In the reviewed articles, quinpirole is used as a dopamine D2 and D3 receptor agonist to induce persistent behaviour in animal models. Dopamine has been related to perseverative behaviour. The perseverative behaviour was observed in an open field with objects of different shapes and sizes. The main structures studied with this methodology are the orbitofrontal cortex, striatum, thalamus, basolateral amygdala and nucleus accumbens. The animal models studied comply with the face, predictive and construct validity.
El quinpirol en los artículos revisados, tiene como objetivo inducir comportamiento perseverante en el modelo animal, ya que este es un agonista de la dopamina a través de receptores D2 y D3. Se ha relacionado el papel de la dopamina con el comportamiento perseverante. Para registrar el comportamiento perseverante el aparato utilizado es el campo abierto con reforzadores (objetos de diferentes formas y tamaños). Las principales estructuras estudiadas con esta metodología son la corteza orbitofrontal, núcleo estriado, tálamo, núcleo acumbens y la amígdala basolateral. Los modelos animales estudiados, cumplen con la validez de constructo, apariencia y predicción.
Obsessive compulsive disorder (OCD) has been extensively studied using animal models, and has focused on the induction of this behaviour in rats (perseverative behaviour) mainly using dopamine agonists (D2/D3) such as quinpirole (QP) and serotonin agonists (5HT1A) such as 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT).
In this review, we focused on quinpirole because of its role as a dopamine D2 and D3 receptor agonist that induces perseverative behaviour in animal models. Dopamine has been related to perseverative behaviour.
We studied the use of QP as an inducer of perseverative behaviour (PB) in animal models from a methodological standpoint, considering different ways to validate the model, as well as the neurological system studied, the device for evaluating the induced behaviour, the type of animal used and the results obtained.1
Methodologies used in the study of OCD in animal models using quinpiroleThe aim of the reviewed studies is to determine the role of dopamine in the development of perseverative behaviour in animal models designed to show its association with OCD.
In some cases, OCD must be studied using animal models, and for this purpose experimental studies with different strategies are used. de Haas observed the behaviour of rats immediately after administration of QP and recorded their behaviour in the open field for 50min. Other authors administered QP directly into target structures by intracranial injection, for example, orbitofrontal cortex (OFC), nucleus accumbens (NA) and basolateral amygdala (BLA),2 and another authors previously injured the structure and then compared behaviour between groups.
de Haas et al. used two different phases for their design, the first consisting of 2 groups, 1 treated with QP and another with 0.9% physiological saline solution (SS). They recoded the behaviour of both groups for 1h and immediately after treatment. In a second phase, the treatments are stopped, but SS is still administered to both groups, thus resulting in four groups; SS-SS, QP-QP, QP and QP-SS-injection free. All groups underwent stereotactic micro-dialysis of the NA.
Other researchers7 formed 2 groups, 1 treated with QP and 1 with SS, and conducted the experiment in 3 phases, the first being induction of PB; a second phase consisting of training intracranial self-stimulation (ICSS) of the cortico-striatal-thalamocortical circuit (reward pathway), and in the third phase the pattern of behaviour was recorded while carrying out the ICSS. In this study, the frequency and velocity at which the rat returns to its home base (HB), and the frequency with which it visits more places before returning to HB are evaluated.
Ahmad, meanwhile, developed a different approach to the first part of the experiment, which consisted of applying 8 injections to 2 different groups; 1 treated with QP and another with 8-OH-DPAT at a dose equal to QP (chronic). In the second part (injections 9 and 10), they switched groups and applied the treatment following the 8-OH-DPAT scheme (acute challenge). After each treatment, the behaviour of the rats was recorded on videotape for 55min, evaluating locomotive activity by measuring the distance and registering the paths travelled.3
Quinpirole as an inducer of persistent behaviour (OCD)It has been shown that perseverative behaviour in rats is similar to obsessive-compulsive disorder in humans and can take many forms. To induce PB in animals, researchers have used QP, a dopamine receptor agonist (D2/D3) with the following action.
Dopamine acts at different locations of the central nervous system, involving different circuits, such as cortex, striatum, globus-pallidus, substantia-nigra, thalamus, cortex and cortex, striatum, globus-pallidus, subthalamic nucleus substantia-nigra, thalamus, cortex. The stimulating effect of dopamine in the aforementioned circuits facilitates activation of the thalamus-cortex circuit; however, activation of D2 receptors in striatopallidal neurons inhibits projections, causing decreased motor activity.4
QP exerts its effect on the neurotransmission of several brain regions, including the OFC. This effect is produced by reducing basal acetylcysteine concentrations in the NA, thus causing glutamatergic changes that induce changes in acetylcholine (ACh), which modulates postsynaptic receptors and their connections. This may be related in some way to the process of care approach.4
Chronic administration of QP in laboratory animals causes repetitive behaviour, deficiency in performance, decreased precision for tasks, increase omissions and decreased development for completing tasks.4
Repeated administration of D2 dopamine agonists causes receptor supersensitivity in a process known as receptor imprinting, which is the mechanism responsible for some behaviours generated after administration of QP. This receptor supersensitivity is known to occur in some psychiatric disorders such as schizophrenia, OCD and dyskinesias associated with neurological motor disorders. Similar disorders are also known to occur in QP-sensitized animals, in which stereotypes, antinocioception, locomotive activity, impaired memory and learning, and even atypical vertical leaps are observed. Strangely, alterations that reduce locomotive activity for the first 15min following application and then increases it after the second hour post-administration4 have also been observed
In animal models of OCD/dopamine, exposure to QP is generally chronic, at a dose ranging from 0.2 to 6mg/kg, with an average of 2.3mg/kg and mode of 0.5mg/kg. QP is usually administered twice weakly, and the treatment lasts 4, 5 and up to 8 weeks, being 4 weeks the most frequently used scheme. The drug is usually given by subcutaneous injection in the inter-scapular region, but is also used intraperitoneally. It is used as a dilution in SS.1–7
Animal modelsThis can be defined as the experimental preparation that takes place in a species with the purpose of studying a phenomenon that occurs in other species. This concept is also developed in psychiatry, which has widely used the rat as a model of different diseases, particularly, OCD. We found that most authors prefer this species, but the use of mice is also proposed.1,5
Type of animals used as a model for the study of OCD using quinpirole as inductor of persevering behaviourTo study QP-induced OCD, there is no preference for the use of a specific strain of rat; the use of male Long Evans and Wistar strains is more extensive, but it is interesting to note that de Haas used female mice and studied 2 different strains (A/J and C57BL-6). The use of mice is rare; male rats are usually used and females are avoided because of the relationship between hormones and behaviour.6
The weight of rats varies from 160 to 300g. None of the studies reviewed reversed the observation cycle and a 12-h light/dark cycle was used in all.1–7
Validity of animal models for the study of OCD using quinpiroleDeveloping a valid animal model requires the signs in the induced animals to be at least reasonably similar to those observed in the modelled disease, and that the treatment is effective in reversing those signs.1
To achieve such validity, the model must have face, predictive and construct validity.1
Face validity refers to a phenomenological similarity between the model and the simulated disorder. Ideally the model should reproduce the disorder condition from its aetiology, symptoms, treatment and physiological bases. The predictive validity means that the performance of the model tests are able to predict the performance of the modelled conditions, and construct validity establishes a theoretically rational reference and depends on the degree of homology between the modelled behaviour and the behaviour model.1
Animal models using QP for studying OCD meet face, predictive and construct validity as follows.
Face validityThe use of QP to model OCD in animals complies with face validity criteria, since the induction of persistent behaviour is achieved in all cases in rats, which is similar to that observed in OCD, and is different from the groups treated with SS.1–7
Construct validityEvidence has confirmed that QP is a dopamine agonist (D2/D3). This drug induces changes in brain neurotransmission areas such as OFC, NA, thalamus and globus pallidus, structures directly related to anxiety disorders in humans, particularly OCD.
Predictive validityTo achieve predictive validity in QP/OCD models, selective serotonin reuptake inhibitors (SSRIs) and antipsychotics are used. Exposure to these drugs in animals after induction of PB with QP re-establishes their normal behaviour. Control groups were also formed in which only SS is given and compared with the QP group, observing differences in the behaviour of both groups.5,6
Another way to achieve predictive validity is by forming QP-induced PB groups, and SS-treated groups with normal behaviour. Subsequently, a crossover is made, which reverses induced behaviour in QP groups and produces PB in the groups treated with QP.2,6
Neurological systems in OCD targeted in animal models using quinpiroleThe neurological systems studied using QP include the orbitofrontal cortex, basolateral amygdala, the accumbens nucleus and the so-called reward circuitry or security-motivation, composed by cortex, striatum and thalamus, which is a circuit that involves neurotransmitters, dopamine (D2/D3), and serotonergic 5HT1A.1–7
Devices used to record quinpirole-induced persistent behaviour (OCD) in animal modelsThe device used in all the reviewed articles was the open field, but with variations in its design.
The open field can be designed with an area of 80cm×80cm×80cm without walls, elevated 60cm off the ground. The surface is divided in 25 locales and incorporates 4 objects measuring 4cm×4cm×4cm, 2 of them metallic and pyramid-shaped, 1 box-shaped transparent shelter made of PET (polyethylene terephtalate), and 1 made of polycarbonate.5
A variant of the open field without walls and 60cm elevation consists of a 160cm×160cm surface divided into 25 locales. Objects are also used, but unlike the above model, glass boxes measuring 8cm×8cm×7.5cm are placed in the corners and open midfield.2 There is a third variant of the elevated open field with no walls and an area of 160×160×160cm which uses only 2 objects of indeterminate size. These 3 devices are constructed of Plexiglas. Using the same surface dimensions, the design can have blue-surfaced 60cm high walls, divided into 25 locales. This design also has four objects consisting of 8cm×8cm×7.5cm Plexiglas boxes (2 placed at the corners and 2 in the centre of the open field).3
The main differences between open field devices concerns the presence or absence of walls and the number of objects, most of which are made of Plexiglas, although other materials such as glass are also used. Dimensions also vary greatly, ranging from 80cm×80cm to 160cm×160cm, the latter being by far the most common (3 of 5 devices).3
Another open field design uses glass for the base. It has a 140cm×140cm surface, with 20cm high walls and 4 Plexiglas objects of different sizes and colours, placed in different positions.7
To record the behaviour in the open field, video and computer systems such as Ethovision 3.1®, Multi Channel Systems Gmbh Germany® and STG4004® computer interfaces are used.1–7
The quinpirole D2/D3 agonist and its role in the development of perseverative behaviour in animal modelsStudies have shown that QP-induced behaviour in mice is strain-dependent. Therefore, there is a genetic factor that influences the presence of PB.5 Compulsive behaviour has also been shown to be regulated by different brain nuclei, and the basolateral amygdala has no influence on persevering behaviour.2
The NA inhibition is the mechanism by which QP induces PB, so it is probably the negative feedback site. It has also been shown that OFC is related to focusing on achieving a goal in the open field.2
Increasing NA dopamine levels by administration of QP through microdialysis in rat brains suggests that QP mimics only the persevering part of the PB in rats.6
The use of d-amphetamine as a chronic treatment to reverse the behavioural effects of QP suggests that QP induces changes in the reward circuit and also increases the locomotion of treated animals.7
Combined use of QP and 8-OH-DPAT has a synergic effect on PB induction in rats; the 8-OH-DPAT being more the more effective component. Each of these drugs induces the same behaviour by different specialized neural circuits.3
ConclusionsWe conducted an extensive search for studies of quinpirole and PB published over the past 5 years, and obtained only seven references. This shows that very little information has been published on this subject in recent years, although we believe that this information gives important insight into the effect of quinpirole on OCD.
Various experiments have shown by different means that dopamine has a role in PB, and that serotonin has also a role in the ideation that leads to such compulsive behaviour.
Persistent behaviour in mice could be influenced by genetic factors, since PB expression is not only determined by QP. This suggests that bias may exist in animal models of OCD. Given that other studies use different strains of rats (Wistar, Long Evans and Spraw Dale), all treated with QP, it is particularly important to check the internal validity of these studies.5
All experiments using QP record behaviour in the open field with objects and their respective variants. They also use a computerized system for recording this behaviour.1–7
Some studies in animal models (rat) using QP do not specify the sex of the animals, and we believe this may be a factor of bias, since behaviour is probably influenced by oestrogen.
None of the studies analyzed changed sleep-wakefulness cycle in the rats. This raises the possibility of bias because 5HT1A increases during sleep8 and decreases during wakefulness, contrary to dopamine that increases during wakefulness and decreases during sleep.9 Failure to invert the cycle might have caused dopamine9 to be increased and interact in synergy with QP, thus affecting the predictive validity of the model.
Regarding the validation of the model used in the studies, we have found that the rat – QP-Open Field trilogy is a valid model for the study of OCD and the involvement of dopamine in this anxiety disorder.
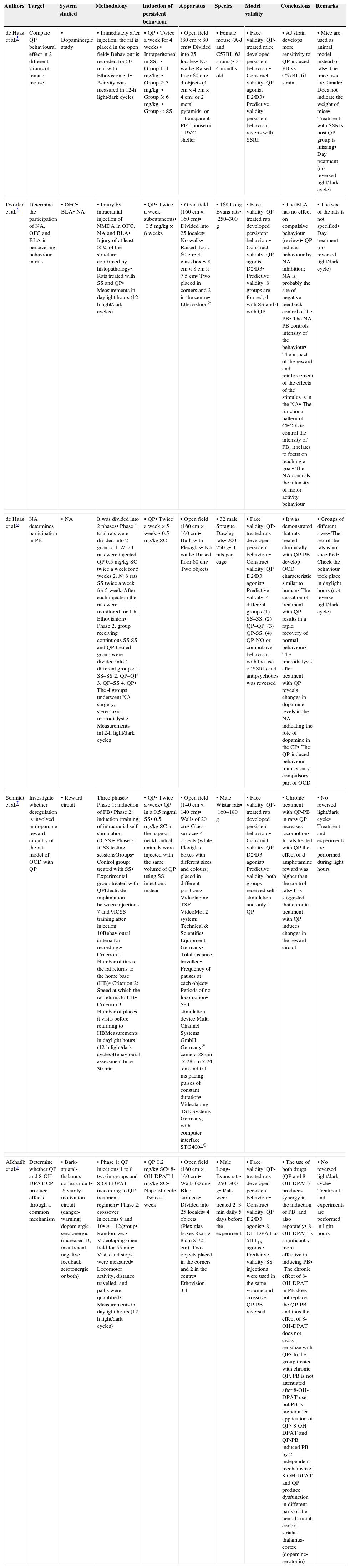
The studies analyzed are compared in Table 1.
Animal models for the study of OCD using dopaminergic and open field.
| Authors | Target | System studied | Methodology | Induction of persistent behaviour | Apparatus | Species | Model validity | Conclusions | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| de Haas et al.5 | Compare QP behavioural effect in 2 different strains of female mouse | •Dopaminergic study | •Immediately after injection, the rat is placed in the open field•Behaviour is recorded for 50min with Ethovision 3.1•Activity was measured in 12-h light/dark cycles | •QP•Twice a week for 4 weeks•Intraperitoneal in SS,•Group 1: 1mg/kg•Group 2: 3mg/kg•Group 3: 6mg/kg•Group 4: SS | •Open field (80cm×80cm)•Divided into 25 locales•No walls•Raised floor 60cm•4 objects (4cm×4cm×4cm) or 2 metal pyramids, or 1 transparent PET house or 1 PVC shelter | •Female mouse (A-J and C57BL-6J strains)•3–4 months old | •Face validity: QP-treated mice developed persistent behaviour•Construct validity: QP agonist D2/D3•Predictive validity: persistent behaviour reverts with SSRI | •AJ strain develops more sensitivity to QP-induced PB vs. C57BL-6J strain. | •Mice are used as animal model instead of rats•The mice used are female•Does not indicate the weight of mice•Treatment with SSRIs post QP group is missing•Day treatment (no reversed light/dark cycle) |
| Dvorkin et al.2 | Determine the participation of NA, OFC and BLA in persevering behaviour in rats | •OFC•BLA•NA | •Injury by intracranial injection of NMDA in OFC, NA and BLA•Injury of at least 55% of the structure confirmed by histopathology•Rats treated with SS and QP•Measurements in daylight hours (12-h light/dark cycles) | •QP•Twice a week, subcutaneous•0.5mg/kg×8 weeks | •Open field (160cm×160 cm)•Divided into 25 locales•No walls•Raised floor, 60cm•4 glass boxes 8cm×8cm×7.5cm•Two placed in corners and 2 in the centre•Ethovishion® | •168 Long Evans rats•250–300g | •Face validity: QP-treated rats developed persistent behaviour•Construct validity: QP agonist D2/D3•Predictive validity: 8 groups are formed, 4 with SS and 4 with QP | •The BLA has no effect on compulsive behaviour (review)•QP induces behaviour by NA inhibition; NA is probably the site of negative feedback control of the PB•The NA PB controls intensity of the behaviour•The impact of the reward and reinforcement of the effects of the stimulus is in the NA•The functional pattern of CFO is to control the intensity of PB, it relates to focus on reaching a goal•The NA controls the intensity of motor activity behaviour | •The sex of the rats is not specified•Day treatment (no reversed light/dark cycle) |
| de Haas et al.6 | NA determines participation in PB | •NA | It was divided into 2 phases•Phase 1, total rats were divided into 2 groups:1. N: 24 rats were injected QP 0.5mg/kg SC twice a week for 5 weeks2. N: 8 rats SS twice a week for 5 weeksAfter each injection the rats were monitored for 1h. Ethovishion•Phase 2, group receiving continuous SS SS and QP-treated group were divided into 4 different groups:1. SS–SS2. QP–QP3. QP–SS4. QP•The 4 groups underwent NA surgery, stereotaxic microdialysis•Measurements in12-h light/dark cycles | •QP•Twice a week×5 weeks•0.5mg/kg SC | •Open field (160cm×160cm)•Built with Plexiglas•No walls•Raised floor 60cm•Two objects | •32 male Sprague Dawley rats•200–250g•4 rats per cage | •Face validity: QP-treated rats developed persistent behaviour•Construct validity: QP D2/D3 agonist•Predictive validity: 4 different groups (1) SS–SS, (2) QP–QP, (3) QP-SS, (4) QP-NO or compulsive behaviour with the use of SSRIs and antipsychotics was reversed | •It was demonstrated that rats treated chronically with QP-PB develop OCD characteristic similar to human•The cessation of treatment with QP results in a rapid recovery of normal behaviour•The microdialysis after treatment with QP reveals changes in dopamine levels in the NA indicating the role of dopamine in the CP•The QP-induced behaviour mimics only compulsory part of OCD | •Groups of different sizes•The sex of the rats is not specified•Check the behaviour took place in daylight hours (not reverse light/dark cycle) |
| Schmidt et al.7 | Investigate whether deregulation is involved in dopamine reward circuitry of the rat model of OCD with QP | •Reward-circuit | Three phases•Phase 1: induction of PB•Phase 2: induction (training) of intracranial self-stimulation (ICSS)•Phase 3: ICSS testing sessionsGroups•Control group: treated with SS•Experimental group treated with QPElectrode implantation between injections 7 and 9ICSS training after injection 10Behavioural criteria for recording:•Criterion 1. Number of times the rat returns to the home base (HB)•Criterion 2: Speed at which the rat returns to HB•Criterion 3: Number of places it visits before returning to HBMeasurements in daylight hours (12-h light/dark cycles)Behavioural assessment time: 30min | •QP•Twice a week•QP in a 0.5mg/ml SS•0.5mg/kg SC in the nape of neckControl animals were injected with the same volume of QP using SS injections instead | •Open field (140cm×140cm)•Walls of 20cm•Glass surface•4 objects (white Plexiglas boxes with different sizes and colours), placed in different positions•Videotaping TSE VideoMot 2 system; Technical & Scientific•Equipment, Germany•Total distance travelled•Frequency of pauses at each object•Periods of no locomotion•Self-stimulation device Multi Channel Systems GmbH, Germany® camera 28cm×28cm×24cm and 0.1ms pacing pulses of constant duration•Videotaping TSE Systems Germany, with computer interface STG4004® | •Male Wistar rats•160–180g | •Face validity: QP-treated rats developed persistent behaviour•Construct validity: QP D2/D3 agonist•Predictive validity: both groups received self-stimulation and only 1 QP | •Chronic treatment with QP-PB in rats•QP increases locomotion•In rats treated with QP the effect of d-amphetamine reward was higher than the control rats•It is suggested that chronic treatment with QP induces changes in the reward circuit | •No reversed light/dark cycle•Treatment and experiments are performed during light hours |
| Alkhatib et al.3 | Determine whether QP and 8-OH-DPAT CP produce effects through a common mechanism | •Bark-striatal-thalamus-cortex circuit•Security-motivation circuit (danger-warning) dopamiergic-serotonergic (increased D, insufficient negative feedback serotonergic or both) | •Phase 1: QP injections 1 to 8 two in groups and 8-OH-DPAT (according to QP treatment regimen)•Phase 2: crossover injections 9 and 10•n=12/group•Randomized•Videotaping open field for 55min•Visits and stops were measured•Locomotor activity, distance travelled, and paths were quantified•Measurements in daylight hours (12-h light/dark cycles) | •QP 0.2mg/kg SC•8-OH-DPAT 1mg/kg SC•Nape of neck•Twice a week | •Open field (160cm×160cm)•Walls 60cm•Blue surfaces•Divided into 25 locales•4 objects (Plexiglas boxes 8cm×8cm×7.5cm). Two objects placed in the corners and 2 in the centre•Ethovision 3.1 | •Male Long-Evans rats•250–300g•Rats were treated 2–3min daily 5 days before the experiment | •Face validity: QP-treated rats developed persistent behaviour•Construct validity: QP D2/D3 agonist•8-OH-DPAT as 5HT1A agonist•Predictive validity: SS injections were used in the same volume and crossover QP-PB reversed | •The use of both drugs (QP and 8-OH-DPAT) produces synergy in the induction of PB, and also separately•8-OH-DPAT is significantly more effective in inducing PB•The chronic effect of 8-OH-DPAT in PB does not replace the QP-PB and thus the effect of 8-OH-DPAT does not cross-sensitize with QP•In the group treated with chronic QP, PB is not attenuated after 8-OH-DPAT use but PB is higher after application of QP•8-OH-DPAT and QP-PB induced PB by 2 independent mechanisms•8-OH-DPAT and QP produce dysfunction in different parts of the neural circuit cortex-striatal-thalamus-cortex (dopamine-serotonin) | •No reversed light/dark cycle•Treatment and experiments are performed in light hours |
Abbreviations: BLA, basolateral amygdala; OFC, orbitofrontal cortex; PB, persevering behaviour; HB, home base; ICSS, intracranial self stimulation; SSRIs, selective serotonin reuptake inhibitor; NA, nucleus accumbens; QP, quinpirole; SC, subcutaneous; SS, physiological saline; OCD, obsessive compulsive disorder; 5HT1A, serotonin; 8-OH-DPAT, 8-hydroxy-2-(di-n-propylamino) tetralin, QP-PB, quinpirole-induced preserving behaviour.
The authors declare that they have no conflict of interests.