National Institutes of Health (PubMed), Literatura Latino-Americana e do Caribe em Ciências da Saúde (Lilacs) e Cochrane. Os descritores foram selecionados com o uso do Medical Heading Terms (MeSH): “vitamin D” OU “vitamin D deficiency” E “anemia, sickle cell” E “child” E “adolescent”. A busca limitou-se aos artigos em inglês, espanhol e português, com data de publicação até abril de 2014.
Síntese dos dadosForam selecionados 11 estudos, entre os 18 encontrados. A pesquisa revelou que os níveis séricos de vitamina D em crianças e/ou adolescentes com anemia falciforme encontram-se baixos em seis de 11 artigos analisados. Essa frequência de deficiência de vitamina D em pacientes com anemia falciforme excede a do grupo de comparação saudável. A baixa ingesta de vitamina D, a sazonalidade, a exposição solar, o metabolismo aumentado próprio da hemoglobinopatia e o aumento da idade são fatores associados à deficiência. Houve associação entre deficiência significativa de vitamina D e fraqueza óssea e crises dolorosas. Há correlação positiva entre aumento dos níveis de vitamina D por meio da suplementação e a capacidade funcional física.
ConclusõesA deficiência de vitamina D em crianças e adolescentes com doença falciforme é prevalente e necessita de mais estudos para evidenciar a sua relação com comorbidades e possíveis benefícios da suplementação da vitamina D.
Sickle cell disease is caused by a mutation resulting from an exchange of nitrogenous bases in the sixth codon of the beta-globulin hemoglobin gene, generating an abnormal hemoglobin called hemoglobin S (HbS).1 The manifestations of sickle-cell disease are due to the presence of HbS, of which molecules are organized into polymeric beams when deoxygenated and give the RBC an elongated and rigid conformation, called a “sickle-shaped red blood cell”.2 After the sickling process, the red blood cells begin to show changes in membrane proteins and increased expression of adhesion molecules that, consequently, lead to red blood cell adhesion to the endothelium. This process triggers an inflammatory phenomenon, activation of coagulation, hypoxia, ischemia and local infarction, in addition to reduced RBC survival.2 Sickle-cell disease is one of the most common genetic diseases in Brazil and in the world.3 The chronic hemolysis, anemia, and vaso-occlusive phenomena that occur in patients with the disease are triggers of an accelerated metabolism, and this process leads to a basal metabolic rate 20% higher in patient with sickle-cell anemia than in the normal population.1,4 However, there are no records of methodologies and equations to estimate the energy expenditure in children with sickle-cell anemia.5
Children with sickle cell anemia have a higher risk of developing nutritional deficiencies due to reduced appetite,6 poor dietary intake of nutrients7 and infectious complications, which demands greater attention from health professionals. Among the vitamins, vitamin D must be carefully evaluated in children with sickle-cell anemia. This is due to the high concentration of melanin in the skin, low levels of physical activity,8 and low food intake.7,9 Children with sickle-cell anemia are more likely to develop vitamin D deficiency when compared to the healthy controls.10 Calcium and vitamin D are important for bone metabolism, and the low calcium intake leads to a reduction in the ideal bone mass peak in children and adolescents with sickle-cell anemia, which determines growth failure.11 Vitamin D deficiency, in turn, is associated with increased respiratory infections, muscle weakness and increased risk of falls and microlesions.12 Additionally, in children with sickle-cell anemia, whose bones are affected by infarction, osteoporosis and osteonecrosis, vitamin D deficiency may worsen bone condition.13
Considering these facts, this study aimed to carry out an integrative literature review to analyze the frequency of vitamin D deficiency and its consequences in children and adolescents with sickle-cell anemia.
Literature reviewFor the literature review, data searches were carried out in the MEDLINE databases; at the US National Library of Medicine and the National Institutes of Health (PubMed) interface; Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS), and the Cochrane Library. To make up the search strategy, the descriptors were selected using Medical Subject Heading Terms (MeSH). Those terms were used for the search in the MEDLINE database, and subsequently adapted and translated into Spanish and Portuguese for the search in other databases. The following parameters were used: “vitamin D” OR “vitamin D deficiency” AND “anemia, sickle cell” AND “child” AND “adolescent”.
Inclusion criteria for the selection were articles published in English, Portuguese and Spanish, with a study population in the age range of children and adolescents, younger than 18 years old, with sickle-cell disease. Exclusion criteria were animal studies and human studies that did not include the predetermined age range and that had no association with sickle-cell anemia. No time limit was considered for the bibliographic search. The reference search was carried out from December 2013 to April 2014.
To eliminate article duplication, documents were ordered by titles and authors, and those that appeared more than once were excluded. For the selection of studies, a review of titles and abstracts was performed. After the first review, 18 articles were selected: 2 from the Cochrane Library database, 15 from PubMed and 1 from LILACS database. After the initial selection, a new text evaluation was carried out in full texts, resulting in the exclusion of 7 items. These articles were excluded, as they did not contemplate the age range described in the inclusion criteria. At the end of the evaluation, we selected one article from the Cochrane database and 10 from the Pubmed database.
The studies were entered in a Microsoft Office Excel 2007® spreadsheet with recorded information on title, authors, journal, year of publication, objective, study design, population, level of evidence, main results and conclusions of the study. The developed studies had their experimental protocols approved by the Institutional Review Board of the respective institutions. Because this is a review and update on the subject, the present study was not submitted to the Institutional Review Board at our institution.
The aforementioned hierarchization based on the level of evidence was carried out following the classification methodology according to study design, which categorizes the studies in seven levels.14,15 In the first level, the evidence is derived from systematic review or meta-analysis of all relevant randomized controlled clinical trials; at level 2, from at least one randomized, controlled and well-designed clinical trial; in level 3, evidence from well-designed clinical trials, without randomization; level 4, evidence from well-designed cohort and case-control studies; level 5, results of systematic review of descriptive and qualitative studies; level 6, evidence derived from descriptive or qualitative study, and level 7, evidence from experts’ opinions and/or reports of expert committees.
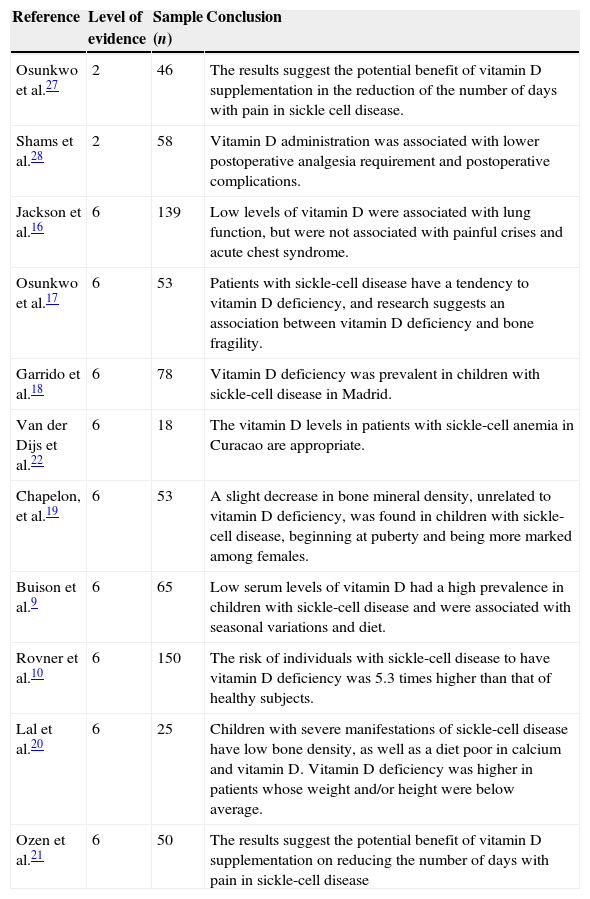
The final review sample consisted of 11 articles, of which representations are shown in Table 1.
Summary of selected articles for the review: sickle cell disease and vitamin D.
| Reference | Level of evidence | Sample (n) | Conclusion |
|---|---|---|---|
| Osunkwo et al.27 | 2 | 46 | The results suggest the potential benefit of vitamin D supplementation in the reduction of the number of days with pain in sickle cell disease. |
| Shams et al.28 | 2 | 58 | Vitamin D administration was associated with lower postoperative analgesia requirement and postoperative complications. |
| Jackson et al.16 | 6 | 139 | Low levels of vitamin D were associated with lung function, but were not associated with painful crises and acute chest syndrome. |
| Osunkwo et al.17 | 6 | 53 | Patients with sickle-cell disease have a tendency to vitamin D deficiency, and research suggests an association between vitamin D deficiency and bone fragility. |
| Garrido et al.18 | 6 | 78 | Vitamin D deficiency was prevalent in children with sickle-cell disease in Madrid. |
| Van der Dijs et al.22 | 6 | 18 | The vitamin D levels in patients with sickle-cell anemia in Curacao are appropriate. |
| Chapelon, et al.19 | 6 | 53 | A slight decrease in bone mineral density, unrelated to vitamin D deficiency, was found in children with sickle-cell disease, beginning at puberty and being more marked among females. |
| Buison et al.9 | 6 | 65 | Low serum levels of vitamin D had a high prevalence in children with sickle-cell disease and were associated with seasonal variations and diet. |
| Rovner et al.10 | 6 | 150 | The risk of individuals with sickle-cell disease to have vitamin D deficiency was 5.3 times higher than that of healthy subjects. |
| Lal et al.20 | 6 | 25 | Children with severe manifestations of sickle-cell disease have low bone density, as well as a diet poor in calcium and vitamin D. Vitamin D deficiency was higher in patients whose weight and/or height were below average. |
| Ozen et al.21 | 6 | 50 | The results suggest the potential benefit of vitamin D supplementation on reducing the number of days with pain in sickle-cell disease |
In the selected studies, vitamin D deficiency was treated as an object of primary or secondary research, when associated to the main subject of the study. According to Dietary Reference Intakes, vitamin D deficiency occurs when serum 25-hydroxyvitamin D levels are <11ng/mL.11
Seven of the nine articles analyzed serum vitamin D levels in children and/or adolescents with sickle-cell disease and showed a deficiency of this nutrient.9,16–21 Jackson et al., in a cohort of children in whom vitamin D levels were measured, found that 96.4% had vitamin D deficiency and, of these, 64.0% had severe deficiency (<10ng/mL); 1.4% had insufficient levels, and only three children (2.2%) had adequate levels.16 Van der Dijs et al. observed that, when compared to healthy controls, patients with sickle-cell anemia had lower serum calcium concentrations. These levels, however, were not below the reference values. In the same study, there was no difference between the groups regarding the phosphate, parathormone and vitamin D levels.22 However, other studies demonstrated that the frequency of low serum levels of vitamin D in the group with sickle-cell anemia exceeded the frequency found in the healthy group.9,10
Due to the participation of solar radiation in vitamin D metabolism, a review study evaluated seasonal variations, demonstrating higher serum levels of vitamin D in the seasons with higher temperatures, spring and summer.9,16 In another study, however, this variation was not demonstrated.18
Another determining factor for the adequacy of serum levels of vitamin D and calcium is proper food intake. In sickle-cell disease, nutritional needs are greater due to the higher energy requirement.10 Studies evaluating food intake of subjects that had vitamin D deficiency have shown that dietary intake of the vitamin is not appropriate.9,10 The intake of vitamin D was below the recommended values of 200IU (5mg/day).9,11 The low consumption of milk, source of calcium and vitamin D, tends to decline even more with age, leading to increasingly lower serum levels of vitamin with increasing age.9
The difference during the aging process was also demonstrated by Garrido et al., who reported low serum levels of vitamin D in children younger than 5 years, but, in children older than 5 years, this deficiency was even higher. No child older than 5 years showed acceptable levels of vitamin D.18 These results are in parallel with another study, which showed an inverse correlation between the levels of vitamin D and age, that is, the older the age, the lower the levels of vitamin D. In this study, osteocalcin serum levels were lower than those found in healthy children, which may be a consequence of vitamin D deficiency.20 Osteocalcin, which participates in bone mineralization, is synthesized by osteoblasts, and their induction occurs by vitamin D3. Osteocalcin levels are higher during childhood, and their peak occurs during puberty.23 This fact could explain the inverse correlation, as the older the age, the higher the production of osteocalcin.
Serum levels of vitamin D and comorbiditiesSome selected studies assessed the association of vitamin D deficiency with comorbidities in patients with sickle-cell anemia.9,16,17,20,21,24 Jackson et al., in a retrospective study, aimed to evaluate the association of vitamin D deficiency with painful crises and asthma attacks in children with sickle-cell anemia. Although vitamin D deficiency was prevalent in the population, the study found no statistical association between vitamin D deficiency and the number of painful crises and asthma.16 Osunkwo et al. assessed 53 children and adolescents with sickle-cell anemia, of which 32% had chronic pain, and 42%, bone fragility. A significant association was observed between vitamin D deficiency and painful crises. The same study also found an association between low vitamin D levels and bone weakness.17
In sickle cell anemia, the bone can be affected by microinfarctions, osteopenia, osteonecrosis, osteoporosis and osteomyelitis.25 Risk factors for the occurrence of osteopenia in sickle-cell disease include delayed puberty and the low competence of bone mass metabolism peak, microinfarctions resulting from vaso-occlusive events, chronic pain with immobilization, and of the efficiency of calcium, vitamin D and other nutrients.20,26
Low levels of vitamin D are associated with the decrease in mineral acquisition by the bone.9,24 Lal et al. observed significant bone density deficit in the proximal end of the femur and lumbar spine in children with severe manifestations of sickle-cell anemia. There was no correlation between the intake of calcium or vitamin D and bone density. In the same study, however, there was a strong negative correlation between vitamin D values and serum levels of alkaline phosphatase, suggesting that vitamin D significantly affected bone metabolism in these patients.20 In the study by Ozen et al., an insufficient intake of vitamin D was observed, which was more significant among patients with osteopenia and osteoporosis, compared to those without alterations, which demonstrates the importance of adequate vitamin D intake for the prevention of comorbidities.21
Vitamin D supplementationThere are few studies that addressed vitamin D supplementation in children and adolescents with sickle-cell anemia. In our review, we found only three studies. The study by Garrido et al., which assessed the status of vitamin D in children with sickle-cell anemia in Spain, observed that vitamin D levels in children younger than 1 year were below appropriate levels, despite the supplementation. The vitamin D supplementation, in this situation, promoted a vitamin deficiency stabilization, preventing the development of a more severe deficiency.18
Osunkwo et al., in a pilot randomized clinical trial, analyzed supplementation with 500mg of calcium and 200IU of vitamin D for a period of 6 months in 46 subjects with sickle-cell anemia, aged 7–21 years. The patients, previously diagnosed with vitamin D deficiency by serum 25-hydroxyvitamin D measurements, were randomly assigned to receive calcium and vitamin D supplementation or placebo. During the study, the recording of painful crises and the assessment of quality of life scores in the physical domain were performed, to which scores were attributed regarding the patient's performance of everyday activities. The results showed an inverse correlation between vitamin D levels and the incidence of pain, demonstrating that the higher the levels of vitamin D, the lower the incidence of painful crises. There were also positive correlations between vitamin D levels and the physical domain scores, demonstrating the benefits of vitamin D for the quality of life of these patients.27
In the study by Shams et al., 58 children with sickle-cell anemia were randomly divided into two groups, one of which received daily supplementation of 400IU of vitamin D for a period of 6 months prior to circumcision surgery, while the other group received no intervention before the procedure. In the postoperative period, the groups were evaluated for the need of analgesia, the presence of pain and the occurrence of complications related to sickle-cell disease, such as cerebrovascular accidents and painful crises, as well as those unrelated to sickle-cell disease, such as fever and infection signs. Vitamin D administration was associated with a lower incidence of postoperative complications associated with sickle-cell anemia, as well as less need for analgesia postoperatively.28
The aforementioned studies bring results from small population samples, with the first one being a pilot study. Additionally, the second study was carried out with male patients only, submitted to minor surgery, which results in limited external validity. Due to the low number of clinical trials included in this review that analyzed vitamin D supplementation in children and adolescents with sickle-cell disease, the benefits of this supplementation for this population are not conclusive yet, demonstrating the need for further studies on this subject.
Final considerationsThe performance of this review and the small number of identified articles shows the scarcity of studies on the nutritional status of children and adolescents with sickle-cell disease and the influence of vitamin D on the clinical profile of these patients. There were no studies carried out in Brazil. It should be noted that the review was performed using the keywords in Portuguese, Spanish and English, and no time limit was established. Although many studies reported on the prevalence of vitamin D deficiency in children and adolescents with sickle-cell disease, its consequences and the effects of supplementation are inconsistent.
According to the literature, vitamin D deficiency is common in this population. It also showed a significant difference in deficiency when the study group was compared with healthy subjects. As for causes, the influence of nutrient intake and higher dietary requirements, seasonality and sun exposure, age and common disease complications, such as bone infarctions, was demonstrated. As for the association between serum levels of vitamin D and comorbidities, the results are still controversial, showing a possible association of vitamin D deficiency with the occurrence of painful crises, decreased physical performance and impaired bone metabolism. In studies in which vitamin D and calcium supplementation intervention was performed, an association between adequate levels of vitamin D and the improvement in the patient's quality of life related to physical performance and reduction of complications associated or not with sickle-cell disease and pain in the postoperative period was demonstrated.
It can be concluded, with this review, that vitamin D deficiency in children and adolescents with sickle-cell disease is frequent, and that more studies are required to provide evidence of the association of vitamin D deficiency with painful crises and bone metabolism, as well as to assess the potential therapeutic benefits of vitamin D supplementation in this population.
FundingThis study did not receive funding.
Conflicts of interestThe authors declare no conflicts of interest.