To present the main results of the literature on genetic polymorphisms in Turner syndrome and their association with the clinical signs and the etiology of this chromosomal disorder.
Data sourcesThe review was conducted in the PubMed database without any time limit, using the terms Turner syndrome and genetic polymorphism. A total of 116 articles were found, and based on the established inclusion and exclusion criteria 17 were selected for the review.
Data synthesisThe polymorphisms investigated in patients with Turner syndrome were associated with growth deficit, causing short stature, low bone mineral density, autoimmunity and cardiac abnormalities, which are frequently found in patients with Turner syndrome. The role of single nucleotide polymorphisms in the etiology of Turner syndrome, i.e., in chromosomal nondisjunction, was also confirmed.
ConclusionsGenetic polymorphisms appear to be associated with Turner syndrome. However, in view of the small number of published studies and their contradictory findings, further studies in different populations are needed in order to clarify the role of genetic variants in the clinical signs and etiology of the Turner syndrome.
Apresentar os principais resultados dos estudos que investigaram polimorfismos genéticos em síndrome de Turner, bem como sua associação com alguns sinais clínicos e etiologia desse distúrbio cromossômico.
Fontes de dadosRevisão bibliográfica feita no PubMed, sem limite de período, com os seguintes termos: Turner syndrome and genetic polymorphism. Foram identificados 116 artigos e, de acordo com os critérios de inclusão e exclusão, 17 foram selecionados para leitura.
Síntese dos dadosOs polimorfismos investigados em pacientes com síndrome de Turner estavam relacionados com déficit de crescimento, que causou baixa estatura, densidade mineral óssea baixa, autoimunidade e anomalias cardíacas, que podem estar presentes com frequências significativas nas pacientes. Também foi verificado o papel dos polimorfismos de único nucleotídeo (SNPs) na etiologia da síndrome de Turner, ou seja, na não disjunção cromossômica.
ConclusõesOs polimorfismos genéticos parecem estar associados à síndrome de Turner. Entretanto, por conta dos poucos estudos publicados e dos achados contraditórios, pesquisas em diferentes populações são necessárias para esclarecer o papel dessas variantes genéticas para os sinais clínicos e a etiologia do distúrbio cromossômico.
The description of female patients with Turner syndrome (TS) was published in 1938 by Henry Turner1; however, in 1930, German pediatrician Dr. Otto Ullrich had already reported a case of an 8-year-old girl with signs suggestive of TS.2 Therefore, this syndrome is also called Ullrich-Turner syndrome.
The disorder has an incidence of 1/2500 girls and clinical signs include lymphedema of hands and feet, short and webbed neck, low hairline at the nape of the neck, cubitus valgus, hypoplastic or hyperconvex nails, micrognathia, high-arched palate, short stature, gonadal dysgenesis, primary amenorrhea, sexual infantilism, infertility, shield chest, breast hypertelorism, cardiac (coarctation of the aorta and ventricular septal defects) and renal anomalies (horseshoe kidney, urethral duplication and unilateral kidney agenesis), multiple pigmented nevi, scoliosis, fourth/fifth metacarpal or metatarsal hypoplasia or both. The following problems may also be present: hearing impairment, arterial hypertension, osteoporosis, obesity, visual disturbances, impaired glucose tolerance, learning disabilities, psychosocial problems and thyroid diseases, among other autoimmune diseases.3
TS is characterized by large phenotypic variability, ranging from the classical form (girls with pubertal development and growth delay) to those with few dysmorphic signs, which are almost indistinguishable from the general population.4 The definitive diagnosis of TS is carried out by analyzing the karyotype, which allows the identification of the individual's chromosomal constitution. TS chromosomal etiology was only elucidated in 1959, when the first patient was investigated by cytogenetics and showed the 45, X chromosomal constitution.5 Brazilian studies in patients with TS showed that the karyotype 45, X, i.e., monosomy of the sex chromosome X, was found in 40–60% of patients with TS, but mosaicisms and karyotypes with structural alterations, mainly isochromosomes, are also found.4,6–8 A retrospective study indicated that better-quality diagnosis has improved the quality of the cytogenetic result of TS, with modification of the proportion between the types of karyotype observed, especially the progressive decrease in the identification of 45, X patients and increased detection of karyotypes with structural aberrations.6
In Brazil, the mean age of TS diagnosis is around 12 years of age,4,7,9,10 and 25.3% and 51.1% of the patients were respectively diagnosed in childhood (1–11 years) and adolescence (12–18 years), on account of the short stature.7 Therefore, it is important to assess girls with short stature, regardless of the presence of typical dysmorphic signs and pubertal delay, by requesting the karyotype analysis to confirm or rule out TS. The authors also point out that neonatologists and pediatricians should be aware about the possibility of genetic syndromes such as TS, considering that the main signs are present at birth, but are not taken into account at that time.7 Thus, medical training improvement aimed to recognize the spectrum of clinical manifestations of this chromosomal syndrome is necessary. In addition to the difficulty of achieving an early diagnosis of this genetic condition, other factors associated with late TS diagnosis include less severe growth retardation, presence of spontaneous pubertal signs, socioeconomic determinants and absence of obvious dysmorphic features.4,10
Early diagnosis of TS is vital, as it also allows the identification of congenital and acquired anomalies, as well as the detection of cases with Y chromosome sequences in the karyotype, which is associated with gonadoblastoma, a tumor with high malignant potential that can be prevented through prophylactic gonadectomy,11 besides allowing hormonal treatments with growth hormone (GH) and oxandrolone12 and estrogen/progestogen12,13 to respectively increase final height and accentuate secondary sexual characteristics at the adequate chronological age, preventing further damage to patient health. Therefore, an early and accurate diagnosis is important for a successful therapeutic approach. It is noteworthy that there is no classical karyotype or phenotype associated with TS, and the diagnosis is not always evident, but should be actively sought, both from a clinical and cytogenetic point of view.6
Additionally, TS is a genetic disorder, and single nucleotide polymorphisms (SNPs) may be involved in its etiology. SNPs, which are defined as a change that can occur in one of every 1000 base pairs, are common in the human genome and are involved in several human diseases. They correspond to genetic alterations present in more than 1% of the population and can be located in different regions of the gene: promoter, coding and non-coding. SNPs in the coding and promoter regions are more likely to alter gene function and, consequently, the formed protein.14
In this context, this study aims to review the main results of the studies investigating genetic polymorphisms in TS, as well as their association with clinical signs and etiology of this chromosomal disorder.
MethodThe present study is a literature review, with a descriptive approach. A survey was carried out in PubMed, in July 2014, without time limit, using the following terms: Turner syndrome and genetic polymorphism. The PubMed database was chosen for this systematic review because it is more comprehensive and internationally used for researches in the health area.
Inclusion criteria were articles closely related to the topic and selection was based on titles and/or abstracts, full article availability and publication in English and/or Portuguese. The ones that did not meet the abovementioned criteria were excluded.
A total of 116 articles were found. Of these, 14 were selected and 102 excluded. Of the excluded ones, 21 contained the term Turner in the list of authors. The reference lists of the included articles were also assessed to identify relevant studies not detected by the electronic search. Based on the established inclusion criteria, we selected three more studies, totaling 17.
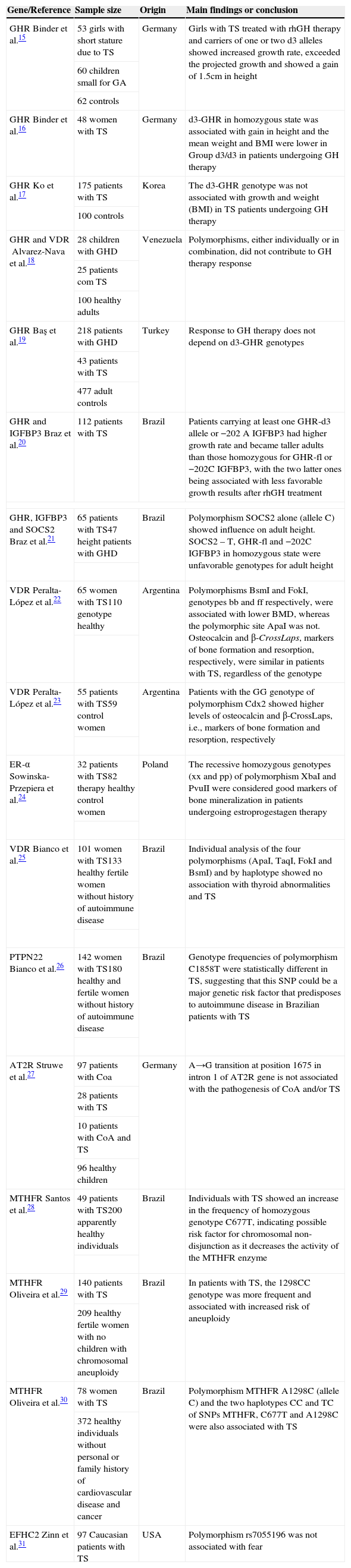
ResultsThe main results of the 17 studies15–31 included in this systematic review are shown in Table 1.
Summary of key findings of 17 studies that evaluated genetic polymorphisms in Turner syndrome.
| Gene/Reference | Sample size | Origin | Main findings or conclusion |
|---|---|---|---|
| GHRBinder et al.15 | 53 girls with short stature due to TS | Germany | Girls with TS treated with rhGH therapy and carriers of one or two d3 alleles showed increased growth rate, exceeded the projected growth and showed a gain of 1.5cm in height |
| 60 children small for GA | |||
| 62 controls | |||
| GHRBinder et al.16 | 48 women with TS | Germany | d3-GHR in homozygous state was associated with gain in height and the mean weight and BMI were lower in Group d3/d3 in patients undergoing GH therapy |
| GHRKo et al.17 | 175 patients with TS | Korea | The d3-GHR genotype was not associated with growth and weight (BMI) in TS patients undergoing GH therapy |
| 100 controls | |||
| GHR and VDRAlvarez-Nava et al.18 | 28 children with GHD | Venezuela | Polymorphisms, either individually or in combination, did not contribute to GH therapy response |
| 25 patients com TS | |||
| 100 healthy adults | |||
| GHRBaş et al.19 | 218 patients with GHD | Turkey | Response to GH therapy does not depend on d3-GHR genotypes |
| 43 patients with TS | |||
| 477 adult controls | |||
| GHR and IGFBP3Braz et al.20 | 112 patients with TS | Brazil | Patients carrying at least one GHR-d3 allele or −202 A IGFBP3 had higher growth rate and became taller adults than those homozygous for GHR-fl or −202C IGFBP3, with the two latter ones being associated with less favorable growth results after rhGH treatment |
| GHR, IGFBP3 and SOCS2Braz et al.21 | 65 patients with TS47 height patients with GHD | Brazil | Polymorphism SOCS2 alone (allele C) showed influence on adult height. SOCS2 – T, GHR-fl and −202C IGFBP3 in homozygous state were unfavorable genotypes for adult height |
| VDRPeralta-López et al.22 | 65 women with TS110 genotype healthy | Argentina | Polymorphisms BsmI and FokI, genotypes bb and ff respectively, were associated with lower BMD, whereas the polymorphic site ApaI was not. Osteocalcin and β-CrossLaps, markers of bone formation and resorption, respectively, were similar in patients with TS, regardless of the genotype |
| VDRPeralta-López et al.23 | 55 patients with TS59 control women | Argentina | Patients with the GG genotype of polymorphism Cdx2 showed higher levels of osteocalcin and β-CrossLaps, i.e., markers of bone formation and resorption, respectively |
| ER-αSowinska-Przepiera et al.24 | 32 patients with TS82 therapy healthy control women | Poland | The recessive homozygous genotypes (xx and pp) of polymorphism XbaI and PvuII were considered good markers of bone mineralization in patients undergoing estroprogestagen therapy |
| VDRBianco et al.25 | 101 women with TS133 healthy fertile women without history of autoimmune disease | Brazil | Individual analysis of the four polymorphisms (ApaI, TaqI, FokI and BsmI) and by haplotype showed no association with thyroid abnormalities and TS |
| PTPN22Bianco et al.26 | 142 women with TS180 healthy and fertile women without history of autoimmune disease | Brazil | Genotype frequencies of polymorphism C1858T were statistically different in TS, suggesting that this SNP could be a major genetic risk factor that predisposes to autoimmune disease in Brazilian patients with TS |
| AT2RStruwe et al.27 | 97 patients with Coa | Germany | A→G transition at position 1675 in intron 1 of AT2R gene is not associated with the pathogenesis of CoA and/or TS |
| 28 patients with TS | |||
| 10 patients with CoA and TS | |||
| 96 healthy children | |||
| MTHFRSantos et al.28 | 49 patients with TS200 apparently healthy individuals | Brazil | Individuals with TS showed an increase in the frequency of homozygous genotype C677T, indicating possible risk factor for chromosomal non-disjunction as it decreases the activity of the MTHFR enzyme |
| MTHFROliveira et al.29 | 140 patients with TS | Brazil | In patients with TS, the 1298CC genotype was more frequent and associated with increased risk of aneuploidy |
| 209 healthy fertile women with no children with chromosomal aneuploidy | |||
| MTHFROliveira et al.30 | 78 women with TS | Brazil | Polymorphism MTHFR A1298C (allele C) and the two haplotypes CC and TC of SNPs MTHFR, C677T and A1298C were also associated with TS |
| 372 healthy individuals without personal or family history of cardiovascular disease and cancer | |||
| EFHC2Zinn et al.31 | 97 Caucasian patients with TS | USA | Polymorphism rs7055196 was not associated with fear |
TS, Turner syndrome; rhGH, recombinant human growth hormone; GH, growth hormone; BMI, body mass index; GHD, GH deficiency; BMD, bone mineral density; CoA, coarctation of the aorta; SNPs, single nucleotide polymorphisms; GA, gestational age.
The present study is a review article on studies about contribution of polymorphisms to the manifestations of TS, its etiology and response to treatment. SNPs shown in Table 1 are related to growth deficit, leading to short stature, low bone mineral density, autoimmunity and heart abnormalities; these characteristics are present at significant frequency in patients with TS. The role of polymorphisms in the etiology of TS, namely, the chromosomal non-disjunction, was also demonstrated.
Short statureOne of the main clinical signs of TS is short stature, which was present in 97% and 100% of patients with TS assessed.7,8 Thus, growth hormone therapy (GHT) is given to these patients to increase their final adult height. GH exerts its biological functions by direct binding to the GHR (Growth Hormone Receptor) or indirectly via IGF-1 (Insulin-Like Growth Factor) in synergy.32 Therefore, polymorphism in the GHR may affect the response to GH therapy in patients with TS.
The GHR gene is located on 5p13.1-p12 and has two different isoforms: a complete or full-length (GHRfl) and one with deletion of exon 3 (GHRd3). Three possible genotypes can be observed: fl/fl, fl/d3 and d3/d3, with the latter being the one with the lowest frequency. The GHRd3 polymorphism was evaluated in seven studies regarding its influence on response to therapy with recombinant human growth hormone (rhGH) in patients with TS. Four studies have investigated this SNP alone15,17,19 and three, its role alone and in combination with polymorphisms in the VDR (vitamin D receptor), IGFBP3 (insulin-like growth factor binding protein) and SOCS2 (suppressor of cytokine signaling) genes, via gene-gene interaction.18,20,21 The results obtained by the German15,16 and Brazilian groups20,21 reported a positive association between the investigated SNPs and growth response to rhGH therapy. The VDR gene is important for height because it interferes with calcium and phosphate homeostasis, influencing skeletal growth.18 Additionally, polymorphisms inherited from the VDR gene can affect IGF signaling and in turn, response to rhGH in patients with GH deficiency and TS. The polymorphisms −202 A/C IGFBP3 and SOCS-2 are involved in the pharmacogenetics of rhGH and negative regulation of GHR signaling, respectively.20,21 In one of these studies, the authors suggest the use of these genetic markers to identify, among patients treated with rhGH, those genetically predisposed to have less favorable results.21 The homozygous state for the d3-GHR allele was associated with weight control and body mass index (BMI) in TS in one study,16 but not in another.17 Regarding growth, polymorphisms in the GHR gene contributed to therapy response alone15 or in combination.20,21 One of the studies20 mentioned that the possible reasons for the lack of correlation in two previous studies included the small number of patients evaluated18 and the fact that few individuals are carriers of the d3 allele.17 The latter study reported that the genotypes of the GHR gene could not be divided in three groups as a consequence of the low frequency of allele d3, which could have led to negative results.17 The three studies that showed negative association of the d3-GHR polymorphism were carried out in Korea, Venezuela and Turkey.17–19 One of them18 reported that the study was limited by sample size. However, the findings were the product of a prospective study18 and the previous researches were retrospective.15–17 The need for prospective studies was reported by two studies.17,20 One way to increase sample size is to perform multicenter studies, such as those carried out by Ko et al.17 and Baş et al.,19 both retrospective. In the first study, the 175 patients with TS were from 20 hospitals in Korea.17 The study published in 2012 reportedly included the largest number of patients among studies carried out so far, but only evaluated 43 patients with TS,19 a number similar to that of non-multicenter studies.15,16 Because of the difficulty in obtaining large and homogeneous samples of patients with TS and GH deficiency, multicenter studies and/or meta-analyses are needed to confirm the role of this polymorphism in the pharmacogenetics of rhGH.21 It is also noteworthy that, in relation to GH treatment in children with TS, two recent studies employing pharmacogenomic methods were published by the same group of researchers seeking to establish an individualized therapy.33,34
Positive associations have been reported only for individuals of the same ethnic origin.15,16,20,21 In spite of the contradictory results regarding GHRd3 polymorphism, a recent review showed the implications of this SNP in clinical practice and concluded it constitutes a predictive factor of better response to hormone replacement therapy in patients with short stature.32 Two other meta-analysis studies35,36 carried out by independent groups, also confirmed the influence of this polymorphism in response to growth in children with short stature treated with rhGH. However, these two meta-analyses included a maximum of three studies of rhGH therapy effect and its relation to genotype d3-GHR in TS, which highlights the need for additional investigations.35,36
Bone mineral densityTS patients have low bone mineral density (BMD), but it can be maintained at normal levels if the patient follows a healthy lifestyle with regular practice of physical exercise, early introduction of an appropriate dose of estrogens (hormone replacement therapy), as well as calcium and vitamin D.3,37 The VDR gene binds to the active form of vitamin D to modulate gene transcription. Vitamin D, in turn, is a steroid hormone which regulates bone metabolism, immune response, cell proliferation and differentiation.38 Vitamin D deficiency is associated with increased risk of fractures, autoimmune diseases, type 1 and 2 diabetes, hypertension and heart disease,38 clinical characteristics present in patients with TS.3 Considering the abovementioned facts, the VDR gene was investigated in four studies, one of them associated with growth,18 two with BMD22,23 and one with thyroid anomalies.25 The BsmI, FokI and Cdx2 polymorphisms were positively associated with bone density, and early detection of these polymorphisms could be useful for predicting severe osteopenia in patients with TS.22,23 Another study showed a positive association between polymorphisms in the ER-α gene (estrogen receptor-alpha) and BMD in patients with TS undergoing treatment with estroprogestagen.24 Patients with TS have estrogen deficiency as a result of ovarian failure, and hypoestrogenism plays a vital role in bone mineralization disorders. Thus, hormone replacement therapy with estrogen contributes to the development of secondary sexual characteristics and optimizes peak bone mass, preventing osteoporosis.39
AutoimmunityTS patients have high risk for autoimmune diseases, with the most common being those related to the thyroid. A descriptive study showed that cardiovascular anomalies (45%), otitis (43%), thyroid dysfunction (33%) and hypertension (26.6%) were the most frequent clinical alterations observed in 42 patients with TS.8 Polymorphisms in the VDR and PTPN22 gene (Protein Tyrosine Phosphatase, Non-receptor type 22) were investigated by the same group of researchers to verify their association with autoimmune thyroid diseases in patients with TS.25,26
The allelic variation of the PTPN22 gene, C1858T, was associated with the risk of autoimmune diseases in Brazilian patients with TS.26 The study published in 2012 reported that one of its main limitations was the small number of patients with TS and thyroid disorders – only 22 – which reduced the statistical power to detect associations between polymorphisms of the VDR gene and autoimmune diseases in TS.25 However, both researches suggest that further studies involving a larger number of patients be carried out to assess whether this association exists or not.
Cardiovascular malformationCardiovascular malformations are the highest contributors to morbidity and mortality in girls with TS, especially as a result of aortic dissection risk and, therefore, they can change life expectancy of these patients.40 The most common are coarctation of the aorta (CoA), bicuspid aortic valve and hypoplastic left heart.3 CoA and bicuspid aortic valve were present in 6.9% and 21%, respectively, of 233 French patients with TS assessed for cardiovascular findings,41 with a 19% frequency being found in another study.9 One gene associated to cardiovascular diseases is AT2R (Angiotensin Type 2 Receptor); however, polymorphism A→G does not seem to be involved in the pathogenesis of CoA in individuals with and without TS.27 A recent study showed that 5.3% of girls with CoA were diagnosed with TS when karyotyping was carried out. The authors conclude that all girls with CoA should undergo karyotyping, with a minimum count of 50 cells at the time of heart disease diagnosis.42
Previous studies have demonstrated that: (1) folic acid prevents congenital heart defects; (2) homocysteine levels have a strong correlation with cardiovascular disease genesis, and (3) female sex hormone deficiency is an important factor for homocysteine increase.30,43 However, polymorphisms C677T and A1298C in the MTHFR (5,10-methylenetetrahydrofolate reductase) gene were not associated with homocysteine levels in Brazilian patients with TS, and these were not higher in patients with the risk haplotype.30 Also in relation to the risk of congenital heart defects, disruption of the folate pathway contributed to the incidence of atrioventricular septal defect in individuals with Down syndrome.44 It would be interesting to carry out a study focusing on TS, due of the high incidence of cardiac abnormalities in this group of patients.
Chromosomal nondisjunctionTS is an aneuploidy caused by chromosomal nondisjunction, which occurs when homologous chromosomes or sister chromatids fail to separate, with advanced maternal age being a risk factor. Genetic polymorphisms that alter enzymes involved in folate metabolism, such as the enzyme methylenetetrahydrofolate reductase (MTHFR) can lead to DNA hypomethylation and increase the risk of chromosomal nondisjunction and thus, of TS. The MTHFR gene is located on 1p36.3 and includes two polymorphisms: substitution of cytosine by thymine at nucleotide 677 (C677T) and adenine by cytosine at nucleotide 1298 (A1298C), resulting in decreased MTHFR activity, interfering with the folate metabolism.
Three studies analyzed polymorphisms in this gene in Brazilian patients with TS.28–30 The results regarding the contribution of these polymorphisms to the etiology of TS are conflicting, as the C677T SNP was associated with TS in one study,28 whereas in the others it was A1298C.29,30 Three independent meta-analyses45–47 positively associated MTHFR C677T and Down's Syndrome: one of them included 28 case-control studies,45 and the other, 22 studies for MTHFR C677T and 15 for MTHFR A1298C.47
The genetic polymorphisms analyzed here seem to influence the severity of the clinical manifestations of TS, as well as response to treatment. In this sense, future studies could indicate their use in clinical practice as molecular markers for diagnosis and prognosis. Another interesting finding is that a detailed analysis of Table 1 shows that 41% (7/17) of the studies on genetic polymorphisms in TS were performed by Brazilian researchers. Brazil has a high degree of miscegenation and genetic polymorphisms are related to ethnic origin. However, only one of the studies briefly mentioned that the country is a heterogeneous nation consisting of populations descended from different ethnicities.30 There is a consensus in the literature on the need to analyze the same polymorphism in different populations, as a genetic association, although valid for a specific population, may not be relevant to individuals from a different ethnic group. Stratification of the groups regarding ethnicity is not so simple, as neither skin color nor region of origin can adequately differentiate a mixed population.48
Another important issue addressed in only a few studies18,25 concerns study limitations in relation to sample size. All of the articles included in this review reported the need for further studies in “Discussion” section, but did not provide a description of possible sources of error and their effect on data. Regarding the study sample, research on genetic polymorphisms in human diseases must have the statistical power defined as the probability of the study to detect an effect when it exists. A critique of the 17 scientific articles analyzed here is that only one of them established the statistical power.25
ConclusionsGenetic polymorphisms seem to be associated with TS, as most studies showed a positive association (11/17). However, considering the few published studies and contradictory findings, probably due to differences in ethnicity of the analyzed samples, studies carried out in different populations are desirable in order to clarify the role of these SNPs for the clinical signs and etiology of this chromosomal disorder. Moreover, multicenter studies with a large number of patients with TS are necessary for the results to have statistical power, which is important in studies with genetic polymorphisms in human diseases.
FundingThis study did not receive funding.
Conflicts of interestThe author declares no conflicts of interest.