Variceal bleeding (VB) is a serious complication in cirrhotic patients. Hepatic pressure venous gradient (HPVG) is the gold standard to assess high risk of VB, but, this is not always available and is an invasive method. Therefore is necessary to explore if non-invasive parameters are useful as predictive factors of high risk of VB.
ObjectiveTo evaluate if low platelet count, spleen size, platelet count/spleen size ratio, portal vein diameter, blood flow velocity of the portal vein, congestion index of the portal vein, and variceal size could be useful as non-invasive parameters for predicting high risk of VB in cirrhotic patients.
Subjects and methodsObservational, cross sectional study, that includes 99 cirrhotic patients with esophageal varices. For predictive analysis we considered as the dependent variable “presence of VB” and the independent variables we tested were: Child-Pugh score, platelet count, spleen size, portal vein diameter, platelet count/spleen size ratio, blood flow velocity of the portal vein, congestion index of the portal vein, variceal size. Univariate and multivariate logistic regression were performed.
Results99 cirrhotics with esophageal varices were included, 56 (56.6%) were female, the mean of age was 57.8±12.2. About variceal size, 54 (54.5%) of patients had large varices. Regarding to occurrence of VB, 46 (46.5%) presented it. In the multivariate analysis, the presence of large varices in the endoscopic study was the best predictor of VB (OR=11.1; 95% CI=3.9 to 32.8, P<0.0001). Portal vein diameter ≥ 13mm results with an OR=5.0; 95% CI=1.1 to 21.7, P=0.03
ConclusionsThe presence of large esophageal varices is the most important predictive risk factor for the occurrence of VB, independently of the class of Child-Pugh. Additionally, the portal vein diameter ≥ 13mm is a non-invasive parameter related to high risk of VB. Therefore, these factors could be used as predictors of high risk of VB when the measure of HPVG is not available.
La hemorragia variceal (HV) es una complicación grave en pacientes cirróticos. El gradiente de presión venosa hepática (GPVH) es el estándar de oro para clasificar pacientes con alto riesgo de HV, sin embargo, este no siempre está disponible y es un método invasivo. Por tanto, es necesario explorar si parámetros no invasivos son útiles como factores predictores de alto riesgo de HV.
ObjetivoEvaluar si la baja cuenta plaquetaria, tamaño del bazo, cociente cuenta plaquetaria/tamaño del bazo, diámetro portal, velocidad del flujo portal, índice de congestión portal, y tamaño variceal podrían ser útiles como parámetros no invasivos para predecir alto riesgo de HV en pacientes cirróticos.
Sujetos y métodosEstudio observacional, transversal, que incluyó 99 pacientes cirróticos con varices esofágicas. Para el análisis predictivo consideramos como la variable dependiente “presencia de HV”, y como variables independientes: estadio de Child-Pugh, cuenta plaquetaria, tamaño del bazo, diámetro portal, cociente cuenta plaquetaria/tamaño del bazo, velocidad del flujo portal, índice de congestión portal, tamaño variceal. Realizamos análisis de regresión logística uni y multivariado.
ResultadosSe incluyeron 99 pacientes cirróticos con varices esofágicas, 56 (56.6%) fueron mujeres, la media de edad fue 57.8±12.2. Respecto al tamaño variceal, 54 (54.5%) tuvieron varices grandes. Respecto a la ocurrencia de HV, 46 (46.5%) la presentaron. En el análisis multivariado, la presencia de varices grandes por endoscopia fue el mejor predictor de HV (RM=11.1; IC 95%=3.9 a 32.8, P<0.0001). El diámetro portal resultó con una RM=5.0; IC 95%=1.1 to 21.7, P=0.03.
ConclusionesLa presencia de varices esofágicas grandes es el factor predictivo más importante de la ocurrencia de HV, independiente de la clase de Child-Pugh. Adicionalmente, el diámetro portal ≥ 13mm es un parámetro no invasivo relacionado con alto riesgo de HV. Por tanto, estos factores podrían ser utilizados como predictores de alto riesgo de HV cuando en GPVH no este disponible.
Portal hypertension is a progressive complication of cirrhosis. Portal hypertension leads to the formation of porto-systemic collaterals. Gastroesophageal varices are the most relevant porto-systemic collaterals because their rupture results in variceal bleeding (VB), the most common lethal complication of cirrhosis, associated with a mortality of at least 20% at 6 weeks.1–4
The most important predictor of variceal bleeding is the variceal size, with the highest risk of first hemorrhage about 15% per year occurring in patients with large varices. Other predictors of hemorrhage are decompensated cirrhosis (Child B/C) and the endoscopic presence of red wale marks.5
Nowadays, the gold standard for assessing portal pressure is the hepatic venous pressure gradient (HVPG), which is obtained by determining the wedged hepatic venous pressure (WHVP) by placing a catheter in the hepatic vein and by inflating a balloon and occluding a larger branch of the hepatic vein, then the WHVP is corrected for increases in intraabdominal pressure by subtracting the free hepatic vein pressure (FHVP) or the intraabdominal inferior vena cava pressure.6
The HVPG have predictive value for the development of gastroesophageal varices,7,8 and the risk of VB.9–11 Patients with an HVPG ≥ 20mmHg (measured within 24hours of VB) have been identified as being at a higher risk for early recurrent bleeding within the first week of admission or failure to control bleeding and a higher 1-year mortality compared to those with lower pressure.12,13 Late rebleeding occurs in approximately 60% of untreated patients, mostly within 1-2 years of the index hemorrhage.14,15 Limitations to the generalized use of HVPG measurement are the lack of local expertise and poor adherence to guidelines that will ensure reliable and reproducible measurements,6 as well as its invasive nature.1 Furthermore, is expensive and not always is available. For this reason, is very important to search for non-invasive, accessible, easy to perform, and reproducible parameters that can help us to identify patients with high risk of bleeding.
Many studies have evaluated non-invasive methods such as, low platelet count, spleen size, portal vein diameter, platelet count/spleen size ratio, and they seem to be predictors related to the presence of large or high grade esophageal varices.16–32 However, there is not sufficient information about if these non-invasive parameters could be used as reliable predictors of high risk of VB that avoid the need for HVPG.
The aim of this study was to evaluate if low platelet count, spleen size, platelet count/spleen size ratio, portal vein diameter, blood flow velocity of the portal vein, congestion index of the portal vein, and variceal size could be useful as non-invasive parameters for predicting a high risk of VB in cirrhotic patients.
Subjects and methodsStudy designObservational, cross sectional study conducted since February 2011 to February 2013, including cirrhotic patients for any etiology, with portal hypertension and presence of esophageal varices documented by an endoscopic study. Patients with portal hypertension with a prehepatic or posthepatic origin, previous therapy with transjugular intrahepatic porto-systemic shunts, or with history of chirurgic derivation of the portal vein, spleenectomy, thrombosis or cavernomatosis of the portal vein, or with diagnosis of hepatocarcinoma were excluded.
Esophageal varices were classified according to Baveno V Consensus33 in small or large varices. We searched for presence or absence of VB. Also patients must have a hepatic doppler ultrasound and laboratory tests to determine Child-Pugh score, and also platelet count, these studies must have been performed with no more than three months of difference regarding to the endoscopic study. Hepatic doppler ultrasound was performed with a Siemens Acuson S2000 equipment with a convex multi-frequency transductor by an expert radiologist, the following measures were performed: Portal vein diameter, blood flow velocity of the portal vein, spleen size (spleen longitudinal axis in centimeters), and with these parameters, were calculated the congestion index of the portal vein, and platelet count/spleen size ratio according to the next formulas:
Congestion index of the portal vein=cross-sectional area of the portal vein (cm2)/blood flow velocity of the portal vein (cm/sec)
Platelet count/spleen size ratio=platelet count (cell/103)/spleen size (mm)
Statistical analysisThe distribution of numerical variables were analyzed with Kolmogorov-Smirnov test, in case of non-normal distribution, logarithmic base 10 transformation was performed to allowed expressed the variables as mean and standard deviation (SD). Qualitative variables were expressed as proportion and percentage. For predictive analysis we considered as the dependent variable “presence of VB” and the independent variables we tested were: Child-Pugh score, platelet count, spleen size, portal vein diameter, platelet count/spleen size ratio, blood flow velocity of the portal vein, congestion index of the portal vein, variceal size. Univariate and multivariate logistic regression were performed. A P value ≤ 0.05 was considered significant. SPSS version 19.0 was employed.
ResultsWe included 99 cirrhotic patients with esophageal varices evidenced by endoscopic study, 56 (56.6%) were female, and 43 (43.4%) were male. The mean of age was 57.8±12.2. According to Child-Pugh score, 46 (46.5%) were Child-Pugh A, 41 (41.4%) were Child-Pugh B and 12 (12.1%) were Child-Pugh C. Chronic alcohol intake was the most common cause of cirrhosis counting for 43 (43.4%) patients, followed by non-alcoholic steatohepatitis in 20 (20.2%) patients, 16 (16.2%) patients with chronic viral hepatitis, 14 (14.1%) patients with cryptogenic cirrhosis, and 6 (6.1%)patients with an autoimmune origin. About variceal size, 54 (54.5%) of patients had large varices and 45 (45.5%) had small varices. Regarding to occurrence of VB, 46 (46.5%) presented it, and 53 (53.5%) did not.
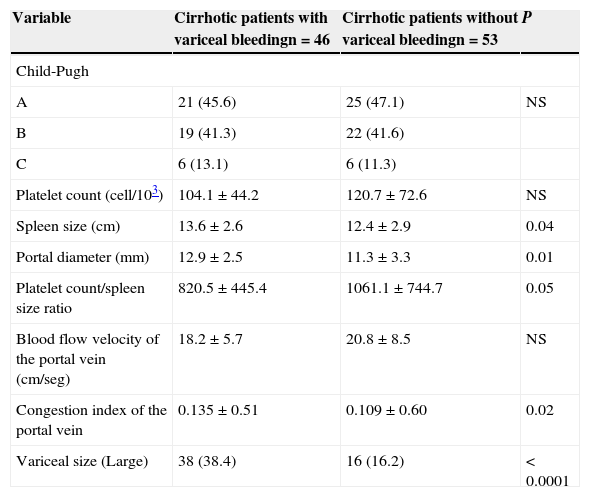
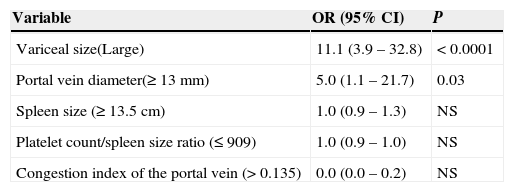
When we compared the characteristics between cirrhotic patients with VB and patients without VB, we found differences between groups regarding to spleen size, portal diameter, platelet count/spleen size ratio, congestion index of the portal vein, and variceal size (see Table 1). However, in the multivariate analysis, the presence of large varices in the endoscopic study was the best predictor of VB (OR=11.1; 95% CI=3.9 to 32.8, P<0.0001) (see Table 2).
Univariate analysis comparing characteristics between cirrhotic patients with and without variceal bleeding.
| Variable | Cirrhotic patients with variceal bleedingn=46 | Cirrhotic patients without variceal bleedingn=53 | P |
|---|---|---|---|
| Child-Pugh | |||
| A | 21 (45.6) | 25 (47.1) | NS |
| B | 19 (41.3) | 22 (41.6) | |
| C | 6 (13.1) | 6 (11.3) | |
| Platelet count (cell/103) | 104.1±44.2 | 120.7±72.6 | NS |
| Spleen size (cm) | 13.6±2.6 | 12.4±2.9 | 0.04 |
| Portal diameter (mm) | 12.9±2.5 | 11.3±3.3 | 0.01 |
| Platelet count/spleen size ratio | 820.5±445.4 | 1061.1±744.7 | 0.05 |
| Blood flow velocity of the portal vein (cm/seg) | 18.2±5.7 | 20.8±8.5 | NS |
| Congestion index of the portal vein | 0.135±0.51 | 0.109±0.60 | 0.02 |
| Variceal size (Large) | 38 (38.4) | 16 (16.2) | < 0.0001 |
Qualitative variables are expressed as frequency and percent. Numerical variables are expressed as mean and standard deviation.
Multivariate analysis to determine predictive non-invasive parameters for high risk of variceal bleeding.
| Variable | OR (95% CI) | P |
|---|---|---|
| Variceal size(Large) | 11.1 (3.9 – 32.8) | < 0.0001 |
| Portal vein diameter(≥ 13 mm) | 5.0 (1.1 – 21.7) | 0.03 |
| Spleen size (≥ 13.5 cm) | 1.0 (0.9 – 1.3) | NS |
| Platelet count/spleen size ratio (≤ 909) | 1.0 (0.9 – 1.0) | NS |
| Congestion index of the portal vein (> 0.135) | 0.0 (0.0 – 0.2) | NS |
OR=Odds ratio.
95% CI=95% Confidence interval.
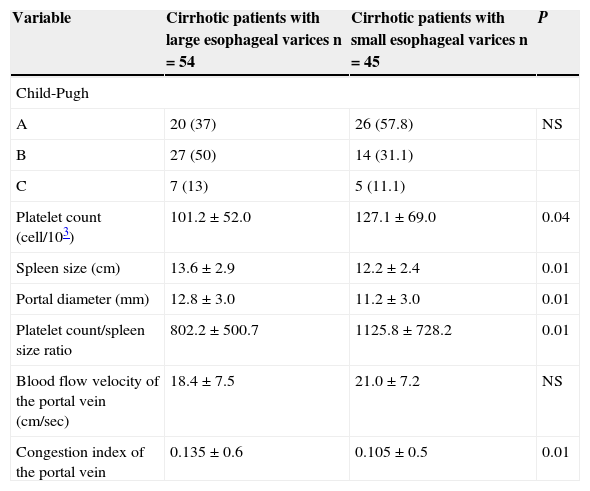
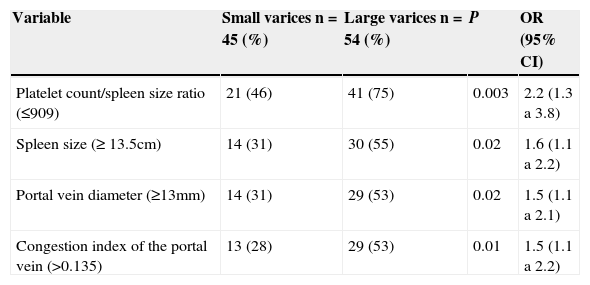
When we evaluated non-invasive parameters: Child-Pugh score, platelet count/spleen size ratio, spleen size, portal vein diameter, portal vein congestion index, as predictive factors of the presence of large esophageal varices, we found that all of them, with exception of Child-Pugh score were predictive factors for the presence of large esophageal varices. The best predictive factor for presence of large esophageal varices was platelet count/spleen size ratio ≤ 909 (OR=2.2; 95% CI=1.3 to 1.8, P=0.003) (see Tables 3 and 4).
Univariate analysis comparing characteristics between cirrhotic patients with large and with small esophageal varices.
| Variable | Cirrhotic patients with large esophageal varices n=54 | Cirrhotic patients with small esophageal varices n=45 | P |
|---|---|---|---|
| Child-Pugh | |||
| A | 20 (37) | 26 (57.8) | NS |
| B | 27 (50) | 14 (31.1) | |
| C | 7 (13) | 5 (11.1) | |
| Platelet count (cell/103) | 101.2±52.0 | 127.1±69.0 | 0.04 |
| Spleen size (cm) | 13.6±2.9 | 12.2±2.4 | 0.01 |
| Portal diameter (mm) | 12.8±3.0 | 11.2±3.0 | 0.01 |
| Platelet count/spleen size ratio | 802.2±500.7 | 1125.8±728.2 | 0.01 |
| Blood flow velocity of the portal vein (cm/sec) | 18.4±7.5 | 21.0±7.2 | NS |
| Congestion index of the portal vein | 0.135±0.6 | 0.105±0.5 | 0.01 |
Qualitative variables are expressed as frequency and percent. Numerical variables are expressed as mean and standard deviation.
Multivariate analysis to identify predictive non-invasive parameters for presence of large esophageal varices in cirrhotic patients.
| Variable | Small varices n=45 (%) | Large varices n=54 (%) | P | OR (95% CI) |
|---|---|---|---|---|
| Platelet count/spleen size ratio (≤909) | 21 (46) | 41 (75) | 0.003 | 2.2 (1.3 a 3.8) |
| Spleen size (≥ 13.5cm) | 14 (31) | 30 (55) | 0.02 | 1.6 (1.1 a 2.2) |
| Portal vein diameter (≥13mm) | 14 (31) | 29 (53) | 0.02 | 1.5 (1.1 a 2.1) |
| Congestion index of the portal vein (>0.135) | 13 (28) | 29 (53) | 0.01 | 1.5 (1.1 a 2.2) |
OR=Odds ratio; 95% CI=95% confidence interval.
VB is one of the most serious complications of the portal hypertension in cirrhotic patients.1 To assess the degree of portal hypertension in patients with cirrhosis, the gold standard is the measurement of HVPG.1,6 Gastroesophageal varices develops in patients with a HVPG > 10mmHg, those with an HVPG > 12mmHg are at risk of VB, and those with an HVPG > 20mmHg are at high risk of rebleeding in the first 24hours after a first episode of hemorrhage.1–13 According to several studies,1,5,7,30–33 variceal size is one of the most important predictive risk factors for VB. In our study, we corroborate that the presence of large esophageal varices is the main independent predictive risk factor for VB in cirrhotic patients, despite of the class of Child-Pugh. However, is important to note that in our study most of patients were classified as Child-Pugh A and B, only a few patients were classified as Child-Pugh C. Other studies have found that Child-Pugh B/C patients have the greatest risk for develop large esophageal varices and VB.1,34,35
Several previous studies have shown independent parameters like splenomegaly,34,36–39 low platelet count,34,37–39 portal vein diameter, 34,40 platelet count/spleen diameter ratio,34,41,42 congestion index of the portal vein 43 as significant predictors for the presence of esophageal varices. In our study, we also found that spleen size, platelet count/spleen size ratio, portal vein diameter, spleen size and congestion index of the portal vein were independent predictors for the presence of large esophageal varices (P=0.01), low platelet count was also found in patients with large esophageal varices (P=0.04). However, when we evaluated these non-invasive parameters as predictors of high risk of VB, only the portal vein diameter ≥ 13mm was a predictive factor (P=0.03) according to our multivariate analysis.
Primary prophylaxis to prevent the first VB is indicated in cirrhotic patients with large varices, either non-selective beta-blockers (NSBB) or endoscopic variceal ligation (EBL) can be used.44 The advantages of NSBB are that their cost is low, expertise is not required for their use, and they may prevent other complications, such as bleeding from portal hypertensive gastropathy, ascites, and spontaneous bacterial peritonitis because they reduce portal pressure. Additionally, many patients prefer to avoid endoscopic procedures because of their invasive nature.45 Since our study corroborates that non-invasive ultrasonographic parameters are capable to identify patients with large varices, and large varices are a main risk factor for VB, we propose that in selected patients, or when endoscopy is not available, non-invasive ultrasonographic parameters could be enough to decide start primary prophylaxis with NSBB.
A limitation of our study is its retrolective character. Furthermore, in our institution we do not count with equipment to perform HPVG, therefore we could not determine it in our patients, as we did not count with the gold standard to prognosis the risk of VB we considered inappropriate to determine the sensibility, specificity, and predictive values for variceal size and portal vein diameter as tools for predict high risk of VB in cirrhotic patients.
Prospective studies to evaluate the non-invasive parameters proposed in this study are necessary to validate our findings. Since we collected the ultrasonographic data from medical records, we had not the opportunity to evaluate the inter-observer concordance.
ConclusionsThe presence of large esophageal varices is the most important predictive risk factor for the occurrence of VB, independently of the class of Child-Pugh. Additionally, the portal vein diameter ≥ 13mm is a non-invasive parameter related to high risk of VB. Therefore, presence of large varices and a portal vein diameter ≥ 13mm can be used as predictors of high risk of VB when the measure of HPVG is not available. However, other non-invasive parameters seem to be not useful and cannot replace the determination of the HPVG.
Our study confirms that in medical centers where endoscopic study is not available, non-invasive parameters such as, portal vein diameter ≥ 13mm, spleen size ≥ 13.5cm, platelet count/spleen size ≤ 909, and the portal vein congestion index > 0.135 could be useful tools to predict the presence of large esophageal varices in cirrhotic patients independently of the class of Child-Pugh. The most useful of them, is platelet count/spleen size ratio. Therefore, these parameters could guide the decision to start primary prophylaxis.
Conflict of interestAll authors declare have not conflict of interest.