To determine the frequency of expression of cancer testis antigens and their clinical correlation in patients with Hodgkin lymphoma.
Methodology of the studyIn this analytical, experimental and ambispective study, the MAGE A-3 and NY-ESO-1 antigen expression was correlated with clinical prognostic variables such as clinical stage, response to treatment, and relapse, in a total of 70 patients diagnosed with Hodgkin's lymphoma at the Hodgkin's Lymphoma Clinic of the General Hospital of Mexico “Dr. Eduardo Liceaga”, from December 2000 to December 2015. Twenty-four patients were evaluated using RT-PCR, following extraction of RNA, to detect MAGE-A3 and NY-ESO1 expression. Cellular RNA was extracted from frozen tissue and controls using trizol (Life Technologies, Paisley, UK). 1μg of RNA was used for cDNA synthesis by M-MLV reverse transcriptase (Life technologies, Paisley, UK).
ResultsWe studied 24 patients with a median age of 28 years, a minimum age of 16 years and a maximum age of 48 years, mostly male. 50% of patients presented complete response to the first line of treatment and 27% of patients presented relapse, 37.5% in relation to the expression of MAGE-A3. Expression of the NY-ESO-1 gene was not found in the study group. Twelve percent of patients died during the study, 8.33% of whom were also positive for MAGE-A3 (p=0.264.95% CI). No significant correlation was found between MAGE-A3 expression and major clinical prognostic variables.
ConclusionAlthough the expression of MAGE-A3 in the study group was 37.5% (higher than reported in international studies), we found no correlation with the main clinical prognostics variables. Considering that the expression of MAGE-A3 in the cases studied does not confer prognostic value, making it impossible to use as a prognostic tool in peripheral blood, we are leaving the doors open to continue with this line of research, possibly increasing the number of patients as well as prolonging the follow-up time.
Determinar la frecuencia de expresión de los antígenos testiculares de cáncer y su correlación con la clínica en pacientes con linfoma Hodgkin.
Metodología de estudioEstudio de tipo analítico, experimental, ambispectivo, La expresión de antígenos MAGE A-3 y NY-ESO1 fue correlacionada con variables de pronóstico clínico tales como estadio clínico, respuesta a tratamiento, recaída, de un total de 70 pacientes diagnosticados con linfoma Hodgkin en la clínica de linfoma Hodgkin del Hospital General de México “Dr. Eduardo Liceaga” en el periodo comprendido entre DIC 2000 a DIC 2015. Fueron evaluados 24 pacientes a los que se les realizo mediante RT PCR previa extracción de RNA la expresión de MAGE A-3y NY-ESO1, el RNA celular fue extraído del tejido congelado y de los controles por medio de trizol (life technologies, paisley, uk) se utilizo 1μg de RNA para la síntesis de cDNA por medio de la reverso transcriptasa M-MLV(life technologies, paisley, uk).
Resultadosse estudiaron 24 pacientes con una mediana de edad de 28 años, con una mínima de 16 años y una edad máxima de 48 años, predominio de sexo masculino, 50% de pacientes presento respuesta completa a primera línea de tratamiento y un 27% de pacientes presentaron recaída, en relación con la expresión del gen MAGE A-3 fue de 37.5%, no encontrando en el grupo de estudio expresión para el gen NY-ESO1, El 12% de pacientes fallecieron en el periodo de estudio, de estos 8.33% fueron también positivos para MAGE A-3 (p=0.264,95% IC). No se encontró correlacion significativa entre la expresión de MAGE A-3 y las principales variables clínicas pronosticas.
ConclusiónA pesar de que la expresión de MAGE A-3 en el grupo de estudio fue de 37.5% (mayor a lo reportado en estudios internacionales), no encontramos correlacion con las principales variables clínicas pronosticas, considerando que la expresión de MAGE A-3 en los casos estudiados no confiere valor pronóstico, por lo que no es posible utilizarlo como herramienta pronostica en sangre periférica, dejamos puertas abiertas para continuar con esta línea de investigación considerando el incrementar número de pacientes así como prolongar el tiempo de seguimiento.
Hodgkin's lymphoma (LH) is a neoplasm characterised by lymphatic infiltration of both Hodgkin cells and Reed-Sternberg cells surrounded by an inflammatory cell infiltrate composed of plasma cells, eosinophils and histiocytes.5 Factors associated with its onset have been described as familial factors, immunosuppression and association with viruses, the association with Epstein Barr virus being reported in more than 50% of cases.6
The majority of patients present with lymphadenopathy, with cervical lymphadenopathy being the most frequent, followed by axillary and, less frequently, inguinal lymphadenopathy, extra-nodal manifestations, either by direct invasion or haematogenous dissemination, more frequently involving the spleen, lung and liver, and less frequently involving the bone marrow. Systemic symptoms occur in up to one third of patients, and include fever, night sweats, weight loss and chronic itching.
The diagnosis is made by excisional biopsy of the affected lymphatic tissue. Risk stratification is performed using the Ann Arbor staging system based on the involved lymph node area, the presence or absence of bulky mass, extranodal disease and present B symptoms.
The standard of treatment includes the use of the ABVD chemotherapy regimen (doxorubicin, bleomycin, vinblastine and dacarbazine) with or without 20–30Gy radiotherapy (RT), achieving 98% survival rates.22 Other regimens are associated with increased toxicity (BEACOPP).8 Factors for determining therapy include the histological type, clinical stage (poor prognosis III and IV, presence of bulky mass and constitutional symptoms).24
The role of PET-CT (positron emission tomography) in patients with Hodgkin's lymphoma is significant initially in staging (interval PET-CT) for the most aggressive therapeutic decision, as well as the PET-CT at end of treatment to evaluate the response to treatment (total response, partial response, stability of the disease, progression or relapse) and to determine whether or not to add consolidation radiation therapy.7,9 Its cost is a limitation for the use of it in our environment.
Overall post-treatment survival is good. Late toxicity associated with treatment is the main disadvantage, with secondary malignancies (myeloid leukaemia, myelodysplastic syndrome, lung cancer and breast cancer) and cardiovascular diseases (left ventricular failure and coronary artery disease) being the most frequent. Post-relapse alternatives include high-dose chemotherapy, autologous haematopoietic stem cell transplantation, and the use of monoclonal anti-CD30 antibodies.
Since 1998 the International Prognostic Index (IPI), or Hasenclever index, has been useful in identifying populations at risk of therapeutic failure, including clinical and biochemical parameters (albumin<4g/dl, stage IV, age >45 years, haemoglobin<10.5g/dl, white blood cells>15×103/μl and lymphopenia, masculine gender), classifying progression-free survival at 5 years according to the number of risk factors: 0 factors: 84%, 1 factor: 77%, 2 factors: 67%, 3 factors: 60%, 4 factors: 51%, 5 factors: 42%.
The lactate dehydrogenase test linearly reflects the tumour load. Despite advances in imaging studies with regard to Hodgkin's lymphoma, there are few molecular markers used to identify populations at risk of treatment failure or for follow-up of response to treatment. Current knowledge about the existence of circulating tumour cells attributes up to 90% of cancer-related deaths to metastatic disease in solid tumours.
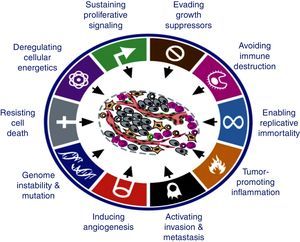
Tumour cells must pass through the endothelium and circulate through the bloodstream until they are either eliminated by immune response mechanisms or find an appropriate microenvironment in which they reside in a latent state, and eventually acquire the ability to proliferate at a later time. Only one out of 10,000 circulating cancer cells is able to form metastases (HALLMARK 2013, Fig. 1).
The clinical utility of circulating tumour cells depends on their availability in peripheral blood, which allows the study of fundamental aspects in the diagnosis, staging and prognosis of malignancies, methods based on the detection of free circulating DNA, RT-PCR and quantitative RT-PCR, demonstrating greater sensitivity when compared with flow cytometry.
There are approximately 100 cancer testis antigens (CTAs). These antigens are molecules which are normally expressed in germ cells. MAGE-A3 is a CTA whose expression is restricted to germ cells in the testis (primary spermatocytes, spermatogonia and trophoblasts), abnormally expressing in diverse tumour cells.10
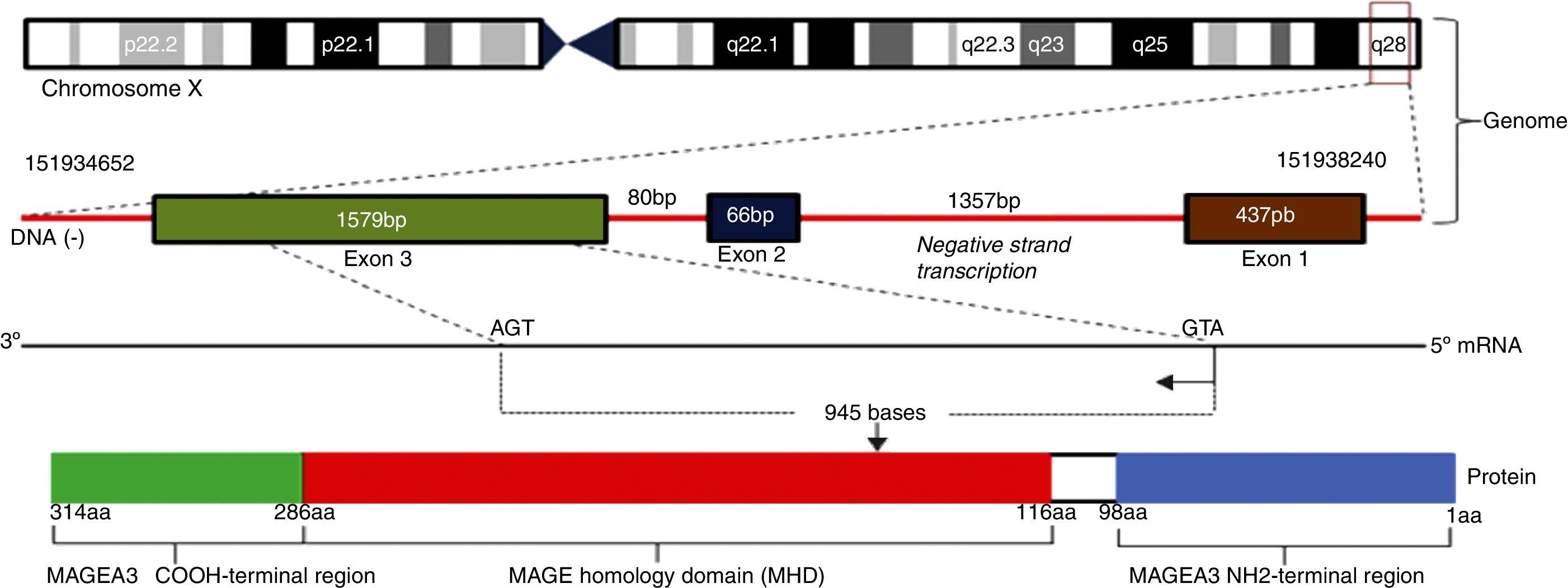
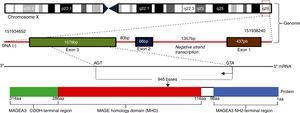
MAGE-A was initially identified in melanoma. All the genes of the MAGE-A family have a protein encoded by a single exon, preceded by several non-coding exons. They belong to a group called MAGE class I, having similar functions and characteristics (60–98% similarity in their coding sequence) (Fig. 2).
The MAGE type I family includes the subgroups MAGE-A, MAGE-B and MAGE-C. They are expressed in tumour cells of the lung, head, neck, bladder and breast, as well as gastric cancer, colorectal cancer, and haematologic malignancies such as lymphoma, leukaemia and myeloma, among others. The MAGE type II family includes the subgroups MAGE-D, MAGE-E, MAGE-F, MAGE-G, MAGE-H, MAGE-I2 and Necdin, which are coded by genes present in the X chromosomes, described in the q28 (MAGE-A genes), p21.3 (MAGE-B), q26 (MAGE-C), and P11 (MAGE-D) regions.11,14 The function of these antigens is not fully known. It has been well linked to protein ubiquitination processes including AMPK (AMP-activated protein kinase), which has critical tumour suppressing activities in both humans and experimental models, so that being ubiquitinated can promote delayed tumour growth through stimulation of the mTOR pathway, inhibition of autophagy, and stimulation of metabolic activity. Its anti-apoptotic properties (inhibits p53) may explain the persistence of minimal residual disease in some malignant cancers, including Hodgkin's lymphoma, where the potential for cure is very high.
The frequency of expression is highly variable in different cancer types, and cancers considered to have high expression of these antigens include: melanoma, ovarian cancer and lung cancer, while some haematopoietic malignancies, kidney, colon and pancreatic cancer are considered to have low frequency of expression.15,16 Exceptions have been reported regarding haematologic malignancies, such as in multiple myeloma where the expression of the CT7/MAGE-C1 antigen is high, as is the expression of CT45 in Hodgkin's lymphoma.
As for immune recognition, the A3 melanoma antigen family (MAGE-A3) was the first human tumour-associated antigen found to be specifically recognised by CD8+ T-cells. MAGE-A3 is coupled to MHC class I. The absence of expression in normal tissues assures an immune response against the tumour. Other cancer testis antigens are the BAGE, GAGE, LAGE and NY-ESO-1 genes, which together with MAGE are also recognised in various cells transformed neoplastically by CD8 (+) T-lymphocytes.29,30
MAGE gene expression is regulated through a methylation mechanism.12 Its expression is inhibited in normal tissue by epigenetic mechanisms of transcriptional repression through hypermethylation of the promoter gene. Of the known cancer testis antigens, those most frequently expressed in tumours are MAGE-A1, MAGE-A3, NY-ESO-1, SSX-2 y SSX-4.
NY-ESO-1 induces spontaneous immune responses of the host in up to 50% of the patients with malignancies that express it. It is considered one of the most immunogenic.4 Being an important candidate for immunotherapy, initially identified in oesophageal cancer due to its name, its expression has been demonstrated by mRNA reverse transcriptase polymerase chain reaction (RT-PCR) in 30% of melanoma, lung, and bladder cancers, 42% of breast cancers and 65% of medullary thyroid carcinomas, among others.30 A direct relationship between NY-ESO-1, tumour evolution and antibody titre has been observed, demonstrating its ability to induce immune response mediated by both CD4+ and CD8+ lymphocytes. Its use is known in various immunotherapies against cancer, such as malignant melanoma, in which the recombinant NY-ESO-1 protein was injected intradermally.
There have been studies that evaluated the expression of these antigens (MAGE-A3) in different haematologic malignancies. In 2010, Han et al. reported the detection of circulating tumour cells in patients with non-Hodgkin's lymphoma, using MAGE-A3 gene expression in peripheral blood, with expression in 47.3% of patients studied (total of 95 patients), finding no relation to survival. A reduction of MAGE-A3 expression was reported following effective chemotherapy, suggesting the MAGE-A3 gene as a tumour marker in non-Hodgkin's Lymphoma, without conferring prognostic value.1
In 2011 Inaoka et al. reported, in a Brazilian population (total of 38 patients), the expression of the MAGE family in 21% of patients with Hodgkin's lymphoma. Considering this expression as a target for immunotherapy, expression was evaluated for the MAGE-A family (18%), as well as MAGE-C1/CT7 (13.2%), MAGE-C2/CT10, NY-ESO-1 and the GAGE family, with the first two being the most frequently associated with an adverse prognosis.12
MAGE-A3 is considered a promising tumour-associated antigen for the selective targeting of malignant cells with immunotherapy. Its usefulness has been mentioned in patients with post-transplant multiple myeloma, using MAGE-A3/Poly-ICLC immunisation followed by adoptive transfer of autologous T-cells from prepared and co-stimulated vaccines. In a phase II clinical trial, T-cell infusions were well tolerated. 90% of patients had local reactions at the vaccine site. Overall survival at 2 years was 74% (95% CI, 54–100%) and 56% had 2 years of event-free survival (95% CI, 37–85%). A high frequency of T-cell responses to the specific vaccine was concluded in this case.28 Similar results were reported in a phase II clinical trial using antigen-specific immunotherapy to generate an immune response against MAGE-A3, to eradicate its expression by tumour in 182 patients with small cell lung carcinoma. The average follow-up was 44 months. 35% of patients receiving a recombinant MAGE-based vaccine showed clinical benefits based on immunotherapy.
In the Haematology Department at the General Hospital of Mexico, a line of research into genes of the MAGE family was pursued for purposes of monitoring and prognostic evaluation in patients with haematologic malignancies. In 2005 a study was conducted and published by Dr Rozen Fuller E. on MAGE-A gene expression and its prognostic correlation in acute lymphoblastic leukaemia, with a total of 47 patients, reporting the existence of a prognostic relationship between the disease and the presence of MAGE-A3. In 2007, a new study was conducted by Dr Olarte et al., evaluating MAGE-A3 expression in patients with diffuse large B-cell lymphoma. With a total of 28 patients, a statistically significant association was observed between MAGE-A3 expression and advanced stages, high LDH levels, poor response to treatment and lower survival.2,13 In 2015, Mendoza Salas et al. studied the frequency of cancer testis antigens in chronic myeloid leukaemia (CML). A total of 10 samples from healthy individuals and 65 bone marrow samples from patients with CML were analysed, reporting a presence of MAGE-A3 of 32.3%, with MAGE-A4 having a greater presence of up to 63%.3 In 2016 De la Cruz Rosas et al. reported the presence of MAGE-A3 in 21% of patients with multiple myeloma, associating it with resistance to therapy, progression and reduction in survival.
We have outlined the importance of knowing the expression of cancer testis antigens in different haematological malignancies, as tumour markers, prognostic factors and targets for immunotherapy in different haematological neoplasms. We have proposed this study because the expression of the cancer testis antigens MAGE-A3 and NY-ESO-1 in patients with Hodgkin's lymphoma in Mexico is unknown. General objective: To determine the frequency of expression of the cancer testis antigens MAGE-A3 and NY-ESO-1 and their clinical correlation in patients diagnosed with Hodgkin's lymphoma in the Hodgkin's lymphoma clinic of the General Hospital of Mexico “Dr. Eduardo Liceaga” from December 2000 to December 2015. Methodology: Our study is analytical, experimental and ambispective. Independent variables: Expression of MAGE-A3 and NY-ESO-1. Variables dependent on clinical prognosis: Clinical stage, response to treatment, relapse. A total of 24 patients were included, from whom peripheral blood samples were drawn with prior informed consent.
We included patients over the age of 16, with histopathological (lymph node) diagnosis of Hodgkin's lymphoma corroborated by immunohistochemistry (Cd15, Cd30, Cd20, and EBV markers) in the indicated period. Informed consent was obtained for the drawing of peripheral blood for the study. We excluded patients who were not followed up in our centre and eliminated patients whose peripheral blood samples were insufficient for the expression of the genes.
Hypothesis: If the expression of the cancer testis antigens MAGE-A3 and NY-ESO-1 is found in patients with Hodgkin's lymphoma, then this may be related to relapse and treatment failure within a short follow-up time.
Expression of the genes was evaluated by reverse transcriptase polymerase chain reaction (RT PCR), with testicular tissue being our positive control, to assess mRNA expression of cancer testis antigens: K562 Cell Line derived from a patient with chronic myeloid leukaemia (CML). Our negative control consisted of peripheral blood samples from healthy donors, from which we obtained mononuclear cells (lymphocytes, monocytes, blasts) using the Ficoll-Hypaque gradient method.
The mononuclear cell separation procedure was done with the Ficoll-Hypaque gradient method with a Ficoll density of 1.077g/cm3; mRNA isolation was performed by alkaline lysis using the Trizol/Invitrogen reagent; integrity (2% agarose gel) was carried out by molecular biology experts from the molecular biology laboratory area of the General Hospital of Mexico. The quantification and purity of the RNA and detection of cancer testis antigens by polymerase chain reaction, are detailed below.
Quantification and purity. Nucleic acids efficiently absorb ultraviolet light due to the presence of aromatic nitrogenous bases along the DNA strands. The UV absorption of DNA is a characteristic of the molecule, which is used efficiently to determine its concentration. Each of the bases has its own unique absorption spectrum and therefore contributes differently to the total property of UV absorption of a DNA molecule.
The absorbencies used are 260nm and 280nm. At 260nm the nucleic acids reach their maximum light absorption. Proteins have a maximum absorption at 280nm (mainly by tryptophan residues). Readings at this wavelength can show if there is protein contamination. The calculation of the A260/A280 ratio is a common way to express the purity of genetic material. Depending on the nucleic composition, a value of 1.65–1.9 indicates a pure sample.
Detection of cancer testis antigens by polymerase chain reactioncDNA synthesisThe cDNA of healthy donors, as well as that of patients with lymphoma and controls, was synthesised from 2μg total RNA. The final cDNA volume was 20μg. The RNA was mixed with 1μl of oligo(dT) 12–18 primer (INVITROGEN, Carlsbad, CA) and 1μl of 10mM dNTPs (Applied Biosystems, Roche). The mixture was incubated at 65°C for 5minutes and placed on ice. We added 4μl of 5× buffer (Tris–HCl 250mM, KCl 375mM MgCl2 15mM), 2μl of DTT (0.1M), and the corresponding volume of H2O, and the mixture was incubated at 37°C for 2min. Then 1μl of M-MLV RT (200U) (INVITROGEN, Carlsbad, CA) was added and the mixture was incubated at 37°C for 50min. The enzyme was inactivated by incubating at 70°C for 15min.
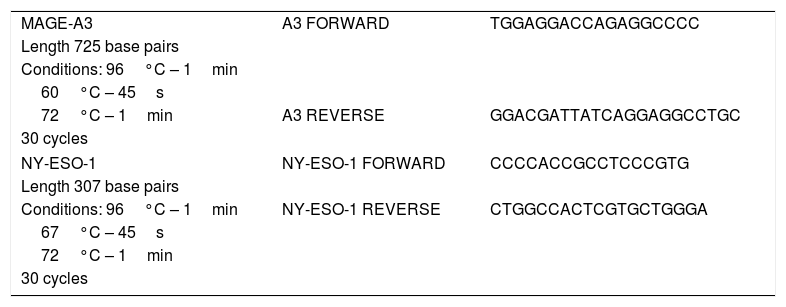
For the PCR, a final volume of 10μl was used, in which 10mM Tris–HCl, 50mM KCl, 1.5mM MgCl2, 100μM dNTPs, 0.2U Taq DNA polymerase, (INVITROGEN, Carlsbad, CA) 0.5μM sense primer, 0.5μM antisense primer and 1μl cDNA were mixed. The primers for the MAGE-A3 and NY-ESO genes were described in Olarte, 2012.27 The same sequence of primers was used to amplify the samples of patients at the General Hospital of Mexico. As a control, the GAPDH constitutive gene was used. The sequence of primers used as well as the expected length of the PCR product can be found in Table 1.
Sequence of primers.
| MAGE-A3 | A3 FORWARD | TGGAGGACCAGAGGCCCC |
| Length 725 base pairs | ||
| Conditions: 96°C – 1min | ||
| 60°C – 45s | ||
| 72°C – 1min | A3 REVERSE | GGACGATTATCAGGAGGCCTGC |
| 30 cycles | ||
| NY-ESO-1 | NY-ESO-1 FORWARD | CCCCACCGCCTCCCGTG |
| Length 307 base pairs | ||
| Conditions: 96°C – 1min | NY-ESO-1 REVERSE | CTGGCCACTCGTGCTGGGA |
| 67°C – 45s | ||
| 72°C – 1min | ||
| 30 cycles | ||
Standardisation was carried out using various concentrations of primer and alignment temperatures, and was performed with testicular tissue, and the K562 cell line, which are the positive controls. The same amplification was corroborated in healthy donors where there was no amplification of genes.
Ethical aspectsThis study complies with institutional ethical standards and the General Health Law on human experimentation, as well as the Declaration of Helsinki, with amendment by the General Assembly of Tokyo, Japan in 1983.
Statistical analysisStatistical analysis was performed using the SPSS software, version 2.0. Initially, descriptive statistics were used to establish means, medians and ranges. The Pearson correlation coefficient was established between qualitative variables (lactate dehydrogenase and RQ-PCR for MAGE-A3 and NY-ESO-1, etc.). The chi-square test was used for the hypothesis test between the MAGE-A3 and NY-ESO-1 expression and was considered significant at or below 0.05 (95% CI).
ResultsA total of 24 patients were analysed, with male patients predominating at 66%, with an average age of 28 years. The predominant histological strain was the mixed cell type in 66%, followed by nodular sclerosis in 29.1%, and 4% rich in lymphocytes.
The diagnosis of classical Hodgkin's lymphoma requires the identification of Reed-Sternberg cells in the appropriate cellular environment and with their own immunohistochemical characteristics. Reed-Sternberg cells are positive for CD30 and CD15, negative for CD45 and EMA, and sometimes positive for B markers. What is expected in terms of positive expression is a predominance of CD30 against CD15. The presence of CD15, CD30 and CD20 was evaluated in our immunohistochemical study. CD30 was the most frequent in 71% of patients, followed by CD15, with CD20 positive in one third of patients. The presence of the Epstein Bar Virus (EBV) corresponded to 50% of patients.
With regard to clinical stages, 66% were in advanced stages (III and IV). With the Hasenclever prognostic index, we evaluated variables with an adverse prognostic impact. We divided the scores into two groups: the 0–2 group had a 5-year progression-free survival rate greater than 67%, while the 3–5 group had a survival rate of less than 60% at 5 years. We found that 33% will have an adverse prognosis with a calculated survival rate of less than 60%. LDH numbers are directly related to tumour size. Figures greater than 250mg/dl represent an adverse manifestation in terms of prognosis and follow-up, being a risk factor for relapse. The majority of our patients, 54%, presented figures greater than 250mg/dl.
The ABVD chemotherapy regimen was used as first line treatment in 87% of patients. Only one patient received an initial regimen with MOPP and 2 patients did not initiate any treatment regimen due to their clinical conditions. Fifty percent received radiation therapy. Fifty percent showed a complete response to the first line of treatment, evaluated with PET CT at the end of treatment, recording a relapse percentage of 27%.
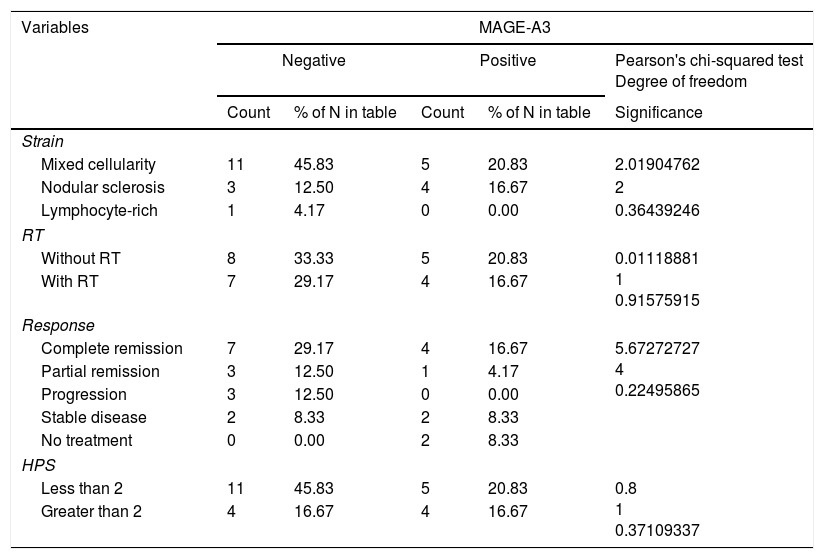
The expression of MAGE-A3 was 37.5% in our patients. In relation to NY-ESO-1 in the analysis of testis antigens by PCR, no expression was found in any patient for this gene. When the MAGE-A3 expression was correlated, the main clinical variables were: response to treatment (p=0.224, 95% CI), Hasenclever prognosis score (>2 or <2) (p=0.371, 95% CI), clinical stage (p=0.689, 95% CI) and relapse (p=0.807, 95% CI). No statistically significant correlations were found. Sixty-six percent of the MAGE-A3 positive cases were positive for Epstein Bar and 44% also presented high LDH figures, as shown in Table 2.
Correlation of prognostic variables with MAGE-A3 in patients with Hodgkin's lymphoma.
| Variables | MAGE-A3 | ||||
|---|---|---|---|---|---|
| Negative | Positive | Pearson's chi-squared test Degree of freedom | |||
| Count | % of N in table | Count | % of N in table | Significance | |
| Strain | |||||
| Mixed cellularity | 11 | 45.83 | 5 | 20.83 | 2.01904762 |
| Nodular sclerosis | 3 | 12.50 | 4 | 16.67 | 2 |
| Lymphocyte-rich | 1 | 4.17 | 0 | 0.00 | 0.36439246 |
| RT | |||||
| Without RT | 8 | 33.33 | 5 | 20.83 | 0.01118881 1 0.91575915 |
| With RT | 7 | 29.17 | 4 | 16.67 | |
| Response | |||||
| Complete remission | 7 | 29.17 | 4 | 16.67 | 5.67272727 4 0.22495865 |
| Partial remission | 3 | 12.50 | 1 | 4.17 | |
| Progression | 3 | 12.50 | 0 | 0.00 | |
| Stable disease | 2 | 8.33 | 2 | 8.33 | |
| No treatment | 0 | 0.00 | 2 | 8.33 | |
| HPS | |||||
| Less than 2 | 11 | 45.83 | 5 | 20.83 | 0.8 1 0.37109337 |
| Greater than 2 | 4 | 16.67 | 4 | 16.67 | |
Of all patients, 12% (3 patients) died in the follow-up period. The majority of these were also positive for MAGE-A3 (p=0.264, 95% CI), although it was not statistically significant, most likely due to the small number of patients.
Discussion of the resultsThe American Cancer Society has estimated 8500 new cases of Hodgkin's lymphoma. As of 2004, there were 935 new cases in Mexico with a higher incidence in males. In terms of descriptive statistics, we could observe some characteristics of our study group associated with an adverse prognosis, among which we mentioned LDH levels above 250mg/dl (the higher value, the larger the tumour size), absence of the CD15 marker and advanced clinical stage at diagnosis (clinical stages III and IV), with a calculated survival rate of less than 60% at 5 years according to the international prognostic index (Hasenclever index).20
Most of the patients received the ABVD regimen (the current standard treatment regimen) as first-line treatment, and half of them also received radiation therapy. The final response to treatment was complete in 50% of cases. This figure is far from what we expected, if we consider the ideal uptake target (80%) of these patients in a localised stage (stages I–II) for a survival rate greater than 92%.21,25
In the United States and Europe, the ratio of Hodgkin's lymphoma to the Epstein Barr virus is 40%. What is reported in Mexico does not differ greatly from international reports.21 The predominant strain is nodular sclerosis in more than 60% of cases. Previous reports in reference centres in Mexico match our data. It should be noted that in 2011, mixed cellularity was reported by the National Cancer Institute (INCAN) and the Salvador Zubirán National Institute of Medical Science and Nutrition (ICMNNSZ) as the most frequently found strain.19
MAGE-A3 expression was found in 37.5% of our patients, which was higher than that reported in international studies, such as the 2011 study by Dr Riguel J Inaoka at the Department of Pathology of the Federal University of Sao Paulo; in 38 patients with Hodgkin's lymphoma a lower rate of expression than ours was reported, at 20%. It should be noted that in their cases they found greater positivity in advanced stages compared to the initial stages of the disease, coinciding with our study.23
In relation to NY-ESO-1 in the analysis of testis antigens by PCR, the fact that its expression was not found may be related to the fact that it is more of an intracellular marker. Therefore, its identification is much more probable in solid tumours such as oesophageal, liposarcoma and others.23
The estimated mortality of Hodgkin's Lymphoma in the United States is 1300 cases each year. The majority of patients diagnosed with Hodgkin's lymphoma are expected to have disease-free survival exceeding 80% at 5 years of follow-up. The one-year survival rate for all patients diagnosed with Hodgkin's disease reaches 92%, with 5-year and 10-year survival rates being around 86% and 80%, respectively, and the mortality rate in our study does not differ from what is expected at the international level and is close to the national level. In a study conducted at the INCMNSZ, in 156 patients studied a total of 15 fatal events were reported, with complete remission in 61.6% of patients.25
No significant correlation was found between MAGE-A3 expression and the major clinical prognostic variables. There was a trend associating the expression of MAGE-A3 cancer testis antigens with mortality, advanced stage and relapse, but the association was not statistically significant. Therefore, we could not use it as a prognostic tool in our patients with Hodgkin's lymphoma. The ideal prognostic marker in cancer is one that allows us to ask questions such as: Can the patient achieve cancer remission or not? What is the best treatment? What was the treatment response? Could the patient have a relapse after having achieved a complete response and can this be anticipated before clinical signs become evident? Therefore, an ideal marker must be highly specific for a particular tumour, it must allow the detection of cancer even with hidden disease, i.e. it should be present even in the early stages, and lastly, it must be very sensitive to avoid false negatives. In our study, MAGE-A3 and MY-ESO-1 did not fully comply with these characteristics.
ConclusionsThe expression of cancer testis antigens (MAGE-A3) was 37.5% in peripheral blood in patients with Hodgkin's lymphoma. No statistically significant relationship was found between MAGE-A3 and clinical prognostic parameters in patients with Hodgkin's lymphoma. It is not possible to use this tumour marker as a prognostic tool in peripheral blood. We consider it important to study the expression of MAGE-A3 antigens in our population with malignant neoplasms, in particular Hodgkin's lymphoma. We recommend continuing with this line of research and opening the doors to new studies that increase the number of patients and prolong the follow-up periods.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis study was conducted with the support of the Army of Nicaragua, the Government of Mexico through the fellowship of excellence granted through the Secretary of Foreign Affairs, and the support of CONACYT with project number 162269, as well as the Research Directorate of the General Hospital of Mexico with registration numbers DIC/09/04/03/131, DIC/08/204/04/017, DIC/12/204/05/01.
Conflict of interestThe authors declare that they have no conflict of interests.