Roundworm has a worldwide incidence of 25% of the population and biliary ascariasis accounts for 10–17% of all cases of roundworm infestation, predominantly in developing countries in tropical and subtropical climates. In the bile duct, it causes pyogenic cholangitis (stenosis and pigmented stones), cholecystitis, pancreatitis and liver abscesses.
Clinical casesWe present the cases of three patients who showed symptoms of biliary disease. Only one of the three cases had imaging data (ultrasound) compatible with biliary ascariasis. However the other two cases had biliary disease mimicking cholecystitis and cholangitis and diagnosis was confirmed at the time of surgical exploration.
ConclusionsThe management of biliary ascariasis rests on three main pillars according to severity. It starts with medical management, followed by endoscopic if the conservative option fails, and finally surgical management in selected cases, with a favourable prognosis in the majority of patients.
Áscaris tiene incidencia mundial del 25% de la población y la ascaridiasis biliar representa el 10 a 17% de todos los casos de infestación por áscaris, predomina en países en vías de desarrollo, climas tropicales y subtropicales. En vía biliar produce colangitis piógena (estenosis y cálculos pigmentados), colecistitis, pancreatitis y absceso hepático.
Casos clínicosse muestran casos de tres pacientes por presentar síntomas de patología biliar, solo uno de los tres casos presento datos imagenologicos (ultrasonido) compatibles con ascaridiasis biliar sin embargo los dos casos restantes presentaron patología biliar simulando colecistitis y colangitis, llegando al diagnóstico de certeza en el momento de la exploración quirúrgica.
Conclusionesel manejo de la ascaridiasis biliar reside en tres pilares escalados, comenzando con el manejo médico, seguido del endoscópico en caso de fracaso con el anterior y por último el manejo quirúrgico en casos seleccionados, con un pronóstico favourable en la mayoría de los casos.
Ascaris lumbricoides (roundworm) is a common parasite and a billion people (25% of the world's population) are infected worldwide.1 The infestation is more common in women, with a ratio of 3:1, and mean age at diagnosis is 35. Risk factors are the poor hygiene/dietary habits, living in specific regions like the Kashmir Valley in India,2,3 subtropical and tropical climates such as Latin America (Brazil4 and Ecuador5), poor nutritional status and low educational level. In Mexico, the highest incidence is in the southern states (Chiapas, Tabasco, Oaxaca, Guerrero), where there is greater deprivation in socio-economic terms and from the point of view of and basic urban services.6
Roundworm is the largest intestinal parasite that infects humans. It belongs to the phylum Nematodes, has a cylindrical shape and a diameter of about 5mm; males and females differ in size (males are 15–20cm and females 20–30cm).7
Their life cycle is direct, with humans their sole host. The cycle begins when embryonated eggs are ingested (they contain the infecting larva or second-stage [L2] larva). Once in the intestine, the larvae are released from the egg and reach other organs like the lungs and heart through the bloodstream. From the lungs, after passing through several phases, they migrate from the trachea to the mouth, where they are swallowed. In the small intestine, they become adults (approximately 2 months) and mate; the females lay about 200,000 eggs a day, eliminated with the faeces of the host. Outside, the eggs continue to develop and after a few weeks, the L2 larva or embryonated egg develops.8,9
Usually the infection is asymptomatic, but it can cause a number of clinical conditions. If illness develops, the symptoms depend on the parasite load. It may start with hives, fever, hypersensitivity and pulmonary eosinophilia or Löffler syndrome (acute respiratory illness with cough, expectoration and bronchial rales).10 This may be followed by gastrointestinal symptoms such as vomiting, nausea, abdominal pain, loss of appetite and diarrhoea (intestinal obstruction can sometimes occur due to accumulation of parasites), and liver (biliary roundworm) and/or pancreatic complications.11
Biliary roundworm infection represents an estimated 10–17% of all cases of ascaridiasis.12 This triggers a number of different clinical conditions, including biliary colic (56%), acute cholangitis (24%), acute cholecystitis (13%), acute pancreatitis (6%) and liver abscesses (<1%).12
The usual habitat of Ascaris is the jejunum. However, increasing pressure and concomitant intestinal infection by viruses or bacteria (Escherichia coli, Klebsiella sp. and Pseudomonas aeruginosa) stimulate parasite motility and they then tend to go on the move, having a preference for small openings. There are a number of factors involved in their migration to the biliary tract. One is a history of cholecystectomy, as this condition increases the secretion of cholecystokinin which, along with secretin, lowers the tone of the sphincter of Oddi, facilitating the migration of the parasite into the bile duct; a study involving 9250 cholecystectomies over 11 years showed that 104 (6.2%) had Ascaris invasion of the bile duct.13 A history of manipulation, such as dilation of the sphincter of Oddi in order to examine the bile duct, can lead to later dysfunction with diminished pressure, easing the passage of the parasite. Lastly, pregnancy is a condition that increases the concentration of progestogens, whose physiological action is to relax smooth muscle, including the sphincter of Oddi.14
Roundworms also secret polypeptides that cause allergic reactions that can make the sphincter of Oddi spasm. This allows them to migrate into the bile duct, dragging intestinal bacteria with them, and this triggers biliary stasis and subsequent pyogenic cholangitis, cholecystitis, in some cases pancreatitis and, if they manage to make it as far as the intraparenchymal hepatic ducts, causes local inflammation with necrosis and subsequent liver abscess.15
Another complication of the migration of these parasites is the formation of calculi and stenosis caused by the parasitic secretion of β-glucuronidases, which have the ability to precipitate bilirubin, forming crystals of calcium bilirubinate. If the parasite dies in the bile duct, this causes inflammation of the biliary mucosa, abundant exudation and eosinophilic infiltration, which produces an intense fibrotic reaction with stenosis.16
With the migration of adult worms to the bile duct, the irritation caused by helminth or their excreta results in biliary colic, spasm of the sphincter of Oddi and partial or total biliary obstruction.17,18
The diagnosis is made by identifying eggs in the patient's stools, but it is also necessary to identify the worm by some imaging technique or endoscopy. The imaging technique of choice is ultrasound and it will show long, parallel, linear structures with no posterior echogenic shadowing of 3–6mm in diameter parallel to the axis of the duct,19,20 hyperechoic pseudotumour, movement of tubular structures or spaghetti sign.21 The use of computed tomography in this condition is limited so it is not recommended. Endoscopic retrograde cholangiopancreatography (ERCP) is the best method for demonstrating and resolving biliary roundworm infestation, as it diagnoses by direct visualisation and can be therapeutic at the same time.22 Magnetic resonance imaging with contrast, especially enhanced by a 3T system, provides an excellent option for the diagnosis of this parasitosis in the bile duct23 (Table 1).
Diagnostic aids.
| Diagnostic method | Sensitivity | Specificity | Benefits | Disadvantages |
|---|---|---|---|---|
| Ultrasound | 40–70% | 90% | Non-invasive. Fast. Economical. | Operator dependent, intestinal gas, limitations in the intrahepatic bile duct. |
| Endoscopic retrograde cholangiopancreatography (ERCP) | 100% | 94–95% | Diagnostic and therapeutic. | Invasive. Has to be done before the parasite migrates. |
| Magnetic resonance cholangiography (3T MRI) | 100% | 100% | Non-invasive. | Expensive. Use of advanced technology. |
| Contrasted computed tomography | 54–70% | 80–90% | Non-invasive. |
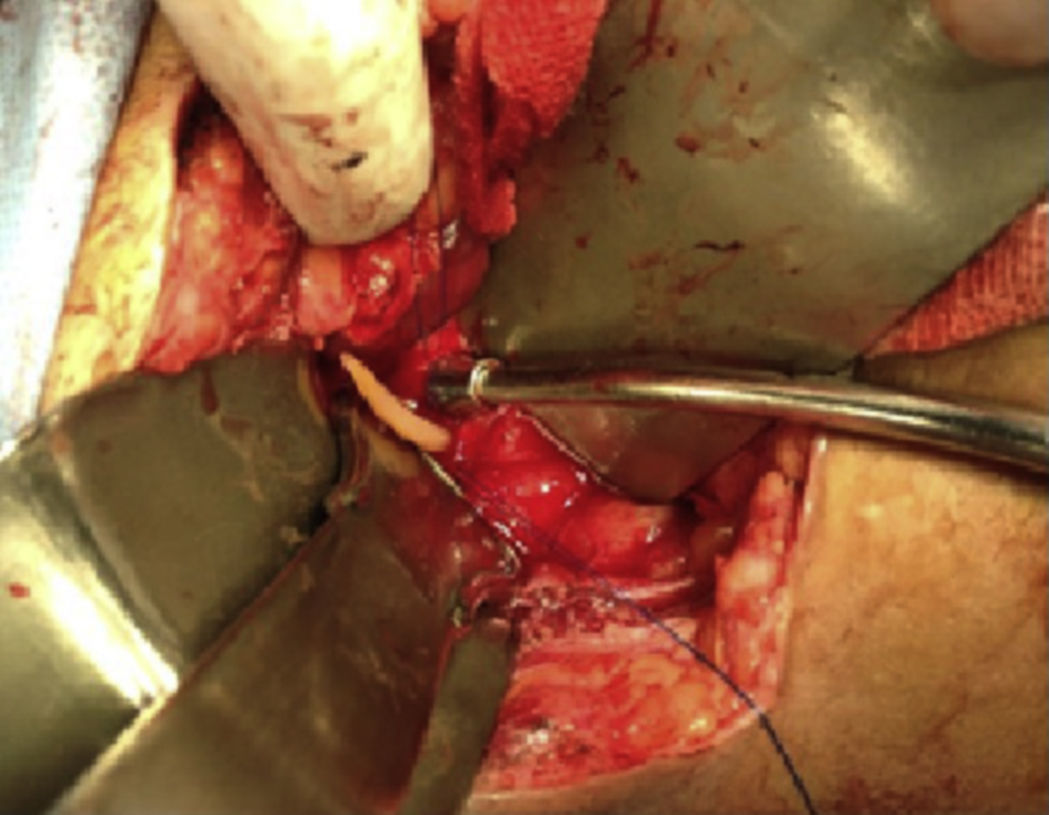
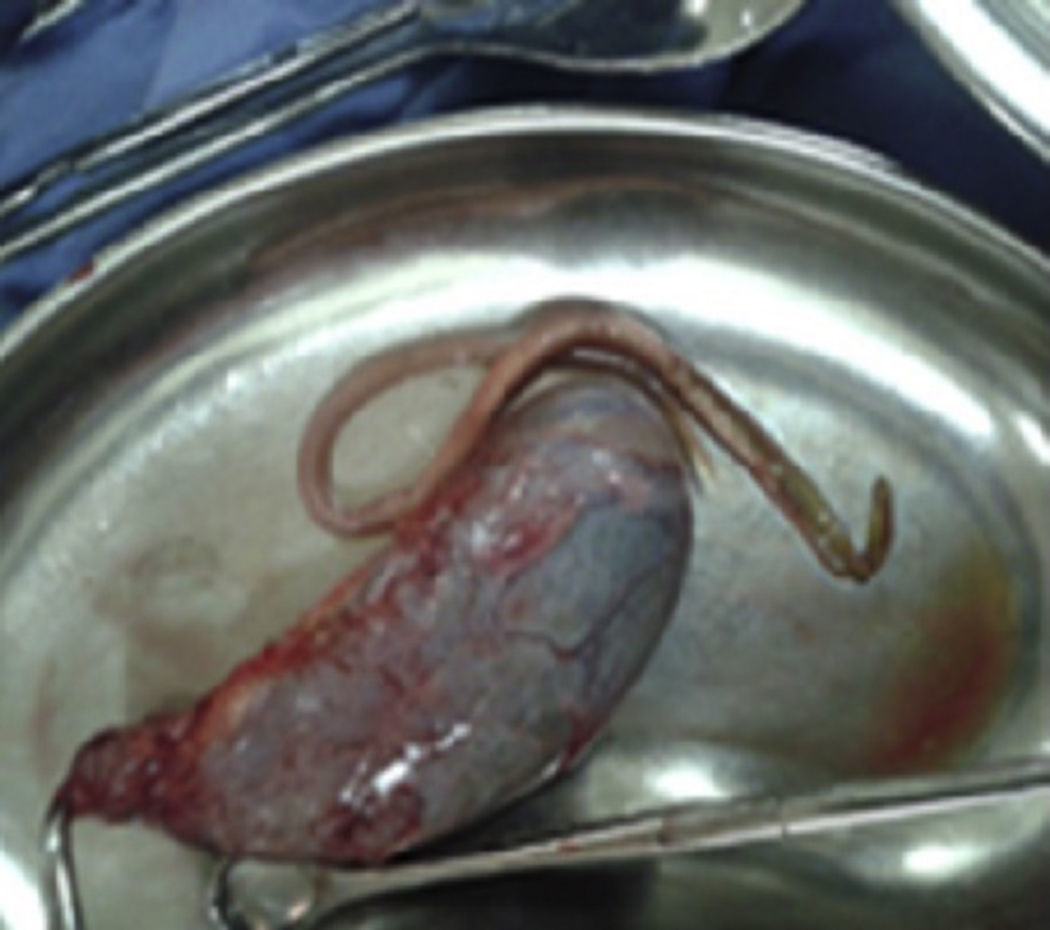
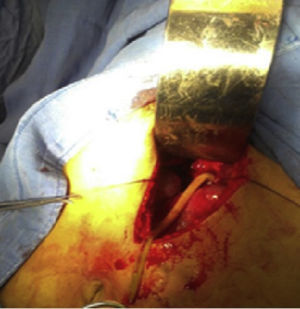
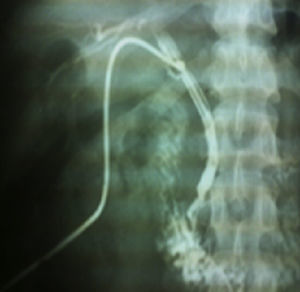
This was a 25-year-old rural woman with low socioeconomic status from the Mixteca Region in Oaxaca. Three months prior to admission, she had suffered from acute cholecystitis and had open cholecystectomy with findings of gallstones. Her symptoms started six days before admission, with colicky epigastric abdominal pain, without radiation, accompanied by nausea, vomiting of gastric contents and fever (not quantified). On physical examination, abdomen distended, peristalsis present, with epigastric and right upper quadrant tenderness, with no signs of peritoneal irritation. On admission, blood count showed leukocytes 9180 per microlitre, liver function tests were within normal parameters, ultrasound of the upper abdomen showed 2 tubular images in the intrahepatic bile duct in the right lobe, extending to the common bile duct. It was decided to do surgical exploration over the previous Kocher-type incision; bile duct dilated, choledochotomy for biliary exploration performed with Randall forceps, finding Ascaris lumbricoides of about 25cm (Figs. 1 and 2). Cholangiography performed with appropriate passing of contrast medium to intrahepatic bile duct and duodenum. The patient made good progress, afebrile, no signs of inflammatory response and 3 days later, due to improvement, was discharged with antiparasitic treatment for the other members of her household.
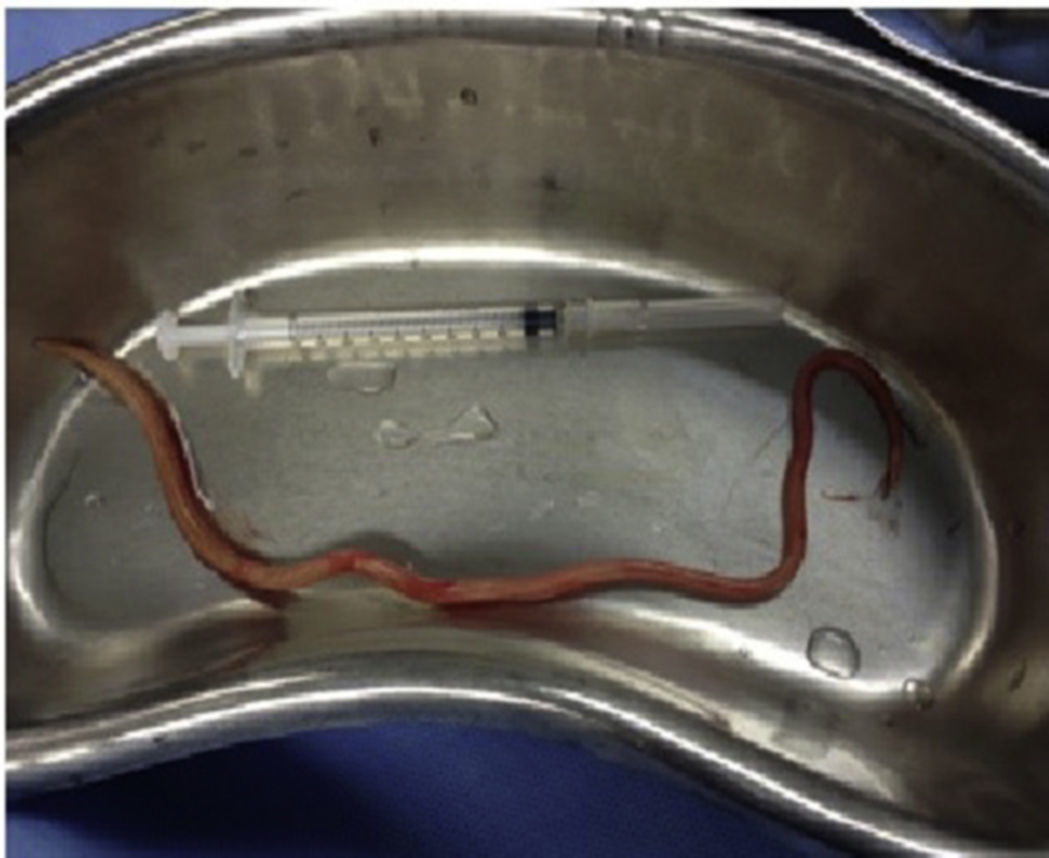
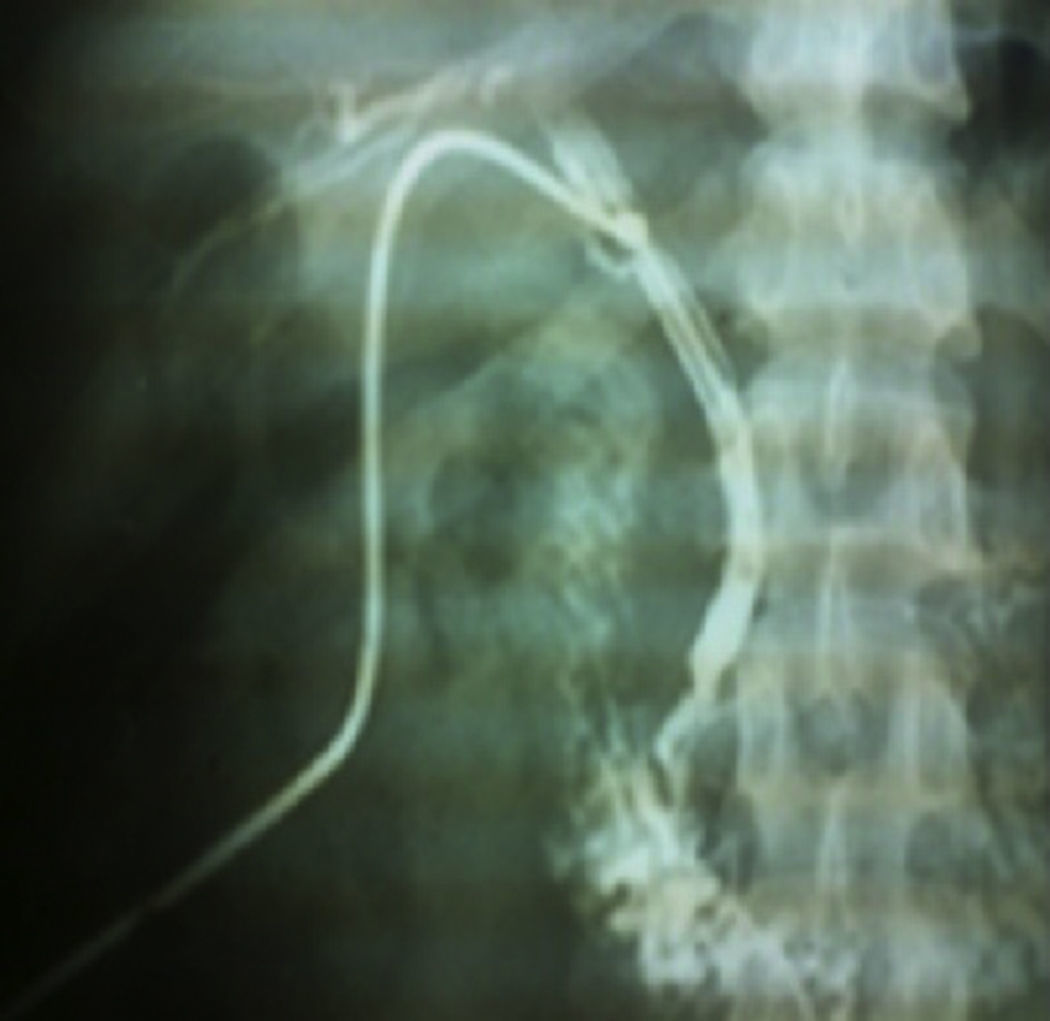
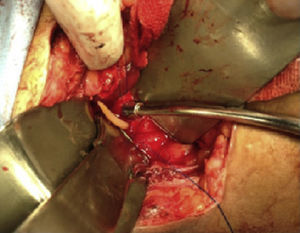
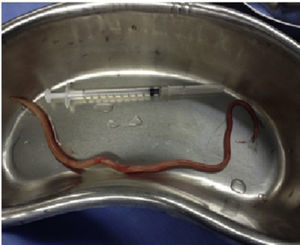
Case report 2This was a 20-year-old woman from San Juan Copala, Juxtlahuaca, Oaxaca, with no relevant previous medical history. Her symptoms started a week prior to admission with colicky, right upper quadrant abdominal pain radiating to the right subscapular region, moderate in intensity, accompanied by nausea and vomiting of gastric contents. On physical examination: positive Murphy's sign. Blood count showed leukocytes at upper limit of normal, with neutrophilia of 87%; elevated liver function tests, with AST 206U/l, ALT 257U/l, alkaline phosphatase 358U/l and bilirubin normal; abdominal ultrasound reported dilation of intra- and extrahepatic bile ducts and biliary sludge and 12mm stone in common bile duct. Cholecystectomy was performed with exploration of bile duct. Gallbladder 13cm×5cm, intestinal parasite 25cm long by 6mm in diameter in the common bile duct (Fig. 3). Common bile duct 2.5cm in diameter, with no evidence of stones in its interior. The parasite was visualised (Fig. 4) in the peri-operative cholangiogram. The patient made satisfactory progress and was discharged due to clinical improvement 24h after surgery, with anthelmintic treatment and follow-up on an outpatient basis.
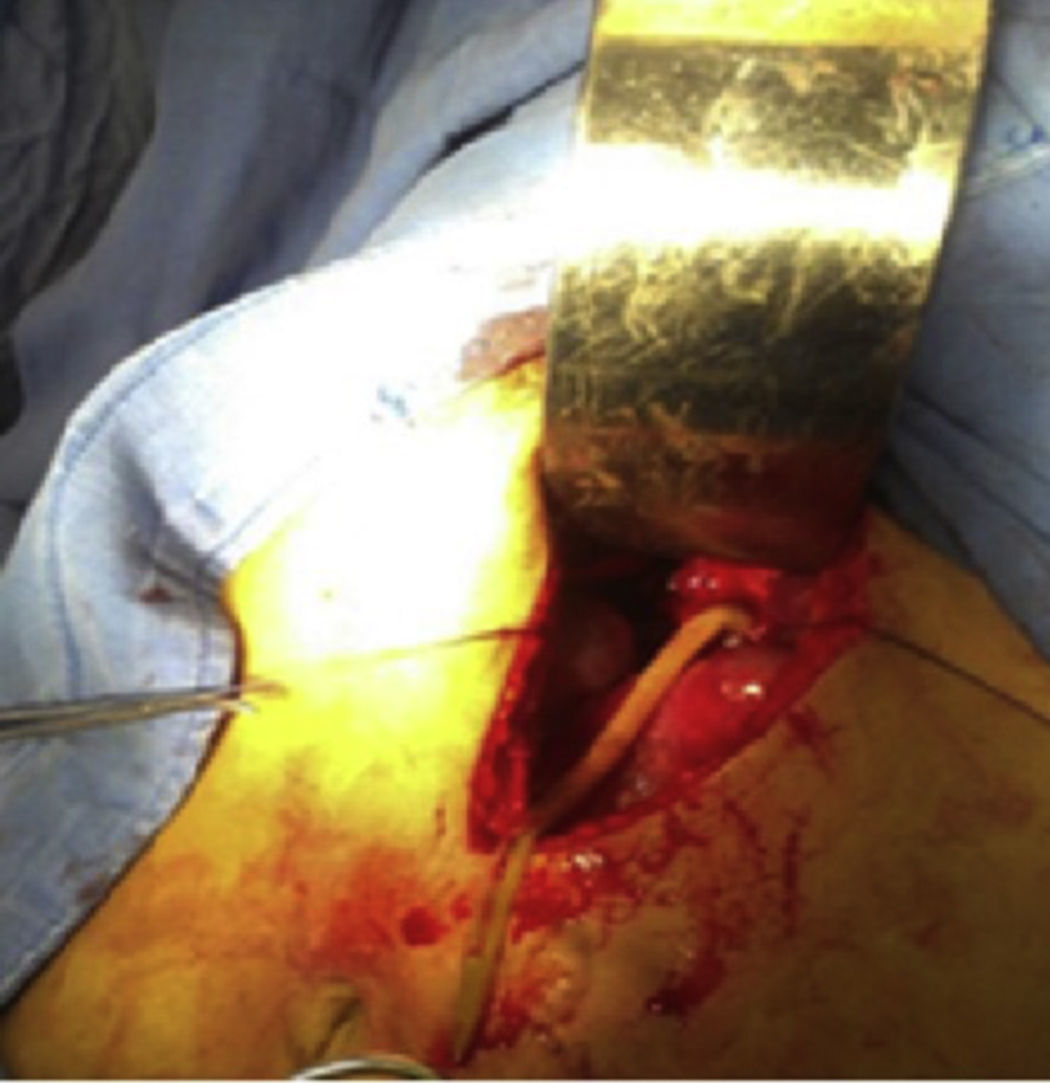
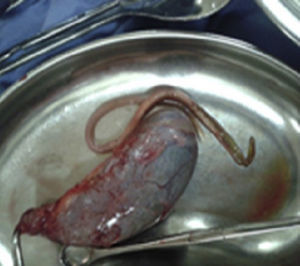
Case report 3This was a 36-year-old illiterate woman who worked in the home, originally from Sierra Sur, Oaxaca, diagnosed with pulmonary tuberculosis aged 34, treated with antituberculosis drugs with favourable outcome. Her symptoms started with multiple, repeated episodes of colicky pain, located in the upper right quadrant, radiating to epigastrium and chest region, associated with nausea and vomiting, managed with analgesics and antispasmodics with partial improvement. Some 48h prior to admission, she developed pain, fever and jaundice, so was admitted to surgery, where laparotomy was performed due to symptoms of cholangitis secondary to probable acute complicated cholecystitis, it being notable that no stones were evident on imaging. The findings were gallbladder (Fig. 5) with no stones inside, common bile duct of 2cm with parasitosis inside; exploration of the bile duct with extraction of parasites was also performed. Her progress was stable with medical management, and at 72h, while still in hospital, she expelled roundworms through her nostrils. The patient is presently under medical follow-up, with no signs of new parasitosis.
DiscussionTreatment of this parasite rests on three main pillars according to severity, starting with medical management based on albendazole (400mg), mebendazole (500mg), piperazine and pyrantel pamoate (preferred in the medical treatment of biliary ascariasis).24 Generally, its mechanism of action is based on selectively damaging the cytoplasmic microtubules of the intestinal cells of the nematodes, but not of the host, causing cell disruption and loss of absorptive and secretory function. Consequently, accumulation of secretory substances occurs in the Golgi apparatus of the parasite, decreasing glucose uptake and depletion of glycogen stores. As many of secretory substances present in the Golgi are proteolytic enzymes that are released intracellularly, the final result is cell autolysis and death of the worm.25 This treatment accompanied by adequate hydration and analgesia resolves the acute condition in 68–80% of patients.26 Preventive drug therapy has a very important role in the management of these helminths, because reinfection seems to be the rule, especially in endemic areas characterised by high marginalisation and lack of health services. The basis of this preventive management is access to potable water, sanitation and hygiene measures in water and food, especially in high-risk groups (school-age children).27
For the complications secondary to migration of the parasite to the bile duct which, according to the Beckingham study28 with 109 patients, included biliary colic in 68%, acute cholangitis in 11%, obstructive jaundice in 5.5%, pancreatitis in 4.5%, choledocholithiasis in 4.5%, acute cholecystitis in 4.5% and liver abscess in 1%, the initial management was always conservative. ERCP can now directly visualise the parasites in the biliary and pancreatic ducts and see linear filling or parallel filiform defects and dilation of the biliary tract (more frequent in the common hepatic).29 This method also allows the resolution of the condition through extraction of the worms with forceps or using a balloon, with rapid improvement in the symptoms; the role of sphincterotomy is still debatable as some authors support its use for the removal of worms, but others attribute the biliary and pancreatic infestation to prior sphincterotomy.30 Endoscopic treatment is used in patients whose symptoms do not respond to medical treatment or in cases of persistence of the parasite for more than three weeks.31
Lastly, surgical treatment is reserved for patients who do not respond to medical or endoscopic treatment, with persistence of symptoms and parasites in the bile duct and patients with roundworm in their gallbladder or acute pancreatitis secondary to roundworm.31
ConclusionsIn general, the prognosis of roundworm infestation is good with medical management and reinfection is treated with prevention measures. For specific complications in the biliary tract, management with a combination of medical and interventional treatment with or without surgery is the best option, with a very low or zero mortality rate and faster reintegration to normal daily life.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflict of interests.