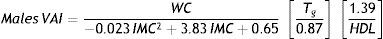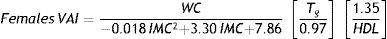Visceral Adiposity Index (VAI) has been strongly associated with adipocytokine synthesis, proinflammatory activity, insulin resistance, dyslipidemia, hypertension and atherosclerosis. VAI is a model that includes and correlates anthropometric and metabolic markers and has been proposed as a risk factor index associated with cardiovascular risk in adults. Some studies conducted in pediatric population have extrapolated VAI calculation in order to predict these abnormalities; nonetheless, no adjustment has been done for pediatric ranges in any of the variables included in the formula. The aim of this study was to design a new sex-specific VAI model adjusted for pediatric population.
Subjects and methodsCross-sectional study, 548 children (290 males and 258 females) aged 3-17 years were included for analysis. Eutrophic, apparently healthy children (n=223) were recruited from 3 public schools located nearby the hospital. Overweight (n=89) and obese subjects (n=236) were patients that regularly attend at the Pediatric Obesity Clinic from “Hospital General de México”. Anthropometric evaluation included weight, height, waist circumference (WC). Body mass index (BMI) was calculated and Tg and HDL measured in a 12hour fasting condition (mMol/L).
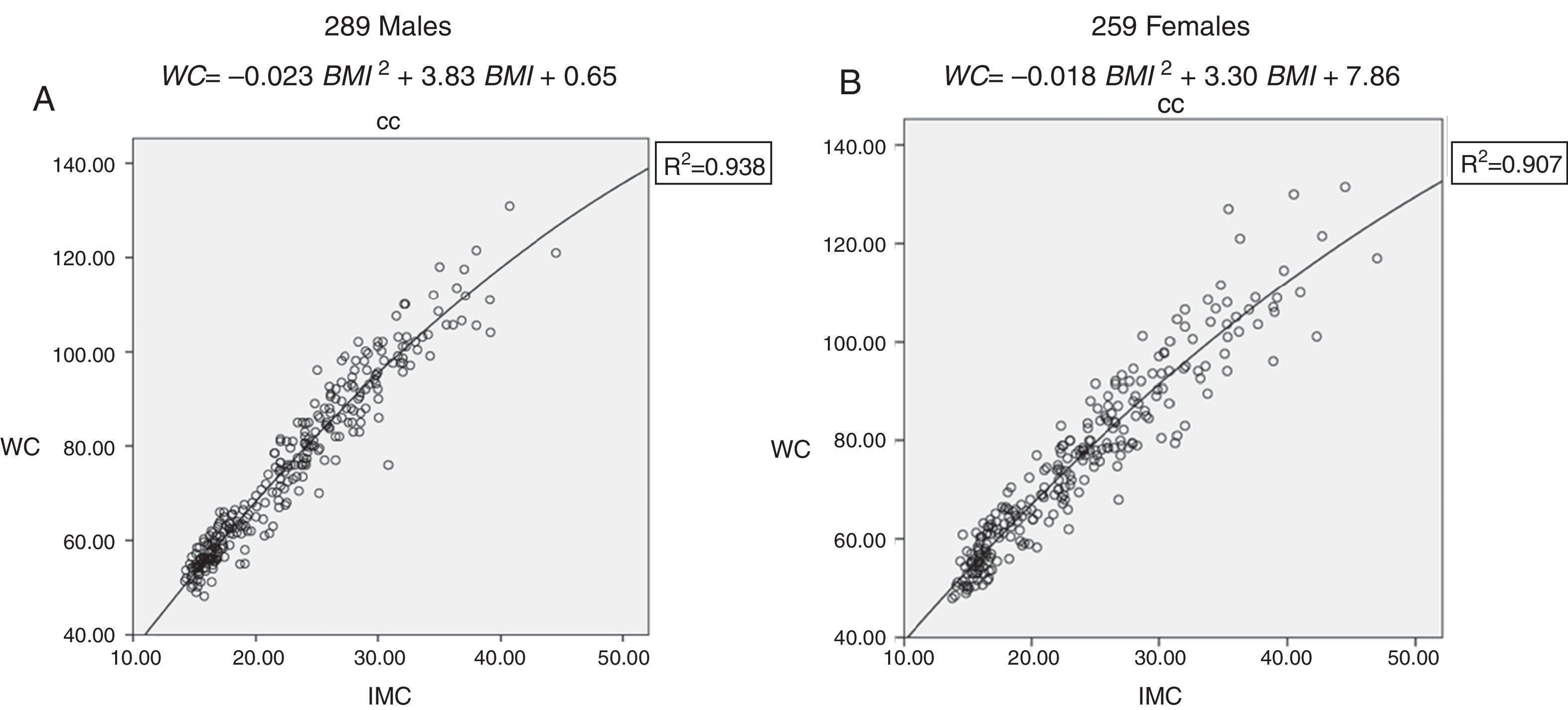
ResultsA non-lineal quadratic regression model accurately explained the relationship between WC and BMI in pediatric population (coefficient of determination R2=0.938; p<0.001 for males and R2=0.907; p<0.001 for females). Medians of triglycerides and HDL-cholesterol values of 223 healthy children were used to calculate the second formula component (Males: median Tg=0.76mmol/l, median HDL-cholesterol=1.37mmol/l; Females: median Tg=0.91mml/l, median HDL-chloesterol=1.42mmol/l).
ConclusionsVAI formula construction seemed to be different in children compared to adults. In the present study we propose a new gender-specific visceral adipose index for pediatric Mexican population living in urban areas that could be further used to predict abnormal cardiometabolic outcomes.
El Indice de Adiposidad Visceral (IAV) ha sido fuertemente asociado con síntesis de adipocitocinas, actividad proinflamatoria, resistencia a la insulina, dislipidemia, hipertensión y aterosclerosis. El IAV es un modelo que incluye y correlaciona marcadores antropométricos y metabólicos y ha sido propuesto como un índice de riesgo cardiovascular en adultos. Algunos estudios realizados en población pediátrica han extrapolado el cálculo del IAV con el fin de predecir estas anormalidades en niños; sin embargo, no se han hecho ajustes para rangos pediátricos en ninguna de las variables incluidas en la fórmula. El objetivo de este estudio fue diseñar un nuevo IAV género-específico ajustado para población pediátrica.
Sujetos y métodosEstudio transversal, se incluyeron un total de 548 pacientes pediátricos (290 varones, 258 mujeres) entre 3-17 años de edad. Niños eutróficos, aparentemente sanos (n=223) fueron reclutados de 3 escuelas públicas localizadas en la periferia del hospital. Sujetos con sobrepeso y obesos (n=89 y n=236, respectivamente) fueron pacientes atendidos regularmente en la Clínica de Obesidad Pediátrica del Hospital General de México. La evaluación antropométrica incluyó peso, talla, circunferencia de cintura (CC). Se calculó el índice de masa corporal (IMC) y se midieron Tg y HDL-colesterol en una condición de 12 horas de ayuno (mMol/L).
ResultadosUn modelo de regresión no-lineal cuadrática explicó con mayor exactitud la relación entre CC e IMC en población pediátrica (coeficiente de determinación R2=0.938; p<0.001 para hombres y R2=0.907; p<0.001 para mujeres). La mediana de triglicéridos y HDL-colesterol de 223 niños sanos se emplearon para calcular el segundo componente de la fórmula (Hombres: mediana Tg=0.76mmol/l, mediana HDL-colesterol=1.37mmol/l; Mujeres: mediana Tg=0.91mml/l, mediana HDL-colesterol=1.42mmol/l).
Conclusionesla construcción de la fórmula del IAV parece ser diferente en niños comparado con adultos. En este estudio, proponemos un nuevo IAV género-específico para población pediátrica mexicana que reside en áreas urbanas, que podría además ser usado para predecir alteraciones cardiometabólicas.
Obesity has emerged as a highly prevalent chronic disease in children, particularly in those living in developing countries. Overweight and obesity prevalence in Mexican pediatric population between 5 and 11 years of age increased up to 37% throughout 2006-2012, according to ENSANUT 20121. Obesity but mainly visceral adiposity increases the risk of having type 2 diabetes (T2D), insulin resistance, dyslipidemia, cardiovascular diseases and non-alcoholic steatohepatitis. Dysfunctional visceral adipose deposition has been described as a morbidity and mortality risk factor even in normal weight subjects. More than two thirds of children 10 years and older who are obese will become obese adults2. Obesity in young adults decreases life expectancy by 5-20 years and confers long-term effects on morbidity3,4. It is imperative to design effective and useful in clinical setting tools to detect early metabolic abnormalities, in order to promote early optimal intervention and prevent cardiovascular and metabolic diseases in children and young adults. Metabolic syndrome (MS) components are a cluster of metabolic and cardiovascular abnormalities that are relatively stable, tend to track from childhood to adulthood and are mainly consequences of insulin resistance. No specific cut-off points of these abnormalities have been standardized for pediatric population5 and few data regarding consequences along life span have been published. Most of the MS definitions in this age group consider abdominal obesity as a the key cornerstone of metabolic derangement since adipose tissue modulates metabolism by releasing NEFAs (Non-esterified fatty acids) and glycerol, hormones (leptin, adiponectin), and proinflamatory cytokines6.
Visceral Adiposity Index (VAI), a gender-specific mathematical index based on simple anthropometric (BMI and WC) and metabolic parameters (TG and HDL), has been proposed by Amato el al7 as a surrogate marker of adipose tissue function and distribution. VAI has shown to be independently associated to cardiovascular and cerebrovascular events, and inversely related to insulin sensitivity. By contrast, these authors found no correlations for these outcomes when WC or BMI were compared independently. Similar data has been reported by other authors regarding several conditions related to insulin resistance and cardiometabolic risk8,9. A protocol conducted in pediatric population has extrapolated VAI calculation to eutrophic and obese children in order to predict insulin resistance and subclinical inflammation10. This study failed to demonstrate VAI superiority over BMI or WC. However, no adjustment was done for pediatric ranges in any of the variables included in the formula. In fact, this correction seems to be suitable since visceral adipose deposition, absolute values of BMI, WC-BMI correlation, as well as triglyceride and HDL-cholesterol levels might be different for pediatric population.
The aim of the present study was to design a new gender-specific VAI model adjusted for pediatric population and based on the original VAI formulation for adults.
Material and methodsSubjectsEutrophic, overweight and obese girls and boys between 3 and 17 years of age were included in the study. Eutrophic and otherwise healthy individuals were recruited from 3 Public Schools nearby the Hospital General de Mexico. An informed consent and assent (Both approved from the IRBs) were obtained from parents and children respectively. Data from overweight and obese subjects were recorded anonymously out of the database of the patients that regularly attend pediatric obesity clinic at Hospital General de México, so no individual informed consent was needed for this group of patients. Obesity was defined when BMI (defined as weight in kilograms divided by the square of height in meters) ≥ 95th Pc, overweight ≥ 85th Pc and <95th Pc and normal weight if BMI ≥25th Pc and < 85thPc according to CDC definitions. Exclusion criteria considered endogenous, endocrine or genetic obesity, T2D, systemic illness, or administration of medications that interfere with glucose or lipid metabolism. Psychiatric conditions and disordered eating behavior were also excluded.
Physical examinationExamination was carried out by trained pediatricians, pediatric endocrinologists and nutritionists previously standardized. Body weight was measured in light clothing and barefoot with a mechanical column scale (to 0.1kg) and standing height with a standard stadiometer board mounted on the wall (to the nearest 0.1cm). WC was measured at the midpoint between the lowest rib and the immediately above point of the iliac crest, using a non-stretchable standardized fiberglass tape and at the end of exhalation. Blood pressure was measured with a sphyngmomanometer. The blood pressure cuff had bladders long enough to encircle at least one-half of the upper arm without overlapping and widths that covered at least two-thirds of the upper arm. Complete physical examination was done in order to detect associated comorbidities.
Biochemical markersBlood samples were obtained after 12 hour fasting and total cholesterol, high-density lipoprotein (HDL-cholesterol), low-density lipoprotein cholesterol (LDL-cholesterol) and Tg determined at the Hospital General de Mexico's Laboratory. The methodology used to analyze blood samples was the following: the reactant HDLD with the SYNCHRON Systems Calibrator was used for lipids determination; SYNCHRON LX system and the UniCel DxC 600/800 system were used for quantitative measurement of the HDL-Cholesterol values on human plasma. This methodology does not require a sample pretreatment.
The GPO (Glycerol Phosphate oxidase) reactant, SYNCHRON System Multi Calibrator, the Triglycerides GPO parameters, the SYNCHRON LX system and the DxC 600/800 Unicel DxC system, were used to determine the quantitative concentration of triglycerides on human plasma. The volume required was 0.3ml of blood sample. The GPO reactant was used to evaluate the triglycerides concentration with an end point method on a determinate time.
Index calculationOriginal VAI index was constructed by two independent components. The first one represents a correlation between WC and BMI. In our study, we decided to include the overall sample of normal weight otherwise healthy, overweight and obese children in order to model the complete equation throughout the BMI spectrum. The second one, was to introduce to the formula the fat function. Tg and and HDL-cholesterol medians of healthy individuals were calculated. Based on the original adult formula, visceral adipose dysfunction was arbitrarily set for Triglycerides values higher than median values of healthy population and HDL values lower than the median values of healthy population.
Statistical analysisUnivariate analysis was conducted and results are presented as frequency, mean and standard deviation of demographic median, anthropometric measures and metabolic syndrome components. Common logarithm base-10 transformation was used for non-normal distributed variables. Lineal correlation between anthropometric variables waist circumference and Body Mass Index was tested with Product-Moment Pearson coefficient, however, the residual analysis suggested a non-linear correlation. A quadratic mathematical model by gender was the best approach using ordinal least squares for data fitness, and was used for regression analysis between WC and BMI to calculate the first VAI formula component. The percentage of covariation (size of effect) was calculated using the coefficient of determination (R2) and a p-value less than 0.05 was considered as statistical significant. The Statistical Package for Social Sciences Software (SPSS 220; Chicago IL) was used for the analysis.
EthicsThe study protocol was approved by the Ethics and Research Institutional Review Boards and Informed consent and assent were obtained from parents and children respectively. The study follow the NOM-012-SSA3-2012 and according to the ICH Harmonized Tripartite Guideline for Good Clinical Practice E6R1.
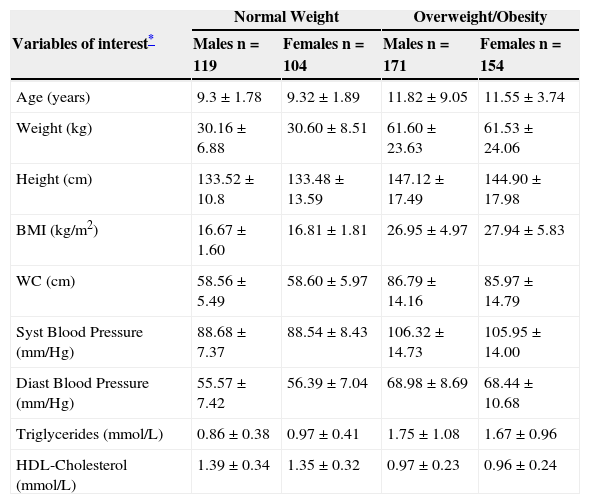
ResultsA total of 548 children (290 males and 258 females) mean age 10.7±5.52: range 3-17 years old were included for the analysis. Variables of interest are shown in Table 1. Eutrophic children (119 males and 104 females) were considered as reference to calculate formula components separately for each gender. Three hundred and twenty five overweight and obese children (171 males and 154 females) and eutrophics were considered to calculate WC-BMI correlation. A wide spectrum of BMI categories (eutrophic, overweight and obese individuals) were considered to design the mathematical regression model that was used to correlate WC and BMI variables (coefficient of determination R2=0.938; p<0.001 for males and R2=0.907; p<0.001 for females fig. 1A and 1B), in order to calculate the first VAI formula component. A non-lineal quadratic regression model instead of a lineal correlation (described for adult population) accurately explained the relationship between waist circumference and BMI in boys (F-ratio=1.05, p<0.05) and in girls (F-ratio=1.04, p<0.05).
Demographic and biochemical variables by weight and sex.
| Normal Weight | Overweight/Obesity | |||
|---|---|---|---|---|
| Variables of interest* | Males n=119 | Females n=104 | Males n=171 | Females n=154 |
| Age (years) | 9.3±1.78 | 9.32±1.89 | 11.82±9.05 | 11.55±3.74 |
| Weight (kg) | 30.16±6.88 | 30.60±8.51 | 61.60±23.63 | 61.53±24.06 |
| Height (cm) | 133.52±10.8 | 133.48±13.59 | 147.12±17.49 | 144.90±17.98 |
| BMI (kg/m2) | 16.67±1.60 | 16.81±1.81 | 26.95±4.97 | 27.94±5.83 |
| WC (cm) | 58.56±5.49 | 58.60±5.97 | 86.79±14.16 | 85.97±14.79 |
| Syst Blood Pressure (mm/Hg) | 88.68±7.37 | 88.54±8.43 | 106.32±14.73 | 105.95±14.00 |
| Diast Blood Pressure (mm/Hg) | 55.57±7.42 | 56.39±7.04 | 68.98±8.69 | 68.44±10.68 |
| Triglycerides (mmol/L) | 0.86±0.38 | 0.97±0.41 | 1.75±1.08 | 1.67±0.96 |
| HDL-Cholesterol (mmol/L) | 1.39±0.34 | 1.35±0.32 | 0.97±0.23 | 0.96±0.24 |
BMI -Body Mass Index, WC - Waist Circumference, Syst Blood Pressure – Systolic Blood Pressure, Diast Blood Pressure – Diastolic Blood Pressure, HDL-Cholesterol – High Density Lipoprotein Cholesterol.
Data show mean±SD.
A quadratic, non-lineal regression model explained the Correlation between WC and BMI.
Correlation between Waist Cirumference (WC) and Body Mass Index (BMI). 1A Correlation for males (coefficient of determination R2=0.938; p<0.001). 1B Correlation for females (coefficient of determination R2=0.907; p<0.001).
Medians of TG and HDL values of the 223 healthy children were used to calculate the second formula component (Males: median TG=0.76 mmol/l, median HDL=1.37 mmol/l; Females: median TG=0.91 mml/l, median HDL=1.42 mmol/l). The obtained formulas for boys and girls were defined as follows:
DiscussionThis study supports a novel index of cardiometabolic risk calculated by each gender. The method includes a sample of eutrophic Mexican population as reference of risk times as an omnibus variable.
Central obesity is mainly related to visceral fat accumulation. Both, subcutaneous and visceral fat had been studied as independent compartments and associated to cardiometabolic comorbidities11. Acquisition of visceral fat during childhood and adolescence has been positively correlated to Total Cholesterol, LDL-Cholesterol, Triglycerides, hyperinsulinemia, and inversely to insulin sensitivity and HDL-Cholesterol levels12,13. Changes in adipose tissue environment and behavior is a result of adipocyte hypertrophy, hypoxia, decreased adiponectin production, increased TNF-¿, IL-6 and several other adipocytokines levels, and is known as “adipose tissue dysfunction”14. VAI has been strongly associated to adipocytokine synthesis, proinflamatory activity, insulin resistance, dyslipidemia, hypertension and atherosclerosis8,9. VAI has been described by Amato as a need to understand and describe adiposity not only as a relationship between weight and height (BMI suggested by Quetelet), but as a matter of fat location and function. As visceral fat drains directly into the portal circulation, it has been linked to many of the comorbidities associated with obesity15. There are only few clinical tools to define this central adiposity. WC has been considered as the most valid measure of regional location of adipose tissue and strongly related to visceral fat and abdominal adiposity16. It has been used as an instrument to detect high risk patients, as it is an independent predictor of insulin resistance, lipid levels, and hypertension17. Nonetheless, a controversial limitation of WC is its inability to make distinction between subcutaneous and visceral fat within the abdominal segment. Although BMI has been routinely used to classify nutritional status13,18 and to evaluate cardiometabolic risk in children and adults from an epidemiology viewpoint, several publications have suggested that abdominal obesity makes a higher contribution than BMI to the probability of morbid events19,20. In fact, BMI did not appeared as a strong predictor for coronary and cardiovascular disease in the Framingham study. Furthermore, waist-to-height ratio (WHtR) has been tested as well as a surrogate index of visceral adiposity and cardiovascular risk in adults and children21. Some meta-analysis conducted in adult population demonstrated that WHtR is superior to BMI in detecting several cardiometabolic risk factors22 and, particularly, T2D23. WC seemed to be a reliable marker of adipose tissue distribution, nonetheless a recent study published by Goodwin et al24, suggests that particularly among adolescents, routine clinical measurements such as BMI and WC might predict well subcutaneous but not visceral fat when compared to body fat distribution measured by Magnetic Resonance Imaging. Moreover, even though visceral fat accumulation could be reliably predicted by WC measurements, controversy has emerged since some authors had described healthy metabolic but obese phenotype in the general population25–27. Large-scale genomewide studies have recently pointed out that obesity related comorbidities might be a consequence of variations in genes encoding proteins that regulate pathway signals involved in adipogenesis, adipocyte differentiation, adipose tissue expandability and distribution as well as ectopic fat dysfunction. Kilpeläinen et al. described that body-fat–decreasing allele near IRS1 is associated with decreased IRS1 expression and with an impaired metabolic profile, including increased visceral to subcutaneous fat ratio, insulin resistance, dyslipidemia, risk of T2D and coronary artery disease and decreased adiponectin levels28. As large scale genomewide studies continue to be identified, the understanding of phenotypic metabolic variations as well as morbid outcomes among normal weight and obese individuals will be clarified.
Visceral adipose index has recently proved to be an indicator of adipose tissue distribution and dysfunction as it has been proposed as a reliable risk factor of cardiovascular (OR (95%CI):2.45 (1.52-3.95) and cerebrovascular (OR(95%CI):1.63(1.06-2.5) events7. A ROC analysis proved in this study a greater sensitivity and specificity of VAI compared to its individual components (WC, BMI, Triglycerides and HDL-cholesterol). Moreover, in women with Polycystic Ovary Syndrome VAI has been used to distinguish metabolically healthy from metabolically unhealthy cases29,30. Oh et al. reported in Korean population that VAI positively correlated with Visceral Fat Area, the visceral-to-subcutaneous fat ratio, and systolic and diastolic blood pressure, and negatively with the Rd value after euglycemic hyperinsulinemic clamp in young women with polycystic ovary syndrome31. Yang et al were able to support the concept that arterial stiffness, measured through brachial-ankle pulse wave velocity, significantly increased across groups with higher VAI levels even in the same metabolic category (p<0.001)8. Several authors have proposed VAI as a marker of metabolic disturbances in other pathologies such as non-alcoholic fatty liver disease and non-alcoholic steatohepatitis, acromegaly, prolactinoma, and Cushing disease32. Few studies have analyzed VAI as a surrogate cardiometabolic risk factor in pediatric population. Al-Daghri et al, conducted a cross sectional study in Saudi children and adolescents and results are inconsistent with previous reports generated for adult population. In that study BMI and WC showed to be superior in terms of association with adipocytokines, blood pressure, HOMA-IR and CRP levels compared to VAI. Interestingly, VAI was independently associated with glucose values. Nonetheless, our study had proposed a novel VAI formula originated from a pediatric population and based on original formula construction that seems to be more suitable for this age group since visceral adipose deposition, absolute values of BMI, WC-BMI correlation, as well as triglycerides and HDL-cholesterol levels differ from those reported for adults. We should be then cautious while interpreting these results.
Visceral adipose index emerged as an empirical-mathematical model originated from observations in normal and overweight, but non obese adult individuals (19-83 year old). A linear equation was extrapolated to correlate WC and BMI. For our study we decided to include normal weight, overweight and obese children in order to map out the overall WC-BMI spectrum. A non-lineal quadratic regression model instead of a linear correlation explained more accurately the relationship between WC and BMI in the overall sample. Quadratic tendency of the equation was evident while BMI increased throughout the morbid obese range suggesting a tendency of WC to reach a steady state level afterwards. This complementary segment of the equation was not evaluated in the study published by Amato et al, since they did not include obese individuals (BMI ≥ 30kg/m2). By the other hand, we should consider childhood as a dynamic process of weight and height gain and exaggerated abnormal hyperplastic adiposity rebound can occur in prepubertal children exposed to an obesigenic milieu. In fact, VAI formula construction seems to be different in children compared to adults. One limitation of this study lies in its cross-sectional design, which does not allow for obtaining cut-off points in order to identify children and adolescents at risk for metabolic and cardiovascular alterations. However, at least for the Mexican population, equation proposed in this study may potentially be used for both clinical and epidemiological applications bearing in mind that they must be validated against other prospective studies. Furthermore, VAI could be a useful tool during intervention and follow-up in obese and non-obese children and could be as well defined as better predictor of early cardiovascular and metabolic risk in all borderline conditions in which overt metabolic syndrome is not present32