The gold standard for the sporotrichosis diagnosis is culture; however, serologic approaches have been recently implemented to aid in the sporotrichosis diagnosis. Nevertheless, the clinical consequences of the introduction of serologic tests are poorly addressed.
AimsTo correlate the results of culture and serology of patients with suspected sporotrichosis.
MethodsA retrospective study of 198 patients with suspected sporotrichosis was conducted. Information about culture isolation of Sporothrix from clinical samples and antibody detection by an enzyme-linked immunosorbent assay (ELISA) were obtained from the medical records of the patients.
ResultsPositive culture and antibody detection was observed in the samples of 84 patients (42.4%). Forty-one samples (20.7%) showed negative results with both techniques and divergent results were obtained in the samples of 73 patients (36.9%). False negative results in the ELISA were observed with 23 patients (31.5%), 78.3% of them with less than 30 days of infection (p=0.0045). Among the initial false positive ELISA in the sera of 50 patients, four samples in culture yielded the growth of Sporothrix, and 27 improved with itraconazole. At the end of follow-up, a diagnosis of proven or probable sporotrichosis was established in 139 patients, and possible sporotrichosis in 11 patients. The treatment of the patients with probable sporotrichosis with antifungal drugs resulted in clinical cure for these individuals.
ConclusionsThese two techniques are complementary in the diagnosis of sporotrichosis, making diagnosis and clinical decision more precise.
El método de referencia en el diagnóstico de la esporotricosis es el cultivo, aunque las técnicas serológicas pueden complementar el diagnóstico. Sin embargo, la interpretación de las pruebas serológicas en la práctica clínica y en el diagnóstico de la enfermedad necesitan un abordaje más eficiente.
ObjetivosCorrelacionar los resultados del cultivo y la serología en pacientes con posibles síntomas de esporotricosis.
MétodosSe realizó un estudio retrospectivo de 198 pacientes con posibles síntomas de esporotricosis. Para establecer el diagnóstico se tuvieron en cuenta el aislamiento de Sporothrix a partir de las muestras clínicas y la detección de anticuerpos anti-Sporothrix realizados por un análisis de inmunoabsorción enzimática (ELISA), datos todos ellos registrados en las respectivas historias clínicas.
ResultadosLos cultivos y la detección de anticuerpos fueron positivos en 84 pacientes (42,4%). Las muestras de 41 pacientes (20,7%) resultaron negativas con ambas técnicas y en 73 pacientes (36,9%) los resultados fueron divergentes. Se obtuvieron resultados falsos negativos en el ELISA en 23 pacientes (31,5%), el 78,3% de ellos con menos de 30días de infección (p=0,0045). De los 50 pacientes con un resultado falso positivo en el ELISA, en 4 de ellos se obtuvo cultivo positivo de Sporothrix y 27 mejoraron con itraconazol. Al finalizar el estudio se estableció un diagnóstico de esporotricosis, que fue probada o probable en 139 pacientes y posible en 11 pacientes. El tratamiento de pacientes con esporotricosis probable con fármacos antifúngicos produjo la cura clínica de estos individuos.
ConclusionesEstos dos métodos son complementarios en el diagnóstico de la esporotricosis y ayudan a la toma de las decisiones clínicas más acertadas.
Sporotrichosis is a worldwide distributed subcutaneous mycosis with a high incidence in Latin America, India, Japan, China, and South Africa.7,11 Several Sporothrix species, a pathogenic thermo-dimorphic fungi, causes this mycosis.16,17 Infection usually follows the traumatic inoculation from an environmental source into the subcutaneous tissue of the patient. In some areas, zoonotic transmission can also occur. In Brazil, scratches and bites of infected cats10 also transmit sporotrichosis, on the great majority of the cases caused by Sporothrix brasiliensis species.7,11 In both transmission scenarios, sporotrichosis outbreaks may occur,7 and a rapid and efficient diagnosis is necessary to treat correctly the infected patients and to promote preventive actions to avoid the spread of the disease to new individuals.
The gold-standard diagnosis of sporotrichosis is the fungal isolation; the mycelial form develops in appropriate culture media at 25–27°C, and the fungal yeast-like form is obtained after subcultures on enriched media such as brain–heart infusion (BHI) agar at 35–37°C.19,27 Although easily performed, this process has limitations, such as culture contamination with other non-pathogenic fungi or bacteria,24,25 or false-negative results due to low fungal burden.27 It can be also time-consuming, requiring 3–4 weeks for complete fungal identification.13 Therefore, alternative diagnostic methods are necessary to improve the diagnosis.9
The most common method for anti-Sporothrix antibody detection is the enzyme-linked immunosorbent assay (ELISA), which has been described using different antigenic preparations with satisfactory sensitivity and specificity.2,5 However, serological tests for several infectious diseases are often negative when the antibody levels are low. Moreover, some discordant results in serologic tests may occur depending on the fungal strain or species used for the preparation of the antigenic extract used in the technique. As a result, false-negative results may occur, as well as cross reactivity.2
Despite the large number of studies evaluating accuracy parameters of enzyme immunoassays for sporotrichosis,2,3,5 their clinical significances are poorly addressed. The aim of this study was to correlate the results of culture and serology in the samples of patients followed up at the Instituto Nacional de Infectologia Evandro Chagas (INI/Fiocruz), Rio de Janeiro, Brazil, an important area of endemic zoonotic sporotrichosis,7,8,19 with their final diagnosis and clinical data.
Materials and methodsEthics: This study was approved by the Research Ethics Committee of INI/FIOCRUZ under the number: 0042.0.009.000-10.
Patients: A cross-sectional study was conducted in patients with suggestive symptoms of sporotrichosis, followed in the Laboratory of Clinical Research in Infectious Dermatology of the INI/FIOCRUZ, between January 2011 and December 2012, using the clinical protocol described elsewhere.1 Information about the time between the beginning of symptoms and medical care, as well as response to antifungal therapy, was recorded. Contact with sick cats, gardening and other professional or daily activities involving soil or plant manipulation were considered on the epidemiologic history of patients. Patients were submitted to serological and mycological tests by ELISA and culture, respectively, at the same time, for the laboratorial diagnosis of sporotrichosis. New biological samples were collected when necessary to clarify discordant results in the diagnosis of sporotrichosis.
Mycologic examination: The samples were collected according to the clinical presentation of sporotrichosis in each patient. They comprised lesion exudate (n=161), sputum (n=7), skin biopsy (n=6), nasal, ocular, or oral swab (n=5), and blood culture (n=4). For 15 patients, the information about the specimen could not be addressed. Processed samples were inoculated in both Sabouraud dextrose agar and Mycosel agar at 25–27°C, for 30 days. If an isolate presenting mycelial morphology compatible with Sporothrix spp. had grown, this fungus was subcultured in BHI agar at 35–37°C for 7 days to verify the dimorphism and to confirm or reject Sporothrix identity. If there was no suggestive growth of Sporothrix colonies after 30 days on cultures, they were classified as negative. Additionally, cultures were checked on a weekly basis for contamination by bacteria or non-pathogenic fungi.
Serologic examination: All the patients included were submitted to an indirect ELISA for the screening of anti-Sporothrix antibodies in the sera against exoantigens of the fungus.2 The serum samples were considered positive when the optical density (OD) was 10% above the cut-off, and negative when the OD was at least a 10% below this value. If the OD of the sample was close to the cut-off value, a second test with the same sample was performed to confirm the result.
Case definition: Sporotrichosis definition followed the criteria of the EORT/MSG consensus established for invasive fungal infections,21 except for the fact that being sporotrichosis an infection that affects mostly immunocompetent individuals, the probable and possible categories were applied to all patients, regardless of their immune status. Therefore, proven sporotrichosis comprised individuals with Sporothrix isolated in culture, probable sporotrichosis included patients with a positive serology and epidemiologic history consistent with sporotrichosis, and possible sporotrichosis comprised patients without positive mycological culture or serological test but with a strong epidemiologic history consistent with sporotrichosis and response to antifungal therapy. The diagnosis of other diseases followed their specific Brazilian guidelines.
Clinical forms: Patients were classified according to the clinical forms used by the institution protocol, which follows the classic classification proposed by Sampaio et al. (1954)22: lymphocutaneous, fixed, disseminated cutaneous and extracutaneous/disseminated.
Statistics: Statistical analyses were performed with non-parametric methods using the GraphPad Prism 7 for analyses. A p<0.05 was considered significant. The agreement between the diagnostic methods was evaluated according to previously described criteria.14
ResultsPatients: Between January 2011 and December 2012, 198 patients fulfilled the criteria for sporotrichosis diagnostic investigation and were included in this study. Their ages ranged from 2 to 78 years (median=43 years). One hundred and fifteen (58.1%) were female and 83 (41.9%), male.
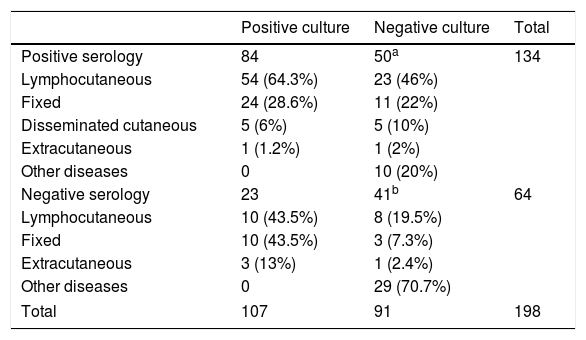
Mycologic and serologic results: Clinical samples were taken from all patients, and underwent both mycologic and serologic tests at the time of their first medical appointment. Positive results were observed in 107 mycologic cultures (54%) and 134 serologic tests (67.7%). Negative culture was observed in 91 (46%) patients, but 50 of them (54.9%) had positive results on antibody detection by ELISA. Table 1 presents all these results, as well as the clinical forms of the illness at the first medical appointment. A fair agreement between these methods was observed (kappa value: 0.239; 95% confidence interval: 0.104–0.373).
Mycologic and serologic results, and clinical forms of 198 patients with suspected sporotrichosis.
| Positive culture | Negative culture | Total | |
|---|---|---|---|
| Positive serology | 84 | 50a | 134 |
| Lymphocutaneous | 54 (64.3%) | 23 (46%) | |
| Fixed | 24 (28.6%) | 11 (22%) | |
| Disseminated cutaneous | 5 (6%) | 5 (10%) | |
| Extracutaneous | 1 (1.2%) | 1 (2%) | |
| Other diseases | 0 | 10 (20%) | |
| Negative serology | 23 | 41b | 64 |
| Lymphocutaneous | 10 (43.5%) | 8 (19.5%) | |
| Fixed | 10 (43.5%) | 3 (7.3%) | |
| Extracutaneous | 3 (13%) | 1 (2.4%) | |
| Other diseases | 0 | 29 (70.7%) | |
| Total | 107 | 91 | 198 |
Clinical and laboratory correlation: We performed a diagnosis of confirmed sporotrichosis in the first medical appointment for 107 patients (54%) based on the isolation of Sporothrix in culture. Eighty-two of them (76.6%) reported contact with cats before the appearance of the lesions, and eighty-four (78.5%) were also positive in the indirect ELISA for anti-Sporothrix antibody detection, including two patients coinfected with the human immunodeficiency virus (HIV). False negative results in the serologic tests were observed in the other 23 patients (21.5%): in eighteen patients the serum samples were collected along the month after the onset of the symptoms or lesions, two were coinfected with HIV, one was an elderly woman with 45 days of evolution of a disseminated infection (skin and adjacent bones) with a history of asthma, and in two patients no reason to explain the false-negative result was found. Information about the time of evolution of the illness at the first appointment could be obtained from the medical records of 98 patients with positive culture, while, for nine patients, this information was not available (one patient with negative serology and eight with positive serology). For the 22 patients with negative results in ELISA and a positive Sporothrix culture, the time of evolution of the disease ranged between 5 and 90 days (median=16 days). The time of evolution of the sporotrichosis in those patients with positive results in ELISA and culture ranged from 7 to 547 days, with a median of 30 days (p=0.0045).Among the 134 patients with positive serology at the time of diagnosis, 50 (37.3%) presented negative culture for Sporothrix, being these patients classified in the group of probable sporotrichosis at the time of diagnosis. The cultures of two of these patients yielded other pathogenic fungi (Exophiala jeanselmei and Microsporum gypseum, one case each); two patients were having antimycotic drugs at the time of sample collection, and in 21 cases the microorganisms isolated in Sabouraud agar or Mycosel agar were bacteria or non-pathogenic fungi.
Probable sporotrichosis diagnosis was maintained as the final diagnosis of 27 patients based on: (a) positive serology, response to antifungal therapy, and epidemiologic history for sporotrichosis (24 patients), or (b) positive serology and response to antifungal therapy (three patients). Nine patients were lost to follow-up, without a documented disease outcome. Four patients (one with a contaminated initial culture and three with no fungal growth) reached the confirmed sporotrichosis diagnosis due to a positive Sporothrix culture in another diagnostic approach: 3 patients in a subsequent appointment (4, 4, and 8 weeks, respectively), and one patient 28 weeks before in another institution. One patient had confirmed sporotrichosis three years prior to the infection evaluated during this study, which was diagnosed as leptospirosis. Medical records of the remaining nine false positive patients in the serological test revealed one patient with phaeohyphomycosis, one patient with leishmaniasis, one patient with toxoplasmosis and dermatophytosis, one patient with a respiratory viral infection, one patient with squamous cell carcinoma, one patient with systemic lupus erythematosus, and three patients referred to other health services without a final diagnosis.
Forty-one patients presented initial negative results in culture and serologic tests. One patient, with advanced AIDS,20 yielded a later positive Sporothrix culture, but other serologic evaluations were not performed. Other diseases were diagnosed in 17 patients: tuberculosis (three patients), bacterial infection (three patients), M. gypseum dermatophytosis (two patients), leishmaniasis (one patient), systemic lupus erythematosus (one patient), lipoma (one patient), hidrosadenitis (one patient), arteritis (one patient), onichomycosis (one patient), excoriation disorder (one patient), herpes simplex virus infection (one patient), and human papilloma virus infection (one patient). Among the other 23 patients, nine improved with itraconazole (100–200mg/day) and one with terbinafine (250mg/day), one presented spontaneous regression of the lesions, and a final diagnosis could not be reached for the last 12 patients.
DiscussionThe gold standard for sporotrichosis diagnosis is the isolation and identification of Sporothrix in culture. This process is simple, but fastidious, requiring some weeks to observe fungal mycelial growth and its further conversion to the yeast form.13,27 This work reports another limitation of the culture diagnostic method, which is the contamination by bacteria or non-pathogenic fungi. Some sporotrichosis patients present secondary infections,13 leading to the growth of bacteria if they are resistant to the antibiotics used in the mycologic culture media, usually chloramphenicol or gentamicin. Also, contamination may occur when samples with an associated microbiota are used for culture, which happens with the skin, the most affected site in sporotrichosis. We strongly suggest a second specimen collection for subsequent cultures in cases the first mycological culture is negative, since some of the herein described patients yielded Sporothrix from their lesions four to eight weeks after the first medical appointment.
Another important limitation for the isolation of Sporothrix in culture is the spontaneous remission of sporotrichosis. This event is rarely reported,4,12 but in Rio de Janeiro, around 10% of patients evolve to this condition.8 Moreover, previous antifungal treatment, which occurred in some patients, can also contribute to lower the fungal burden, difficulting the Sporothrix isolation in cultures from clinical samples.
The ELISA can aid in the diagnosis, but it also has limitations, like cross-reactivity with other diseases such as paracoccidioidomycosis, histoplasmosis, leishmaniasis, aspergillosis, coccidioidomycosis, cryptococcosis, chromoblastomycosis, and tuberculosis.2,5 We identified nine patients in our cohort that were classified as false positive by ELISA, five of them with a final diagnosis of diseases not described before (phaeohyphomycosis, toxoplasmosis, squamous cell carcinoma, viral respiratory infection, and systemic lupus erythematosus), showing that the spectrum of diseases that may cross-react with sporotrichosis serological tests can be wide. To our knowledge, samples from patients with these diseases were never tested on a cohort encompassing the Rio de Janeiro endemic region of sporotrichosis. Patients with systemic lupus erythematosus, however, have been included in the standardization of an ELISA with 100% specificity.3 Nonetheless, we cannot rule out the hypothesis that the patients with positive serologic results herein described have had a previous episode of sporotrichosis. For instance, one patient, diagnosed with leptospirosis at the time of our study that presented sporotrichosis years before, yielded a positive serologic result. The antibody positive detection observed in this case can be better explained by a previous Sporothrix infection rather than cross-reactivity. In paracoccidioidomycosis, IgG serum titres in ELISA are maintained in 67% of patients at least for three years after treatment.23 To our knowledge, there are no studies on the maintenance of antibody titres in sporotrichosis years after infection.
Besides cross-reactivity, this study presents the evolution time of the disease as another factor that may affect ELISA. Humoral immune response in sporotrichosis is driven by IL-4.15 In experimental sporotrichosis, interleukin production depends on the time of infection and clinical forms,18 and a role for antibodies is suggested in later stages of murine sporotrichosis.6 A similar scenario may occur in human sporotrichosis, since most patients with proven sporotrichosis without detectable antibody levels reported less than 3 weeks of infection.
In this study, we found similar proportion of the clinical forms within the different groups, independently of the agreement of both exams (culture and serology). The lymphocutaneous form of sporotrichosis is the main presentation observed in the Rio de Janeiro hyperendemic zoonotic region, and we found the same pattern. Although the group with confirmed sporotrichosis but negative serology presented a lower proportion of the lymphocutaneous sporotrichosis, this difference may be due to a small number of the sample in this group.
A study of Bernardes-Engemann et al. showed that five patients coinfected with Sporothrix and HIV were reactive in an ELISA for sporotrichosis, three of them with high antibody titres, but two with low titres.5 In the cohort herein described, five patients presenting Sporothrix/HIV coinfection were included, and three of them, with a severely impaired immunity, yielded a negative result in the ELISA. Studies including more subjects with this coinfection are necessary for a better comprehension of the humoral response in these patients. The false negative result in the elderly woman can be explained by a lowering of the humoral immunity due to immunosenescence.26
Despite these limitations, the serological methods must be employed in the sporotrichosis diagnosis. The clinical decision to prescribe itraconazole in 27 (13.6%) patients was supported by a positive serologic test, despite the negative culture. All of them presented a very good response to the antifungal therapy. We observed other nine patients with a good therapeutic response to itraconazole, but without mycologic and serologic positive tests. They are likely to be considered false negative in both methods, though we cannot rule out the hypothesis that they had other mycotic diseases that also responded to this broad-spectrum antifungal drug.
In conclusion, because of their limitations and difficulties, both culture and serology tests should be performed whenever possible. Positive serologic tests have a positive impact in the clinical decision to start antifungal therapy in the patients with suspicion of sporotrichosis. Negative cultures should be repeated, if possible, after a few days, as well as the serologic tests, especially when patients have less than one month of symptoms.
Conflict of interestThe authors declare no conflict of interest.
FundingFinancial support for this work was provided by PAPES Fiocruz/CNPq407693/2012-2. R.M.Z.O is supported in part by CNPq (grant number 304976/2013-0).