Lymphoedema is a pathological condition with a low global incidence where protein-rich fluid accumulates in the interstitium, causing inflammation and degenerative changes in the affected limbs. It is divided into primary and secondary; worldwide the most common causes are cancer or its treatment and filariasis infection. It is diagnosed through the patient's medical history and treatment depends on the severity.
Case reportTwenty-one-year-old male with the condition for 8 years, with a gradual increase in volume in the right lower limb, seen in the emergency department due to functional disability; en bloc excision plus split-thickness grafting was performed, fixed with sutures and ABThera, with 85% integration and functional improvement.
ConclusionsLymphoedema has a high socio-economic impact. Its treatment involves several specialists and must be multidisciplinary, who then find the cause and propose management according to the stage. Combined medical and surgical therapy is the best choice for advanced stages.
El linfedema es una condición patológica de baja incidencia mundial, que acumula líquido rico en proteínas en el intersticio, provocando inflamación y cambios degenerativos en las extremidades afectas. Se divide en primario y secundario, la causa global más frecuente es el cáncer o su tratamiento y la infección por filarias. El diagnostico se realiza a través de historia clínica y el tratamiento depende del grado de severidad.
Caso clínicoMasculino de 21 años con padecimiento de 8 años, con aumento progresivo de volumen en extremidad inferior derecha, acude a urgencias por incapacidad funcional; se realizó escisión en bloque más aplicación de injertos de espesor parcial, fijados con suturas y Abthera, con integración de 85% y mejoría funcional.
ConclusionesEl linfedema tiene alto impacto socioeconómico, su tratamiento involucra diversos especialistas, debe ser multidisciplinario, encontrando la causa y según estadio proponer manejo. La terapia médica y quirúrgica en conjunto es la mejor opción para estadios avanzados.
Lymphoedema is a pathological condition in which protein-rich fluid accumulates due to a locoregional dysfunction of the lymphatic vessels.1
Estimating the precise global incidence of this condition is difficult due to unreported and poorly diagnosed cases. However, it known that cancer and its treatment is the most common cause worldwide, followed by filariasis (Wuchereria bancrofti),2 common in developing countries or those with a tropical climate. It is more common in men than in women, with a 2:1 ratio, and most cases are not associated with genetic conditions.3 Several risk factors for developing this disease, which precipitate its clinical expression, have been identified.
It is divided into two groups: primary lymphoedema and secondary lymphoedema. Primary lymphoedema is when it is caused by a malformation of the lymph nodes and vessels with no precipitating cause, which in turn is further divided by the age of onset: congenital4 which has an onset from birth to up to two years of age, shows mutations in the VEGF gene5 and has a frequency that varies between 77% and 94%; praecox or Meige's syndrome, with an onset at puberty or pregnancy before 35 years of age, shows mutations in the FOXC2 gene,6 and has a frequency that varies between 6% and 12%; and tarda which has an onset after 35 years of age and an approximate frequency of 11%. All present more commonly in the lower limbs. Secondary lymphoedema results from diseases in which the natural history or their treatment deteriorates the lymph nodes and vessels. In this group the most common cause is breast cancer, sarcoma, melanoma, or malignant gynaecological tumours or their treatment (lymphadenectomy or radiotherapy)7; other causes include filariasis which affects 90 million people worldwide, recurrent cellulitis and cutaneous tuberculosis, rheumatoid arthritis, sarcoidosis, obesity (BMI>30), and severe trauma.8
The clinical presentation has an insidious onset in cases of primary lymphoedema with no observable triggering cause, starting with soft, distal oedema with a slow progression over the course of years.9 It is important in patients with soft, medical treatment – resistant oedema with no apparent cause to take a full medical history including age at onset and duration of disease, area involved, associated symptoms, personal history of chronic disease (diabetes, hypertension, prior history of cancer and its treatment), recent trips, history of genetic diseases, and history of infections.
The oedema progresses distally and starts to present fat and fibroblast infiltration, which cause irreversible skin hardening. Deformity occurs as well as progressive limitations to mobility in the limb which can affect daily activities. In addition, skin changes such as keratosis and papillomatosis occur and, when the degree of severity is higher, recurrent events such as cellulitis, lymphangitis, and skin breakdown start. In advanced stages, lymphoedema complications such as lymphosarcoma, Kaposi's sarcoma, and lymphoma have rarely been reported.10
There are different classifications of lymphoedema depending on the clinical characteristics (oedema is reversible by elevating the limbs and circumference). The International Society of Lymphoma classifies lymphoedema into four stages from 0–III according to the skin characteristics and reversibility by elevating the limb (Table 1)11. This system is currently the most widely used.
Classification of the International Society of Lymphology.
| Stage | Features |
|---|---|
| 0 | Subclinical condition, no oedema evident. May be present for years before becoming clinical significant. |
| I | Accumulation of protein-rich fluid, reversed by elevating the limb for 24h. No evidence of dermal fibrosis. |
| II | Accumulation of protein-rich fluid that does not resolve by elevating the limbs. There is dermal fibrosis. |
| III | Elephantiasis, skin with trophic changes such as thickening and fibrosed fatty tissue deposits. |
In most cases, a lymphoedema diagnosis is established based on the medical history and physical examination (Stemmer's sign is characteristic; it consists of the inability to form a skin fold after pinching near the first phalangeal joint). However, in some patients in whom the diagnosis is in question, it becomes necessary to use additional tests.12 A Doppler ultrasound should be performed on the limb for diagnostic certainty to check that the deep venous system is working properly. The gold standard for diagnosis in the initial stages of the disease, or if the diagnosis is in question, is lymphoscintigraphy, which shows the flow of nuclear material through the lymph system identifying any defects and their degree. However, its availability is limited to centres with nuclear medicine and by its high cost.13
Computed tomography offers 97% sensitivity and 100% specificity, and therefore it is recommended to use it only in cases where the diagnosis is in question or to establish secondary causes. However, nuclear magnetic resonance (NMR) imaging enables a more detailed image, showing the characteristic honeycomb pattern. Nevertheless, its use is also limited to cases in which the aetiology is uncertain or there is a high likelihood of doubt.14
Once the diagnosis is established, the next step is to classify the disease and then to stage its severity according to the International Society of Lymphology, in order to offer treatment alternatives. Currently medical and surgical treatments are available; medical treatment consists of specialised compression garments, pneumatic compression, and a combination of measures called decongestive lymphatic therapy. This therapy consists of simultaneously applying skin hygiene measures, compression of the affected limb, and decongestive exercises. Despite obtaining good results in studies (mean decreased limb volume of 40–60%), this treatment involves a large number of healthcare personnel, which increases its cost and reduces its availability. Despite its safety and excellent results, it is limited to lymphoedema stages 0 and I.15
Surgical treatment is reserved for advances stages or for those that do not respond to conservative treatment; the cause must be established before considering surgical treatment. The indications for surgical treatment for both primary and secondary lymphoedema are localised primary lesions, lack of medical management, repeated cellulitis events, limited function and self-care, deformity of the affected limb, pain, and decreased quality of life with psychosocial impact.16 The objective of surgical treatment is to alleviate pain, improve quality of life, restore function and self-sufficiency, reduce skin infections, prevent or avoid disease progression, improve cosmetic appearance, and limit deformity, since lymphoedema causes both aesthetic and functional sequelae that limit the function of the affected limbs and has a psychosocial impact because of the deformity it causes.17 The surgical techniques used to treat lymphoedema are classified by their mechanism of resolution into physiological and debulking methods. Physiological techniques are recommended in stages I and II, reserving debulking techniques to those cases in which the skin deformity is significant or to stages III and IV of the disease.18 Once the cause of the lymphoedema is established, there are two considerations to take into account: the first is to measure the excess volume in the affected limb, stage the severity of degree of the disease, and perform a Doppler ultrasound which confirms the health of the venous system.
Currently the objective of physiological techniques is based on creating new lymphatic vessels or creating lymphatico-lymphatic or lymphatico-venous connections using bypass surgery, transposition flaps, or lymph node transfers to the affected sites. The most widely used technique is creating a bypass. This is contraindicated in cases with fibrotic tissue, chronic lymphoedema changes, venous hypertension, recurrent cancer, and lack of trained personnel. There are several types of bypass surgeries including lymphatico-lymphatic, lymphatico-venous, lymphatico-venular, and healthy lymph node transfer.19
In the case of debulking or palliative procedures (removing fibroadipose tissue with or without skin), the intent is not to cure the patient, but rather to decrease pain and improve functionality. There are essentially two techniques, en bloc excision with skin, and liposuction with skin conservation. En block excision is reserved for stages III and IV which present skin deterioration. The disadvantages of the procedure are the volume of bleeding, higher percentage of infections, and the need for additional surgical procedures (need for full- or split-thickness skin grafts, depending on the case). Liposuction has specific contraindications such as the presence of lymphosarcoma, skin fibromas, and skin breakdown. In the palliative techniques category, no technique is superior to the other procedures as each one must be used based on patient characteristics.20 Below we present a case report of a patient diagnosed with severe primary lymphoedema praecox, treated using en bloc excision plus meshed split-thickness grafts in whom the use of VAC therapy (vacuum-assisted closure)-ABThera (due to lack of a conventional system) showed advantages for integrating the grafts over the use of compression bandages.
Case reportTwenty-one-year-old male, originally from and residing in Sierra Oaxaqueña, farmer, illiterate. In terms of family history, the patient reports that in his community (comprised only of family members of differing degrees) there are at least four family members with unilateral oedema of the lower limbs; he reports no other relevant history.
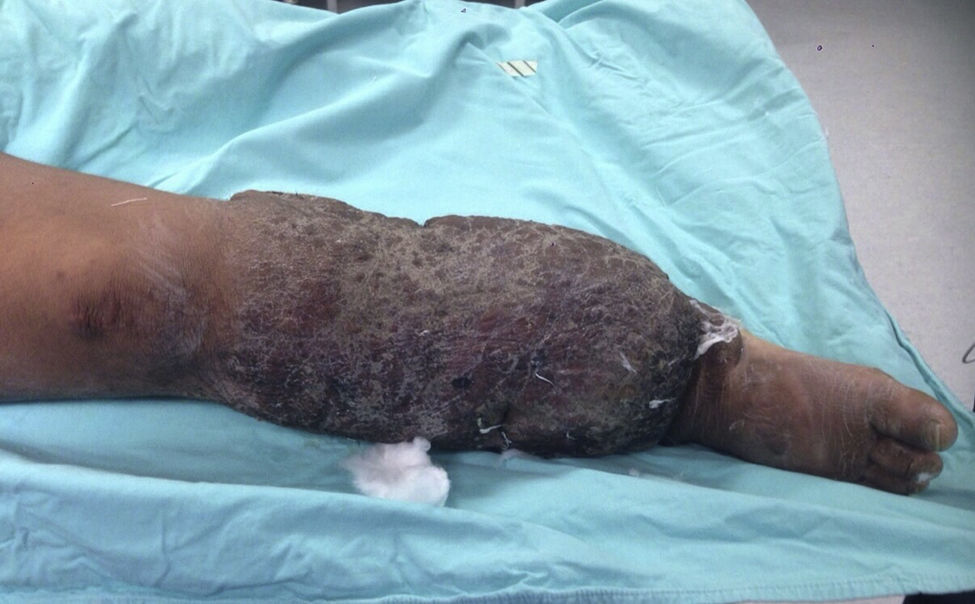
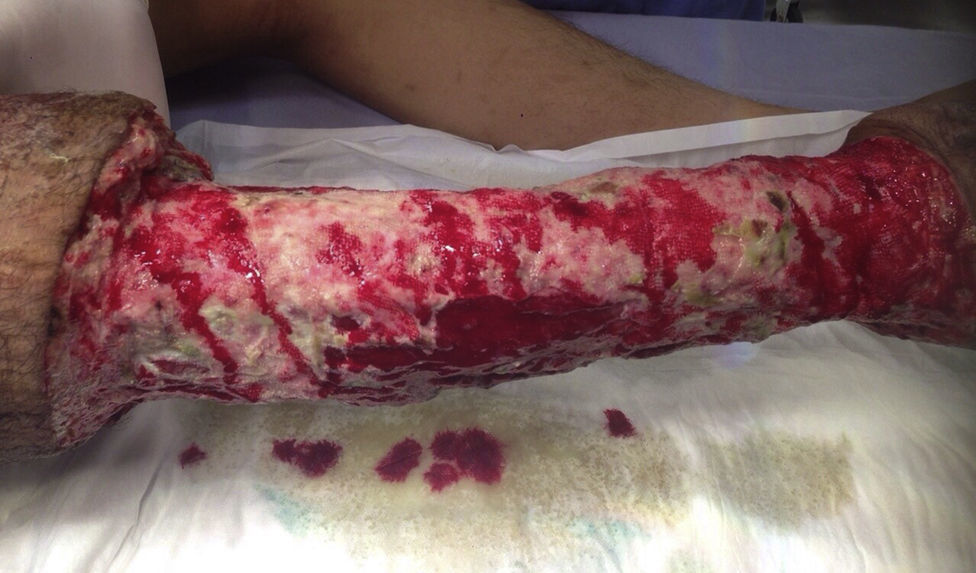
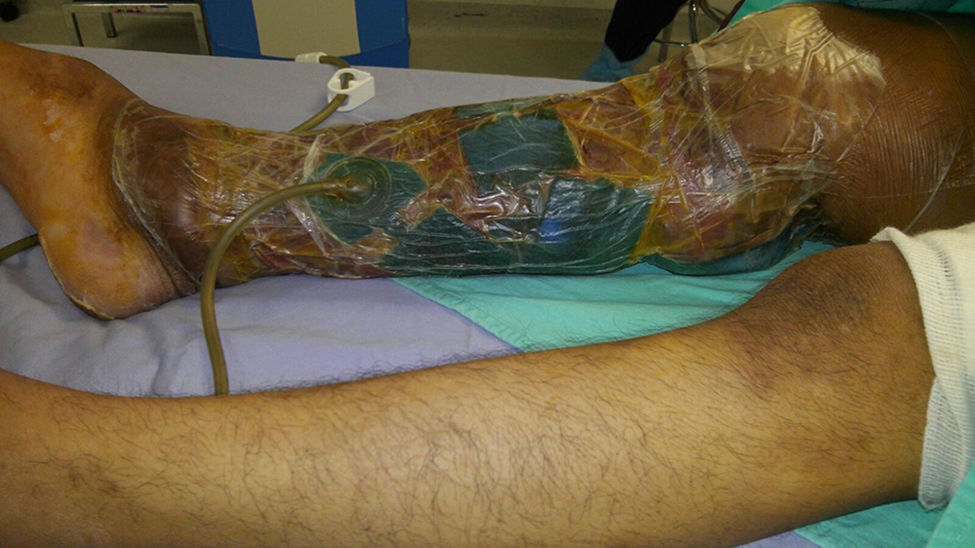
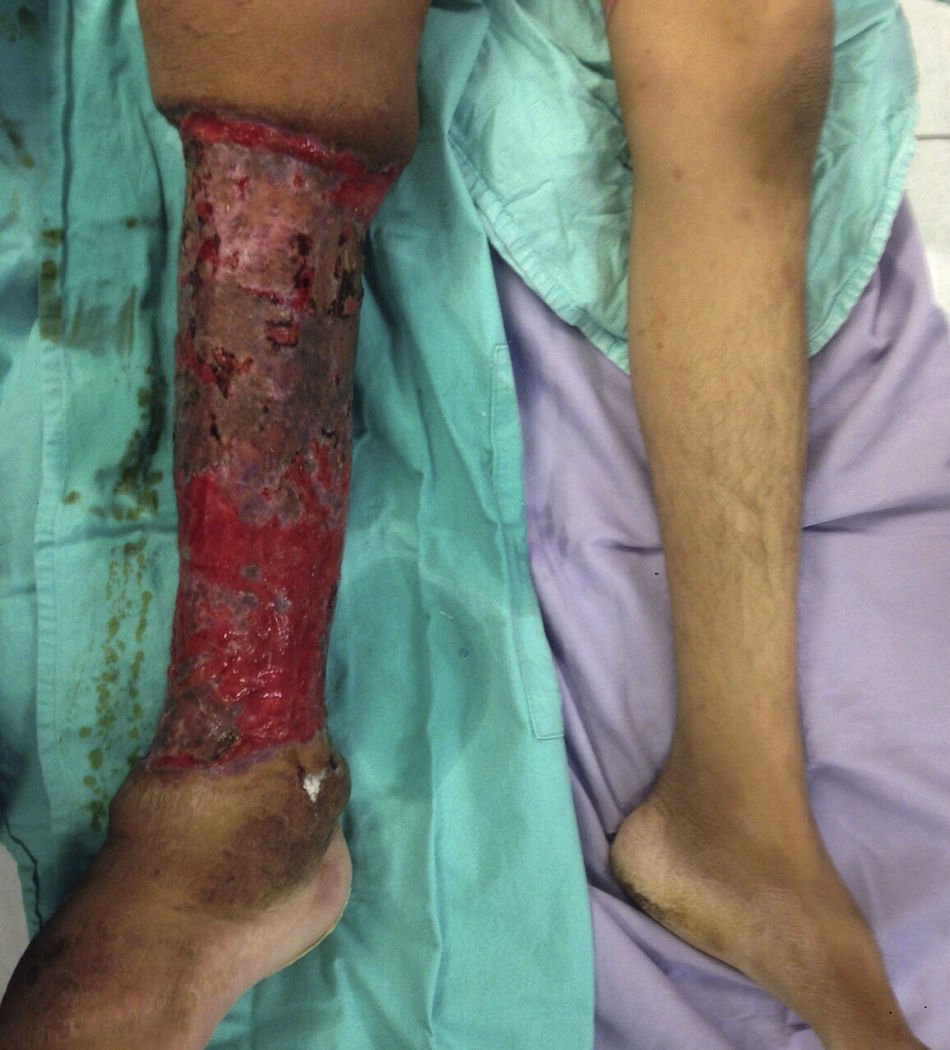
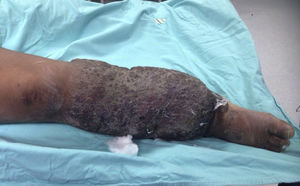
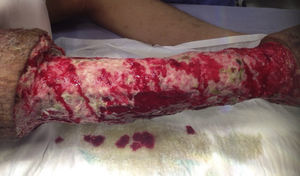
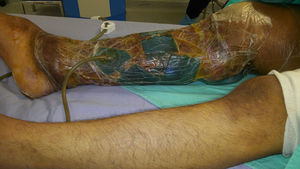
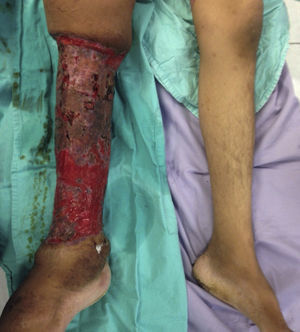
The patient made an unplanned visit to the Accident and Emergency (A&E) Department where he reported that his condition had started 8 years ago with a unilateral increase in the volume of his right left, starting in the malleolar zone and progressing towards the thigh area. The limb reached functional impairment two years ago (due to excessive increase in volume and weight). One week before going to A&E there was an onset of skin breakdown in the posterior region of the leg with discharge of seropurulent material and progressive, insidious-onset pain (VAS: 9 out of 10), which was why he went to A&E. The physical examination showed an increase in the volume of the right leg through the thigh area, with skin ulcers and clear limitations to mobility (Fig. 1). He was assessed by the Department in Infectious Diseases, which ordered a biopsy of the affected tissue and cultures, Doppler ultrasound, and W. bancrofti antigen tests. The cultures and antigen tests were negative. The histopathological report showed chronic dermatitis with lymphatic perivasculitis, and the Doppler ultrasound showed an intact venous system. Afterwards he was assessed by the Department of Cosmetic Surgery, which, with the previous tests and assessments, established a diagnosis of severe or grade III primary lymphoedema praecox. The patient underwent tissue excision on the affected limb with bandages (Fig. 2), withdrawn 5 days later. In a second surgery, meshed split-thickness skin grafts were taken and applied to the en bloc excision area, attached with Monocryl sutures, and covered with a VAC system. It was decided to use this system because the receiving surface presented abundant secretion, and ABThera was used because there was no conventional VAC system (Fig. 3). This system was withdrawn 5 days after it was placed, and 85% graft integration was observed. Therefore, the patient was able to be discharged in a relatively short period, decreasing the number of days in hospital, the number of surgeries, cost, and promoting early reintegration of daily activities (Fig. 4). The patient was sent to physical rehabilitation and after-care measures were given for the limb, with a 30-day follow-up with adequate progress. However, the patient did not come to subsequent follow-up appointments for routine monitoring.
DiscussionLymphoedema is a disease with complex pathophysiological pathways that trigger the accumulation of lymphatic (protein-rich) fluid and this accumulation gradually causes deformity and decreased functionality of the affected limb. Despite the medical advances in this century, treatment for this condition cannot be all medical or surgical since the success rates for medical treatment decrease as the disease advances in severity, requiring surgical intervention in advanced stages to improve functionality and aesthetics. In studies conducted in the United States, it was observed that using bypass techniques in lymphoedema (11 studies including 131 patients) demonstrated a 3.31% reduction in diameter. These studies included patients with upper and lower limb lymphoedema.21 In the upper limb group (6 studies with 79 patients), there was a 2.73% reduction22 and in the lower limb group (5 studies with 52 patients), a 3.52% reduction.23 Of the physiological bypass procedures, the one that was demonstrated to be superior was the lymphatico-venous bypass, with a post-treatment reduction of 48.9% in circumference. However, despite high success rates, if after-care measures are not started on the limb (compression bandage, limb elevation, and reductive massages), within 3 years the lymphoedema returns with the same severity as was previously present. For this reason, physiological techniques must always be accompanied by medical after-care to achieve true long-term success.24 Similarly, debulking and palliative techniques (en bloc excision and liposuction) are procedures that are used in cases of severe limb deformity or full functional incapacity. However, in the case of liposuction if a compressive bandage and skin care are not started, the recurrence rates are very high with a need for later en bloc excision.25 And when it is decided to use en bloc excision, a new intervention is always needed to place skin grafts that cover the area where the skin and subcutaneous tissue were removed.26 Proper immobilisation after the grafts are placed is very important, since excessive movement or elevated secretion from the receiving bed may cause the loss of the skin grafts27; several tools exist to prevent them from moving and to improve the integration percentage, including the VAC system,28 compression bandages, or compression meshes. In our case we used an ABThera system (as at the time of surgery we did not have an adequate VAC system) which contains a plastic cover over sponges. This was helpful for use since upon removing it to verify the integration percentage, we had 85% integration without needing to take and apply a new graft. This decreased costs overall by decreasing the number of surgeries and the length of the hospital stay. Both compression bandages and a conventional VAC system have been used in reported cases with lower graft integration rates due to the propensity for loss by removing the VAC sponges or due to an unsuitable compression bandage. Therefore, using ABThera may be advantageous, by possessing sponges and negative pressure systems with a plastic cover which prevents the grafts from detaching when removing the system.29
ConclusionsLymphoedema is a pathology that involves a large number of healthcare personnel to treat different periods of the disease's natural course, including plastic surgeons, vascular surgeons, general surgeons, infectious disease specialists, pathologists, nurses, physiotherapists, etc. Therefore, it has a high economic and social impact. Combining both medical and surgical treatment has been demonstrated to improve the long-term volume of the limb, its cosmetic appearance, and functionality. Negative-pressure systems are useful for treating this conditions, since they decrease bacterial load, interface between the graft-receiving bed, and immobilise it, which promotes better integration.
Ethical disclosureRight to privacy and informed consentThe authors declare that no patient data appear in this article.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Conflict of interestThe authors declare that they have no conflict of interests.