To evaluate the frequency of infectious complications in children with sickle cell disease (SCD) after surgical splenectomy for acute splenic sequestration crisis.
MethodsRetrospective cohort of children with SCD who were born after 2002 and were regularly monitored until July 2013. Patients were divided into two groups: cases (children with SCD who underwent surgical splenectomy after an episode of splenic sequestration) and controls (children with SCD who did not have splenic sequestration and surgical procedures), in order to compare the frequency of invasive infections (sepsis, meningitis, bacteremia with positive blood cultures, acute chest syndrome and/or pneumonia) by data collected from medical records. Data were analyzed by descriptive statistical analysis.
Results44 patients were included in the case group. The mean age at the time of splenectomy was 2.6 years (1-6.9 years) and the mean postoperative length of follow-up was 6.1 years (3.8-9.9 years). The control group consisted of 69 patients with a mean age at the initial follow-up visit of 5.6 months (1-49 months) and a mean length of follow-up of 7.2 years (4-10.3 years). All children received pneumococcal conjugate vaccine. No significant difference was observed between groups in relation to infections during the follow-up.
ConclusionsSurgical splenectomy in children with sickle cell disease that had splenic sequestration did not affect the frequency of infectious complications during 6 years of clinical follow-up.
Avaliar a frequência de complicações infecciosas em pacientes portadores de doença falciforme (DF) submetidos à esplenectomia cirúrgica, após episódio de sequestro esplênico (SE).
MétodosCoorte retrospectiva de crianças com DF que nasceram após 2002 e que estavam em acompanhamento regular até julho de 2013. Os pacientes foram divididos em dois grupos, casos (constituído pelas crianças com DF que fizeram esplenectomia cirúrgica após sequestro esplênico) e controles (crianças com DF que não tiveram SE e não foram submetidas ao procedimento), a fim de comparar a frequência de infecções invasivas (sepse, meningite, bacteremia com hemocultura positiva, síndrome torácica aguda e/ou pneumonia) por meio de informações obtidas do prontuário. A análise estatística foi descritiva.
ResultadosForam avaliados 44 pacientes com idade média no momento da esplenectomia de 2,6 anos (1-6,9 anos) e com tempo médio de seguimento após esplenectomia de 6,1 anos (3,8-9,9 anos). O grupo controle foi formado por 69 pacientes com idade média do início do seguimento de 5,6 meses (1-49 meses) e tempo de acompanhamento médio de 7,2 anos (4-10,3 anos). Todos receberam a vacina pneumocócica conjugada. Não foi observada diferença significativa entre os grupos em relação aos processos infecciosos durante o período de seguimento.
ConclusõesA esplenectomia cirúrgica nas crianças com doença falciforme e que sofreram sequestro esplênico não se associou ao aumento na frequência de complicações infecciosas após seis anos de acompanhamento clínico.
Sickle-cell disease (SCD) is a type of hemolytic anemia caused by hemoglobinopathy that, due to the substitution of valine for glutamic acid at the sixth position of the beta globin chain, leads to the formation of an abnormal hemoglobin, called “S” hemoglobin (HbS).1 SCD comprises sickle-cell anemia (SCA) and the associations that occur when the hemoglobin S gene is associated with the gene of other hemoglobinopathies, such as SC hemoglobinopathy (HbSC) and HbS-beta thalassemia (HbSβ).1
Among the complications of SCD, splenic sequestration (SS) affects 7.5% to 30% of patients, usually between 3 months and 5 years of age, being the second leading cause of death in the first decade of life.1,2 The mortality rate for SS crisis can reach 12% and may occur in more than 50% of patients.1,3,4 Treatment should be immediate, aiming to restore blood volume through transfusion of red blood cells.1 The prevention of SS recurrence can be performed through periodic transfusions, chronically, or via splenectomy.1,5–8
The effectiveness of chronic transfusion in preventing recurrence is not well established. A study showed that the SS crisis occurs in spite of the reduction of hemoglobin S (Hb) to less than 30%, with the risk of recurrence being similar in patients receiving chronic transfusions compared to the ones who remained under clinical observation.6 However, randomized studies are needed to confirm these data.2 Brousse et al. found that the risk of recurrence was higher when the first SS episode occurred before two years of age and concluded that a more aggressive preventive treatment should be carried out at this age range.9
The performance of a splenectomy early in life is always debatable due to the increased risk of infection by encapsulated bacteria.10 However, it should be considered that children with sickle-cell disease show splenic hypofunction since the first months of life,11 and that advances in the prevention of these infections through prophylaxis with penicillin and conjugate vaccines have decreased the risk.12,13
Therefore, the aim of this study was to evaluate the frequency of infectious complications after surgical splenectomy in children with SCD.
MethodThis is a retrospective cohort study carried out with data obtained from medical records of patients followed in a Pediatric Hematology service. The study population (PG) consisted of children with SCD born from 2002 to 2007, submitted to surgical splenectomy after the first episode of splenic sequestration between January 2003 and February 2009 and who had regular follow-up until July 2013. After the first episode of splenic sequestration, these patients were submitted to a regimen of chronic red blood cell transfusion every four weeks, until the immunization schedule was complete, with the 7-valent pneumococcal vaccine given between 12 and 15 months of age and elective splenectomy being indicated after this prerequisite. The control group (CG) consisted of children with SCD born between 2002 and 2009 who did not have splenic sequestration, were not submitted to surgical splenectomy, and were undergoing regular follow-up at our service until July 2013.
Both groups received penicillin prophylactically, from diagnosis to at least 5 years of age, until they received the booster of the 23-valent pneumococcal polysaccharide vaccine. All patients had adequate and complete immunization schedules for the age regarding the vaccines: 7-valent pneumococcal conjugate, meningococcal, anti-Hemophilus, and 23-valent pneumococcal polysaccharide vaccine. All patients received the conjugate vaccine during the first year of life.
Clinical and laboratory data were obtained from medical records that included patients' routine consultations at the clinic, taking into account the information given by parents/tutors, hospital discharge summary, and test results. The data obtained were: genotype, age at onset of the first splenic sequestration episode, infectious complications (bacterial meningitis, sepsis, positive blood culture [BC], acute chest syndrome [ACS]/pneumonia). For The PG, in addition to the previous data, the age at the time of splenectomy and time of follow-up after splenectomy were collected.
The analysis of the results took variable type into consideration, in case of unrelated samples. Descriptive analysis of the profile of the studied patients was performed. Fisher's exact test was used for the analysis of categorical variables to demonstrate the significance of differences between the PG and the CG in relation to infectious complications. The descriptive level of p<0.05 was considered statistically significant by rejecting the hypothesis of equality.
The study was approved by the Institutional Review Board of Universidade Federal de São Paulo.
ResultsOf the 53 patients submitted to surgical splenectomy during the period of study enrollment, 44 (83%) SCD patients who underwent surgical splenectomy and were submitted to regular follow-up throughout the study period were included. The children's age at the splenic sequestration episode ranged from 2 to 79 months (mean 21.2±16.5 months), and 21 (70.5%) patients were younger than 2 years when the first episode of splenic sequestration occurred, and the age when they underwent splenectomy was 1 to 6.9 years (mean 2.6±1.3 years). As for the genotype, 39 (88.6%) patients were Hb SS carriers, 4 (9.1%) were Hb Sβ0 thalassemia carriers, and 1 (2.3%) was a Hb SC carrier. The time of follow-up after splenectomy was 3.8 to 9.9 years (mean 6.1±1.6 years).
As for the CG, data from 69 patients undergoing regular follow-up were assessed; the age at the start of follow-up was 1 to 49 months (mean 5.6±9.2 months), and the duration of follow-up was 4-10.3 years (mean 7.2±1.9 years). In relation to genotype, 57 (82.6%) patients were Hb SS carriers, 1 (1.4%) was a Hb Sβ0 thalassemia carrier, and 11 (16%) were Hb SC carriers.
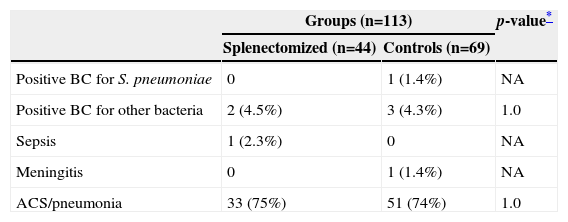
Table 1 shows the infectious complications in children with sickle-cell disease after surgical splenectomy and in children from the control group. No difference was observed between the PG and CG in relation to the infectious processes during follow-up of these patients.
Infectious complications in children with sickle-cell disease after surgical splenectomy and children from the control group.
| Groups (n=113) | p-value* | ||
|---|---|---|---|
| Splenectomized (n=44) | Controls (n=69) | ||
| Positive BC for S. pneumoniae | 0 | 1 (1.4%) | NA |
| Positive BC for other bacteria | 2 (4.5%) | 3 (4.3%) | 1.0 |
| Sepsis | 1 (2.3%) | 0 | NA |
| Meningitis | 0 | 1 (1.4%) | NA |
| ACS/pneumonia | 33 (75%) | 51 (74%) | 1.0 |
BC, blood culture; ACS, acute chest syndrome; NA, data not analyzed.
The infectious agents isolated in blood cultures in the PG were Salmonella spp (1 patient) and Klebsiella spp (1 patient), and in the CG were Escherichia coli (1 patient), S. pneumoniae (1 patient), and Klebsiella spp (1 patient).
DiscussionSeveral studies, as well as the present one, verified that splenic sequestration is more common in children younger than 2 years of age,3–5 with this age range being the one during which infections by encapsulated bacteria, especially Streptococcus pneumoniae, are more prevalent.14 The spleen plays an important role in the defense against these microorganisms, and the removal of this organ is associated with increased risk of infection11; thus, the indication for splenectomy in this age group should always be judicious.
Patients with sickle-cell anemia have splenic hypofunction since the first months of life and undergo self-splenectomy at around 5 years of age,1,11 therefore the risk of infection is increased. Thus, these patients receive prophylaxis with penicillin since the age of 2 months15 and vaccines against encapsulated organisms, such as pneumococcus and Haemophilus.12 The introduction of prophylaxis and vaccines, especially of conjugate vaccines, led to a significant reduction in mortality due to infectious processes in these patients, and in spite of continued need for surveillance and concern about the emergence of pneumococcal strains resistant to penicillin or invasive strains not covered by the vaccine, these preventive measures have been effective so far.12,13 Thus, it can be considered that patients with SCD, surgically splenectomized or not, are at increased risk for infection, but it is of concern whether the complete loss of splenic function early in life after surgical splenectomy could increase the number of infectious complications.
A case series study of sickle-cell patients splenectomized from 1988 to 1992 showed no statistical difference in the incidence of sepsis before and after splenectomy,16 a result similar to that observed by Kalpatthi et al., who performed a retrospective cohort study of 58 patients and also found no difference in the risk of invasive infections before and after surgery.17 The risk of invasive infection, especially pneumococcal, in children with sickle-cell disease is the highest in the first 3 years of life and significantly decreases after the age of 5.18,19 Therefore, following the same patient before and after surgical splenectomy leads to a bias, considering that invasive infections will decrease over the years, even in splenectomized patients, as observed by the abovementioned studies.16,17
Our study compared the frequency of invasive infection among sickle-cell patients at the same age range, with the same time of follow-up, with and without surgical splenectomy, similarly to the study by Wright et al., who also used a control group in their retrospective cohort.20 There was no difference regarding infection rates between the groups in both studies.
The incidence of ACS in patients with sickle-cell disease is approximately 30%.2,21 In the present study, approximately 75% of patients had annotations in their medical files about the previous occurrence of ACS/pneumonia. This is probably due to the different criteria used in emergency departments to characterize such complication. This bias occurred homogeneously in both groups.
The main study limitation was obtaining retrospective data from medical records, which are subject to recall bias and thus prevent the establishment of uniform criteria to define the diagnosis of infectious complications. Prospective studies are necessary to better assess this issue and confirm the results observed to date.
It can be concluded that the indication for surgical splenectomy in children with sickle-cell disease that had splenic sequestration was not associated with an increased frequency of invasive infectious processes during the follow-up period.
FundingThis study did not receive funding.
Conflicts of interestThe authors declare no conflicts of interest.