Haematologic malignancies are generated by alterations in haematopoietic stem cells. Chromosomal rearrangements are present in >50% of patients and are useful as diagnostic and prognostic factors.
ObjectiveIn this study we describe the cytogenetic characteristics observed in patients with haematological malignancies in the Genetics Department during the period 2000–2014.
Material and MethodsThe karyotype was performed on bone marrow (85%) and peripheral blood (15%) with conventional techniques in 9717 samples.
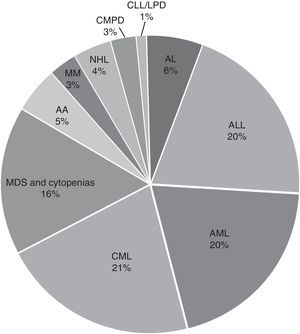
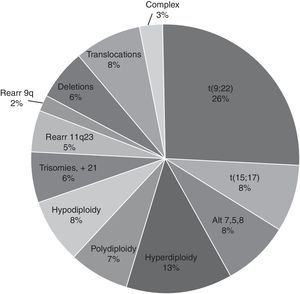
ResultsThe average age was 40 years (range 0.3–95) and the male/female distribution was 50.5%/49.5%. 352 cases (3.6%) were paediatric with a male/female distribution of 59/41%. The diagnosis was: acute leukaemia 4445 (45.7%), CML 2058 (20.4%), and MDS or some form of cytopenia 1573 (16%). Fewer than 5% of samples received were from AA, MM, CMPD, NHL, CLL, LPD and others. The distribution of acute leukaemia was: ALL 44%, AML 43% and unspecified 13%; the predominant subtypes were ALL-L2 at 50.7% and AML-M3 at 54.2%. Only 61% of the 9717 samples were processed. The karyotype was normal in 3956 (66.7%) samples, the rest (1972, 33.3%) had chromosomal abnormalities: 65% structural and 35% numerical. The changes observed most frequently were t(9;22)(q34;q11) 26%, hyperdiploidy/polyploidy 19.3%, diverse translocations 8.4%, hypodiploidy 8%, t(15;17)(q22;q12) 7.8%, and MDS-related disorders (del5q/-5/-7/+8) 7.7%. Different deletions, trisomy, monosomy and/or complex karyotype were present in smaller proportion (<7%).
ConclusionsThe karyotype remains useful to confirm the diagnoses, establish risk-based prognoses, and classify based on risk to patients; for example in cases with t(9;22) in CML or t(15;17) in M3.
Las neoplasias hematológicas son generadas por alteraciones en células progenitoras hematopoyéticas. Presentan rearreglos cromosómicos en >50% de los pacientes de utilidad como factores de diagnóstico y pronóstico.
ObjetivoDescribir las características citogenéticas observadas en los pacientes con enfermedades hematológicas recibidos en el Servicio de Genética durante el período 2000–2014.
Material y MétodosEl cariotipo se realizó en médula ósea (85%) y sangre periférica (15%) con técnicas convencionales en 9,717 muestras.
ResultadosLa edad promedio fue 40 años (rango 0.3 a 95) y la distribución masculino/femenino 50.5/49.5%. Se estudiaron 352 casos (3.6%) pediátricos con una distribución masculino/femenino 59/41%. Por tipo de patología la distribución fue leucemias agudas, 4445 (45.7%), LMC 2058 (20.4%) y SMD o alguna citopenia 1573 (16%). Se recibieron <5% de muestras de AA, MM, SMPC, LNH, LLC, SLPC y otros. La distribución de las leucemias agudas fue: 44% LAL, 43% LAM y 13% sin especificar; los subtipos predominantes fueron LAL-L2 50.7% y LAM-M3 54.2%. Sólo 61% de las 9,717 muestras procesadas fueron factibles para el estudio. El cariotipo fue normal en 3,956 (66.7%) muestras, el resto (1,972; 33.3%) presentó alteraciones cromosómicas: 65% estructurales y 35% numéricas. Las alteraciones observadas con mayor frecuencia fueron la t(9;22)(q34;q11) 26%, hiperdploidía/poliploidía 19.3%, translocaciones variadas 8.4%, hipodiploidía 8%; t(15;17)(q22;q12) 7.8%; alteraciones relacionadas con SMD (del5q/-5/-7/+8) 7.7%. En menor proporción (<7%) se observaron diferentes deleciones, trisomías, monosomías y/o cariotipo complejo.
ConclusionesEl cariotipo sigue siendo de utilidad para confirmar diagnósticos como en los casos con t(9;22) en LMC o t(15;17) en M3 siendo muy útil como auxiliar para establecer pronósticos y clasificar con base en el riesgo a los pacientes.
Haematologic malignancies are generated by alterations in haematopoietic stem cells and include leukaemias, lymphomas, myelomas, aplastic anaemia (AA), myelodysplastic syndromes (MDS) and chronic myeloproliferative disorders (CMPD). In general, lymphomas are classified by the type of cell of origin, while myeloid (AML/CML) or lymphoid (AML/CLL) leukaemias are either acute or chronic. Myelodysplastic syndromes (MDS) are a group of disorders characterised by one or more peripheral blood cytopenias, secondary to bone marrow dysfunction. Generally, CMPDs present a greater amount of mature myeloid cells in peripheral blood and include syndromes that share characteristics of MDS/CMPD and atypical chronic myeloid leukaemia (CML). The latter are diseases of adults with a frequent peak in the fifth and sixth decade of life; their global incidence is about 6–9 per 100,000 inhabitants. Globally, leukaemias and lymphomas are the most frequent haematologic malignancies, representing 2.8% of new cases of cancer. In Mexico, the Population-based Cancer Registry places haematologic malignancies in the first five places.1,2
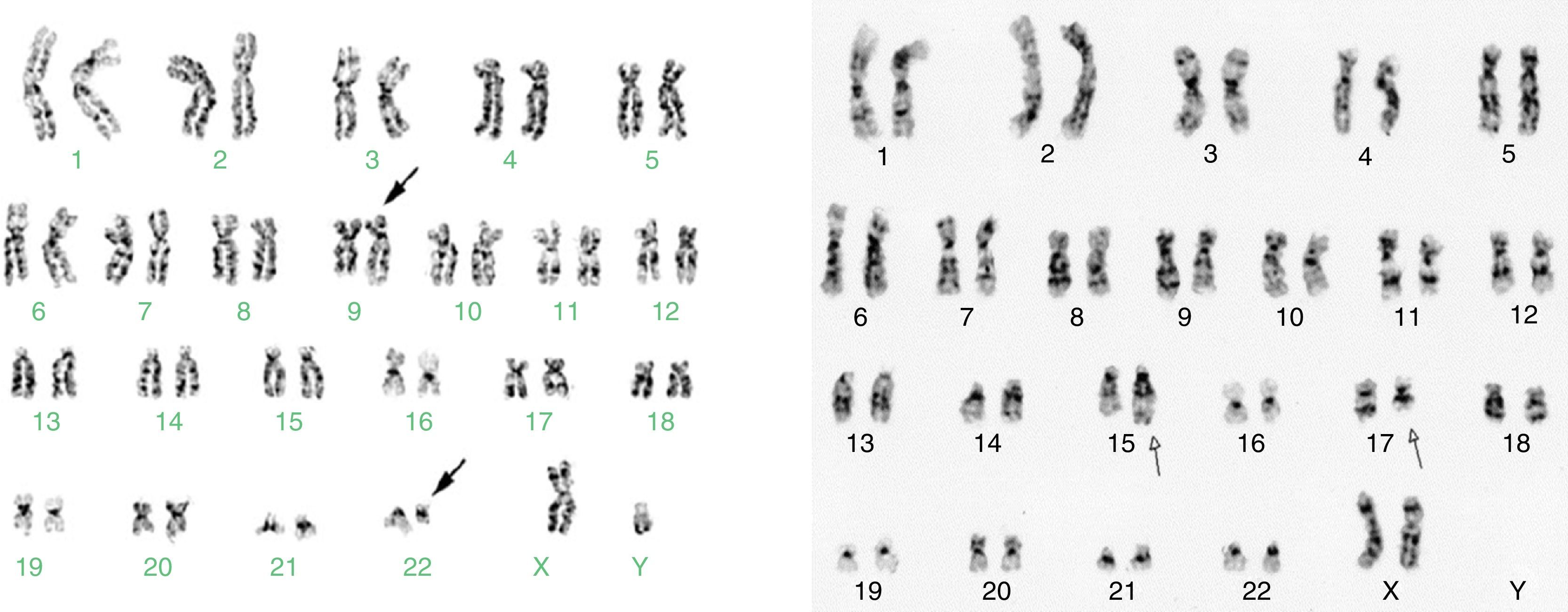
Cytogenetic study in malignant and benign haemopathies is important for the characterisation of the disease, as it contributes to diagnosis and is a well-defined prognostic factor. In more than 50% of haematological malignancies, clonal chromosomal alterations have been characterised based on number of chromosomes (hyperdiploidy, trisomies or monosomies) or the structure of the chromosomes (translocations, inversions, deletions). The first chromosomal rearrangement described in cancer, and currently the best characterised in patients with CML, is t(9;22)(q34;q11), also known as the Philadelphia chromosome (Ph+). The genes involved in the Ph+ rearrangement are ABL and BCR, which, when fused, cause a BCR/ABL oncoprotein with tyrosine kinase activity. This protein is the target of the specific treatment of CML using tyrosine kinase inhibitors. Cytogenetic study of the Ph+ marker is one way to evaluate the effect of tyrosine kinase inhibitors on the leukaemia clone during follow-up of patients with CML.1,3 In patients with AML-M3 or Acute Promyelocytic Leukaemia (APL), all-trans retinoic acid (ATRA) is another target therapy directed against a fusion gene, PML/RARa, caused by the t(15;17)(q22;q21) translocation. The presence of t(15;17) in patients with clinical suspicion of M3/APL is a diagnostic criterion and indicates a candidate for treatment with ATRA.4,5
In ALL, the cytogenetic alterations that present a high risk are t(9;22)(q34;q11), t(4;11)(q21;q23) and hypodiploidy with 30–39 chromosomes (low-hypodiploidy). Complex karyotypes are a risk factor independent of age and white blood cell count. Alterations with a better prognosis in ALL are t(12;21)(p13;q22) translocation, 9p deletions and hyperdiploidy with more than 50 chromosomes. The normal karyotype is reported in 15–45% of all haematological malignancies and is considered to be of intermediate risk. In AML, the low risk alterations are: t(8;21)(q22;q22), t(15;17)(q22;q21), inv(16)(p13q13), rearr(11q23), and high risk alterations are: 3q rearrangements, monosomy 7 and t(1;22)(q10;p10). In MDS the low risk alterations are normal karyotype, del(5q), del(20q) and -Y, and high risk alterations are complex karyotype (>3 alterations) and 7q alterations.5–8
In this report we describe the cytogenetic characteristics observed in patients with haematological malignancies in the Genetics Department during the period 2000–2014.
Patients and methodsPatientsAll karyotype reports registered at the Cytogenetic Laboratory of the General Hospital of Mexico from 2000 to 2014 were included. The patients were referred from the Adult and Paediatric Haematology Departments, as well as from other hospitals and institutions in the country. Patients with diagnoses of acute leukaemia, AML, ALL, MM, MDS, lymphomas, CML, CLL, AA, Fanconi anaemia (FA) and unclassified cytopenias were included. The samples were processed for cytogenetic analysis during the period from January 2000 to December 2014. The study variables included: age, sex, delivery diagnosis, type of sample (bone marrow and/or peripheral blood), study feasibility and karyotype result.
CytogeneticsWe reviewed the karyotype results reported according to the International System for Human Cytogenetic Nomenclature, ISCN.9 We documented the type of sample received for the karyotype, either bone marrow (BM) and/or peripheral blood (PB), the conditions of reception of the sample (diluted, coagulated, haemolysed, etc.), processing and feasibility of the study.
Statistical analysisDescriptive statistics of the demographic variables of interest used measures of central tendency to establish the frequency distribution and the prevalence of chromosomal alterations.
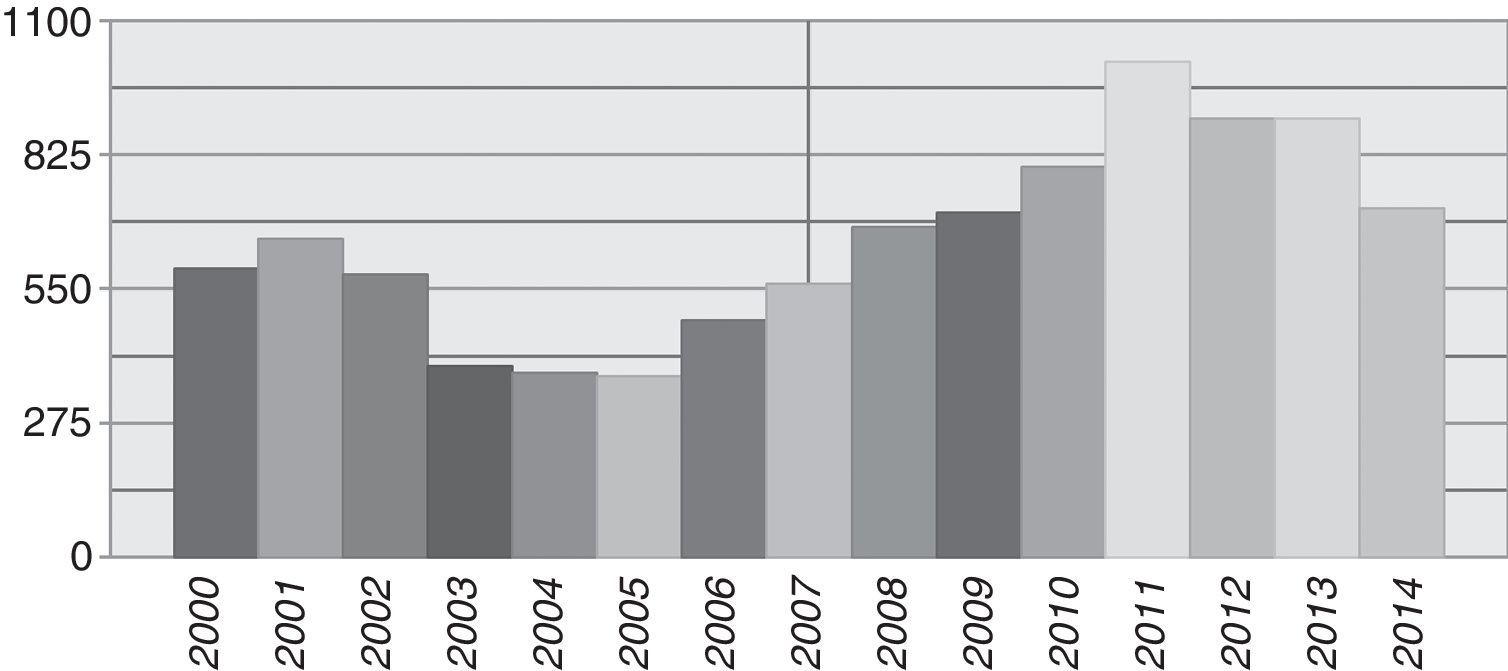
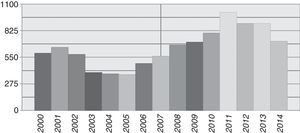
ResultsDuring the period from January 2000 to December 2014, 9717 samples from patients with any type of haematological malignancy were registered at the Cytogenetic Laboratory. The average number of patients received per year was 648 (range 371–1015), with the highest number of samples recorded from 2010 to 2013 (Fig. 1). The average age was 40 years (range 0.3–95) and the male/female distribution was close to equal, at 50.5%/49.5%. A paediatric population was considered to include children under 15 years old; 352 cases (3.6%) were received with a male/female distribution of 59/41%; The average number of children received per year was 23 (range 15–45).
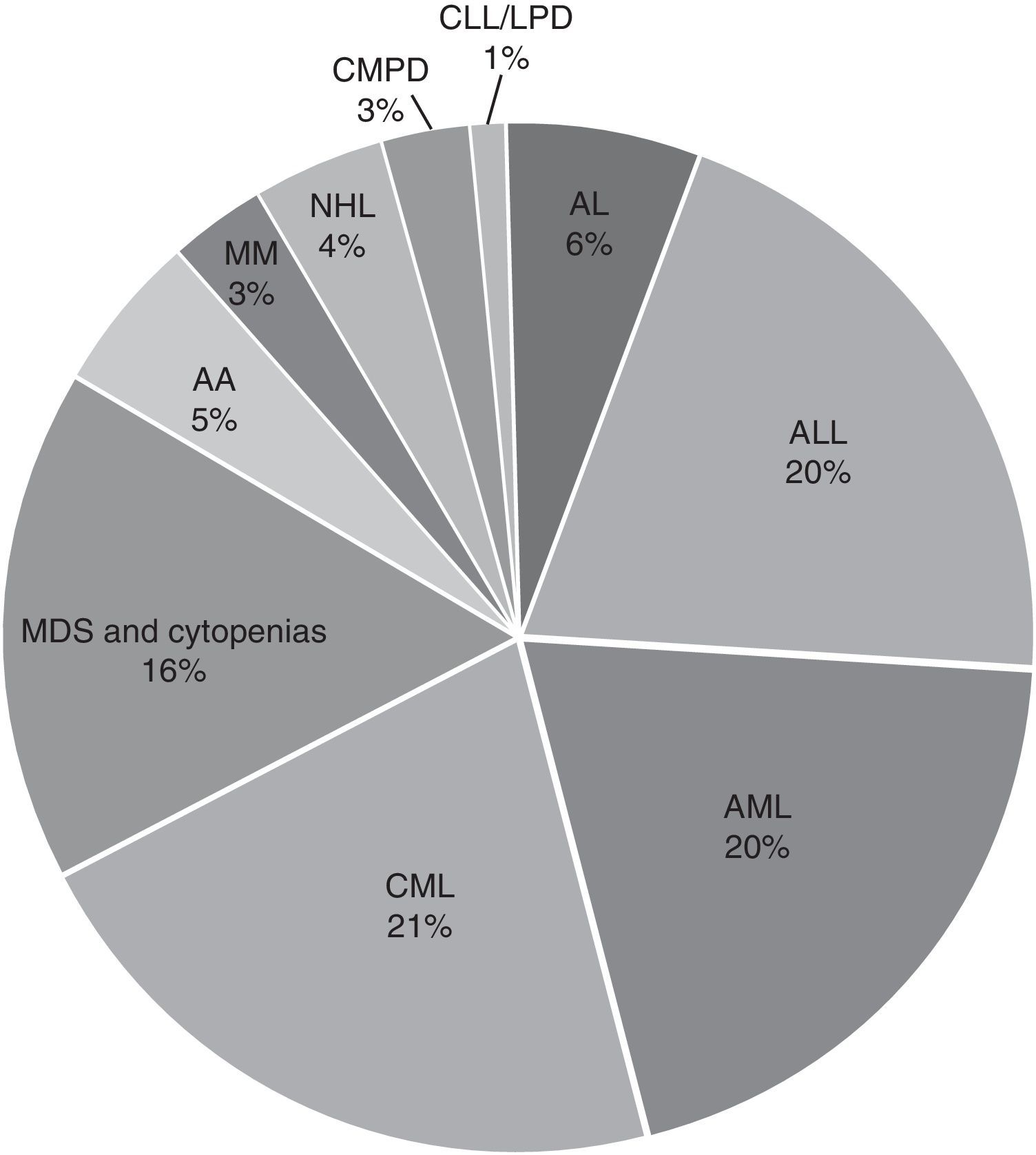
In the general distribution by type of pathology, we found the greatest number of cases for AL, 4445 corresponding to 45.7%, followed by CML with 2058 cases corresponding to 20.4%, and 1573 cases of MDS or any kind of cytopenia corresponding to 16%. Cases with diagnoses of AA, MM, CMPD, NLH, CLL, LPD and others accounted for less than 5% of the cases received. For acute leukaemias, the distribution was 44% ALL, 43% AML and the remaining 13% unspecified as to whether it was lymphoid or myeloid; the predominant subtypes were ALL-L2 at 50.7% and AML-M3 at 54.2% (Fig. 2).
In most cases, the sample was received at the time of diagnosis. Approximately 35% corresponded to follow-up of patients during the evolution of the disease. Of the 2058 cases with CML, 34% corresponded to follow-up samples taken as controls to search for t(9;22)(q34;q11) translocation and to evaluate the treatment effect. In the AML-M3 or LPA subtype as well, approximately 45% of the samples processed were during follow-up to document the disappearance of the clone with t(15;17)(q22;q21).
Acute leukaemia predominated in paediatric patients, with 265 cases (75%) of which 52% were ALL, 12.8% AML, 10.5% without specifying cell line, 14.2% MDS and/or some type of cytopenia, and, of those with <5% incidence, 4.2% CML, 3.7% CMPD and 2.5% NHL.
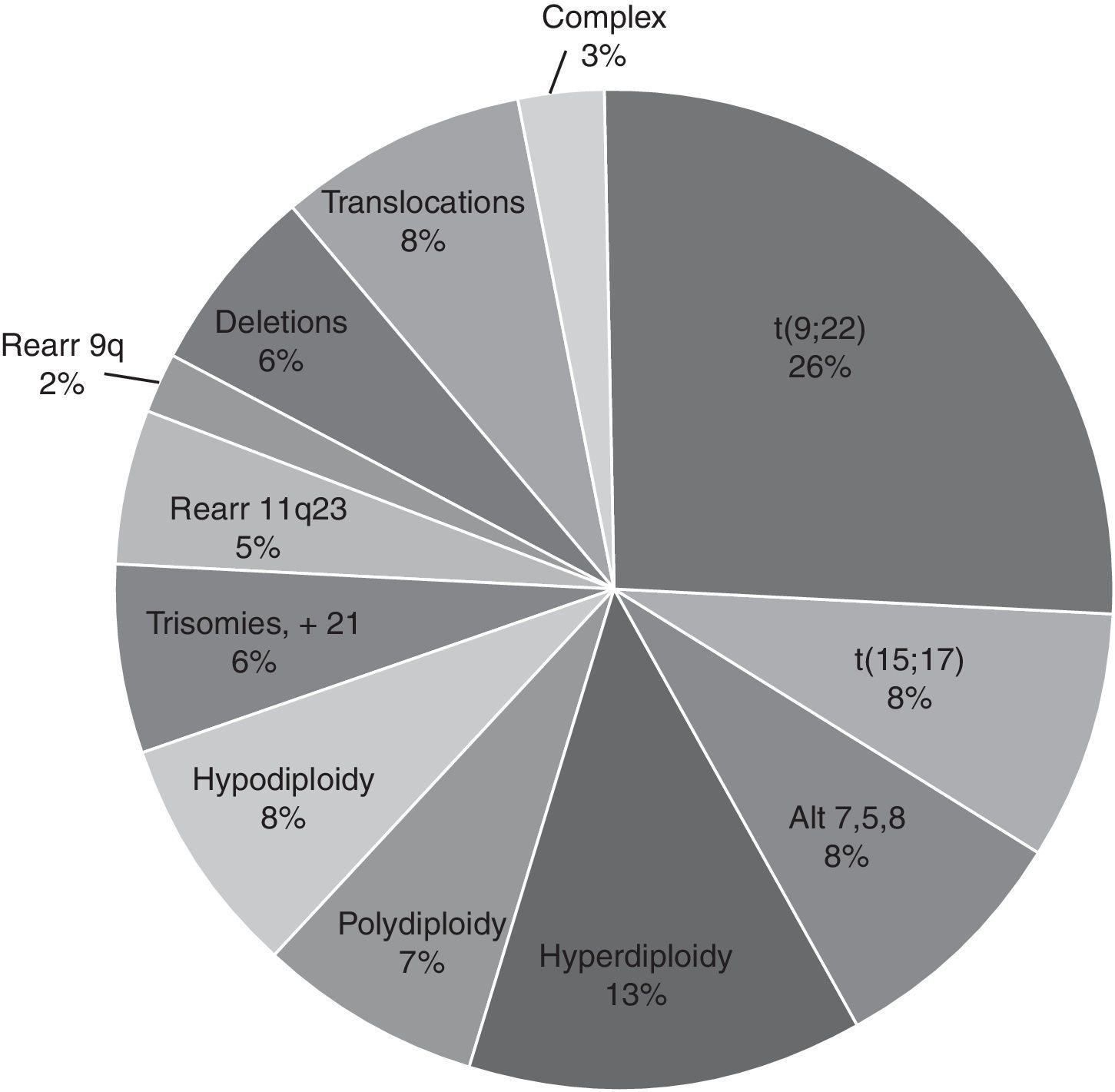
For the cytogenetic study, 85% of the samples were BM and 15% were PB. In 61% of the 9717 samples processed, sufficient material was obtained for the karyotype. In the rest (39%), it was not possible to obtain a result due to various causes related to the condition of the sample or its processing. Of the 3789 samples (39%) that were not assessable by karyotype, 34% were coagulated samples, 30% were diluted or insufficient (<1ml samples), 21% presented contamination of the sample and/or the culture and the remaining 15% were without cellular proliferation and metaphases in the culture. In the samples analysed, the karyotype was normal in 3956 (66.7%) and in the rest (1972, 33.3%) some type of chromosomal alteration was found (Fig. 3). In the cytogenetic analysis, structural alterations prevailed (65%) over the numerical ones (35%) and the alterations observed with the highest incidence were: t(9;22)(q34;q11) [Ph+ chromosome] 26%, Hyperdiploidy/Polyploidy 19.3%; other varied translocations 8.4%, hypodiploidy 8%; t(15;17)(q22;q21) 7.8%; alterations related to MDS (del5q/-5/-7/+8) 7.7% and to a lesser extent (<7%), different deletions, trisomies, monosomies and/or complex karyotypes were observed (Fig. 4).
In the cases with a diagnosis of CML, 52% (1062/2058) had evaluable karyotype, 48% were de novo (prior to receiving treatment) and 52% were in the follow-up phase with treatment. 45% of the de novo cases had t(9;22)(q34;q11) and in 3% of the follow-up cases the Ph+ clone was still present. In ALL, t(9;22)(q34;q11) was present in 20 cases (1%), as well as in <1% of the other chronic myeloproliferative neoplasms (myelofibrosis, thrombocytosis, etc.).
DiscussionIn this study, 9717 samples of bone marrow and/or peripheral blood were collected. Only 61% were evaluable since some were coagulated, haemolysed, diluted or contaminated, and others were not included due to conditions inherent to the disease, the nutritional status of the patient and inadequate bone marrow aspiration.
The total incidence of chromosomal abnormalities was 33%, which is low considering the reports of other series5–7; 37% of the samples had normal karyotype corresponding to patients in follow-up of their disease receiving treatment. This indicates “cytogenetic remission”, or disappearance of the initial abnormal clone. Twelve percent corresponded to patients with a cytopenia originating from infections, medications or other causes. None of these samples had chromosomal alterations; 5% of the samples were AA and presented a normal karyotype.
The most frequent chromosomal alteration was t(9;22)(q34;q11), observed in 45% of cases with de novo CML, in 3% of patients in the follow-up phase, in 1% of ALL and <1% of CMPD. Correlation between the (9;22)(q34;q11) rearrangement and the diagnosis was present in 28% of cases, which reflects what has been reported: most cases of de novo CML and a lower percentage of patients with ALL or CMPD have t(9;22)(q34;q11) translocation.
Of the 961 samples sent with a diagnosis of de novo LAM-M3, only 16% presented t(15;17)(q22;q21) translocation, while the rest presented a normal karyotype or other alterations. It is considered that 98% of cases with M3 have t(15;17)(q22;q21) translocation or one of its variants,4,10 so in these cases we can assume that the diagnosis of M3 was not confirmed or that these were patients in follow-up of the disease with treatment and disappearance of the leukaemic clone. In these cases, it is advisable, before clinical diagnosis of LAM-M3 is certain, to perform a molecular study to identify the PML/RARa fusion gene by means of FISH and/or RT-PCR, techniques that have a higher sensitivity than conventional cytogenetics.
The most frequent cases referred were CML in 21%, AML in 20%, ALL in 20% and MDS in 16%. For other diseases such as CMPD or MM (3%), referral increased in the last five years. This is probably due to a greater global incidence of the diseases or improvements in diagnostic resources to identify them early.
In our institution, the karyotype remains the “gold standard” for confirming diagnoses, as in cases of t(9;22) in CML or t(15;17) in M3. The main utility of cytogenetic studies in acute leukaemias and MDS is to establish prognoses and classify patients based on risk. Likewise, although the karyotype has a low sensitivity, it is also used to evaluate minimal residual disease in patients in clinical remission or post-transplantation of haematopoietic stem cells. The best examples are t(9;22)(q34;q11) translocation as a marker for follow-up in patients with CML treated with tyrosine kinase inhibitors, and t(15;17)(q22;q21) translocation in patients with AML-M3 who received treatment with ATRA.
In conclusion, this review presents the incidence of different cytogenetic alterations in haematologic diseases for a period of 15 years in the General Hospital of Mexico and can contribute to the institutional registry for an improvement in the classification, diagnosis and treatment of these diseases for the benefit of the patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.