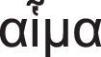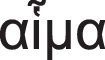Acute leukaemias are characterised by uncontrolled proliferation of immature blood cells with lymphoid or myeloid lineage. Morphological classification is based on the identification of the leukaemia cell line and its stage of differentiation. The first microscopic descriptions dating from the 1930s pointed to 2 different types of leukaemia cells: lymphoid and myeloid. In 1976, the consensus that led to the French-American-British (FAB) classification was achieved. This includes criteria for identifying myeloid and lymphoid leukaemias, and gives a list of morphological subtypes, describing how these affect the patient's prognosis. Today, despite new classifications based on sophisticated studies, FAB classification is widely used by experts due to its technical simplicity, good diagnostic reliability and cost-effectiveness.
Las leucemias agudas se caracterizan por proliferación descontrolada de células progenitoras de estirpe linfoide o mieloide. La clasificación morfológica se basa en la identificación de la línea celular de la célula leucémica y de la etapa de diferenciación. Desde las primeras descripciones microscópicas durante el primer tercio del siglo XIX se plantearon dos tipos distintos de las células leucémicas: linfoides y mieloides. Fue hasta 1976 cuando se logró el consenso que dio origen a la clasificación Franco-Americano-Británica (FAB) que conserva la gran división entre mieloides y linfoides, además de asignar subtipos a partir de características morfológicas, destacando la traducción directa que tienen sobre el pronóstico del paciente. Hoy en día a pesar de contar con clasificaciones que consideran estudios más sofisticados, la clasificación FAB es ampliamente usada por expertos debido a su sencillez técnica, buena fiabilidad diagnóstica y precio económico.
The word leukaemia (originally leukämie in German) comes from the Greek words leukos (λ¿υκóζ): white and haima (
): blood. The modern term leukaemia was first proposed by Rudolf Virchow in 1846.1 Unsurprisingly, the term was suggested after examining infected blood under the microscope, an instrument that was first invented in the 17th century, but only perfected 2 centuries later and used by physicians to identify alterations in tissues and cells.The famous French physician Alfred Armand Louis Marie Velpeau (1795–1867) is credited with providing the first anatomical and pathological description of leukaemia. The patient was a 63-year-old man called Monsieur Vernis, who earned his living selling flowers and lemonade in Paris, and complained of fever, weakness, and an enlarged liver and spleen. Velpeau performed the autopsy on the patient, observing a greatly enlarged spleen weighing 4.5kg and noting that the blood was very thick with a consistency similar to that “of porridge”. Under the microscope, he observed a large quantity of “globules of pus in the blood”. Velpeau reported the histological and anatomical findings in this patient to the Royal Academy of Medicine in Paris, and the article was published in March of 1827. A few years later, in 1839, Alfred François Donné, also a Paris-based physician and an enthusiast of microscopy as a diagnostic tool, described the case of a woman with splenomegaly and increased levels of “mucous globules” in the blood. The case caught the attention of one of Donné’s foreign disciples, the English pathologist John Hughes Bennett (1812–1875). On retuning to his native land, Bennett came across a similar case of organ enlargement and changes in the consistency and colour of the blood, which he described in detail in the Edinburgh Medical and Surgical Journal (1845). Six years later, Bennett dubbed this pathology “leukocytosis”.1,2
Five or six weeks after publication of Bennett's report, a report titled Weisses Blut (white blood, in German) was published in the German journal Neue Notizen aus Gebiete der Natur-und Heilkunde. The article reported the case of a 50-year-old cook by the name of María Straide, with a 4-year history of chronic fatigue and abdominal distension. On physical examination, the patient presented an enlarged, slightly tender spleen, oedema of the lower limbs, and diarrhoea. She had complained of epistaxis that was lasting for 8 days, rash, and weakness. The autopsy showed loss of colour in all organs, “white spots” on the liver, and a greatly enlarged spleen. Microscopic findings were “everywhere in the blood vessels a mass closely resembling pus was found, with colourless corpuscles”. The author of the report was the young 24-year-old German pathologist Rudolf Virchow (1821–1902). These observations gave rise to the term leukaemia (leukämie). Although Virchow was quick to praise Bennett and addressed him with great respect, the appearance of his report so soon after Bennett's, proposing a different name for the same condition, sparked considerable controversy.2,3
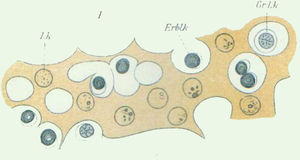
The next major step in the story of leukaemia morphology occurred in 1869, when both blood and bone marrow were studied under the microscope. Studying bone fragments, Franz Ernst C. Neumann (1834–1918), a German pathologist born in Königsberg, pupil of Hermann Ludwig F. von Helmholtz (1821–1894) and Virchow, was the first to show that haematopoiesis takes place in the bone marrow. He hypothesised that blood originates from a common precursor, which he called a stem cell (Stammzelle in German) (Fig. 1).4,5 Neumann also suggested that, in addition to the lymphatic and splenic leukaemias proposed by Virchow, there was another type of leukaemia that originated in the bone marrow. He called this “myeloid leukaemia”, and his theory was later validated by Bizzozero.1,3
The last breakthrough in this brief history of the morphology of leukaemia was spearheaded by Paul Ehrlich (1854–1915). While still a medical student, Ehrlich developed the technique of cell staining, and observed for the first time the cytoplasmic components and different types of nuclei in blood cells, for which he coined the terms acidophils and basophils.6
Morphology of acute leukaemiasAcute leukaemias are characterised by the uncontrolled proliferation of myeloid or lymphoid progenitor cells.1 Their morphological classification is based on the identification of the leukaemia cell line and stage of cell differentiation.1,6
During the latter half of the 20th century, a number of leukaemia subgroups were defined, based on the correlation between their morphology and clinical characteristics, laboratory findings, and response to treatment. A number of classifications were proposed and later discarded as unsuitable for all types of patients. In some of these, the criteria were ambiguous; some used complicated and scarcely reproducible methods, while others used different terminology to describe the same type of leukaemia, thus creating an atmosphere of confusion. In 1976, this situation prompted 7 haematologists to come together to form an international cooperative group that gave rise to the French-American-British (FAB) classification system, which divided leukaemia in 2 types: myeloid and lymphoblastic.7 The classification system can only be used in previously untreated patients, as chemotherapy distorts both normal and malignant cells.8
The following is a summary, with photos, of the FAB acute leukaemia classification system.
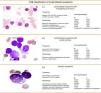
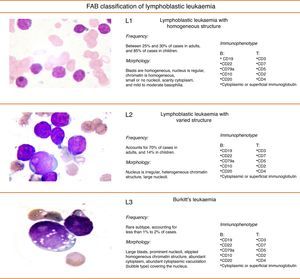
FAB classification of acute lymphoblastic leukaemiaFig. 2 shows the subtypes, morphology, immunophenotye and prevalence of acute lymphoblastic leukaemias (ALL).9
Due to difficulties in distinguishing L1 and L2 subtype cells, the FAB group published an amended version of their classification system in 1981. In this, they proposed a scoring system for identifying L1 and L2 leukaemias on the basis of 4 cytological characteristics: nucleus:cytoplasm ratio, presence of nucleoli, characteristics of the nuclear membrane, and cell size (see Table 1).10
FAB classification of acute lymphoblastic leukaemia.
| Criterion | Classification |
|---|---|
| High nucleus:cytoplasm ratio>75% of cells | + |
| Low nucleus:cytoplasm ratio>25% of cells | − |
| Nucleoli: 0–1 (small)>75% of cells | + |
| Nucleoli: 1 or more (prominent)>25% of cells | − |
| Irregular nuclear membrane>25% of cells | − |
| Megaloblasts>50% of cells | − |
Obtain 1 classification in each category, sum total of signs.
Total classification (−4 to +2): 0 to +2 L1 acute lymphoblastic leukaemia; −1 to −4 L2 acute lymphoblastic leukaemia.
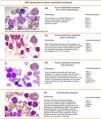
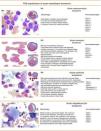
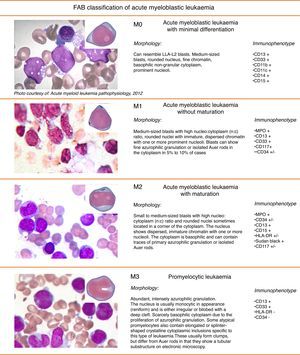
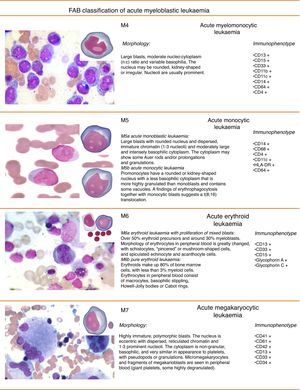
Figs. 3 and 4 show the immunophenotype and morphology of acute myeloblastic leukaemias (AML), together with microscope images and a brief summary.9,11,12
Myeloblastic leukaemia with minimal differentiation, or M0-AML, is particularly difficult to diagnose from the morphological point of view because the blasts present both lymphoblastic and myeloblastic features. They account for only 5% of adult AMLs.13
M1 Acute myeloblastic leukaemiaIn acute myeloblastic leukaemia without maturation (M1), cells are usually monomorphic, with myeloid blasts in peripheral blood and no cells beyond the myeloblast stage of differentiation. Leukocytes are present at diagnosis.
M2 Acute myeloblastic leukaemiaAcute myeloblastic leukaemia with maturation (M2) accounts for around 30% of all cases of AML. Over 10% of cells are at the post-myeloblast stage (promyelocytes, myelocytes and neutrophils). One-third of all cases of AML-M2 are associated with t(8;21) translocation
M3 Acute myeloblastic leukaemiaAcute promyelocytic leukaemia (M3) is usually accompanied by low peripheral blood leucocyte levels, which makes diagnosis difficult. The majority of cells have a very characteristic morphology called atypical promyelocytes (hypergranular cells). Symptoms can include severe haemorrhage due to disseminated intravascular coagulation. The cytogenic change characteristic of AML-M3 is t(15;17), which leads to the fusion of the PML (promyelocytic leukaemic) and retinoic acid receptor-alpha (RARα) genes, resulting in the PML-RARα transcript.
Microgranular variant of AML-M3This accounts for between 15% and 20% of all cases of M3. Prognosis is poorer than normal M3, and leukocytosis is usually found. Morphologically, it is characterised by sparsely granulated leukaemia cells. The cytoplasm is more basophilic that in normal M3, due to a lower concentration of azurophilic granules.
M4 Acute myeloblastic leukaemiaAcute myelomonocytic leukaemia (M4) accounts for 20% of all cases of AML. Clinically, 10% of patients present gingival hyperplasia. This type of leukaemia has a granulocyte component and a monocyte component in varying proportions and different degrees of maturation. Cytochemically, they are positive for chloroacetate esterase, and monocytes are positive for naphthol-AS-d-acetate esterase or α-naphthyl butyrate esterase.
M4Eo variant: this occurs when at least 5% of cells are abnormal eosinophils, presenting monocytoid nuclei and atypical granules
M5 Acute myeloblastic leukaemiaThis represents around 15% of all cases of AML. The leukaemic cells are of a monocytic lineage (monoblasts and promonocytes). This class is divided into: M5a or acute monoblastic leukaemia, in which monoblasts predominate, and accounts for between 5% and 8% of all AMLs; and M5b or acute monocytic leukaemia, in which a high proportion of promonocytes and monocytes are found, in addition to monoblasts. This type comprises 3% to 6% of AMLs.
The monoblastic form is usually associated with t(9;11), t(6;11) and t(8;16) genetic translocations and 11q23 rearrangements (AML with recurrent genetic abnormalities according to the World Health Organization [WHO]).9
M6 Acute myeloblastic leukaemiaThe M6 erythroid sub-type is defined by the FAB as a proliferation of dysplastic erythroid elements with a proliferation of blasts of myeloid origin. The WHO classification characterises 2 sub-types: erythroleukaemia (M6a), described as a mixed proliferation of myeloid and erythroid blasts, and can be secondary to a previous myelodysplastic syndrome. M6a accounts for 5–6% of all AMLs. The other subtype is the pure erythroid variant (M6b), which occurs when dyserythropoiesis is prominent.
M7 Acute myeloblastic leukaemiaAcute myeloblastic leukaemia (AML-M7) represents between 3% and 5% of all AMLs.9
ConclusionsOther leukaemia classifications have now gained prominence, such as that proposed by the European Group for the Immunological Characterization of Leukaemias (EGIL), based on immunophenotyping criteria,14,15 or more recently, the classification drawn up by the World Health Organisation in 2001, updated in 2008, which combines morphology cytochemistry, immunophenotyping, clinical presentation, and genetic abnormalities. The WHO classification is now regarded as the gold standard for leukaemia diagnosis.9,16 Despite the benefits of the FAB classification, molecular biology and immunophenotyping studies are essential for reaching an accurate diagnosis, choosing the right therapy, and establishing a prognosis. For these reasons, these tests are wholly justified, despite the high cost for both the patient and the hospital.17
For specialists, the purely morphological criteria, in other words, Wright's stained smears, of the FAB classification are a powerful diagnostic tool. Browman was one of the first to evaluate intra-observer (64.8% and 70.5%) and inter-observer (63% and 72%) concordance between 2 experts. He found that concordance increased to 89% when cytochemistry studies were added to FAB, and reached 99% when immunophenotyping information is available.18 Similar findings were reported in subsequent studies.19 Curiously, a study performed in our hospital by Sales-Carmona et al. found that inter- and intra-observer concordance was good when using morphological criteria, while the addition of cytochemistry tests actually increased diagnostic disagreement.20
With this review, we hope to give clinicians an introduction to the morphological classification of leukaemias. We believe that far from underestimating these systems or considering them antiquated, haematologists should be trained in their use. After all, as in any human activity, practice makes perfect.
Conflict of interestThe authors declare that they have no conflict of interests.