This study compared the performance of 18F-Florbetapir PET/CT early acquisitions to 18F-FDG PET/CT.
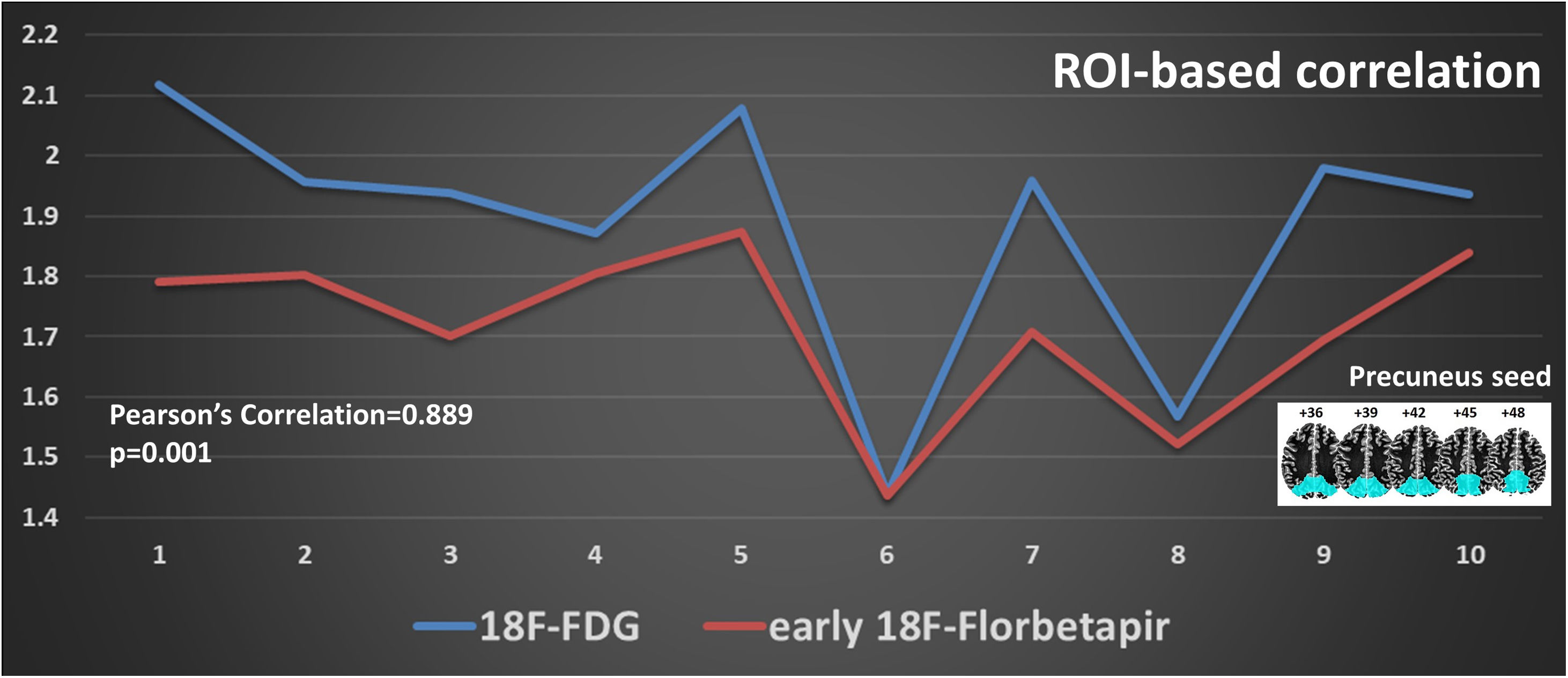
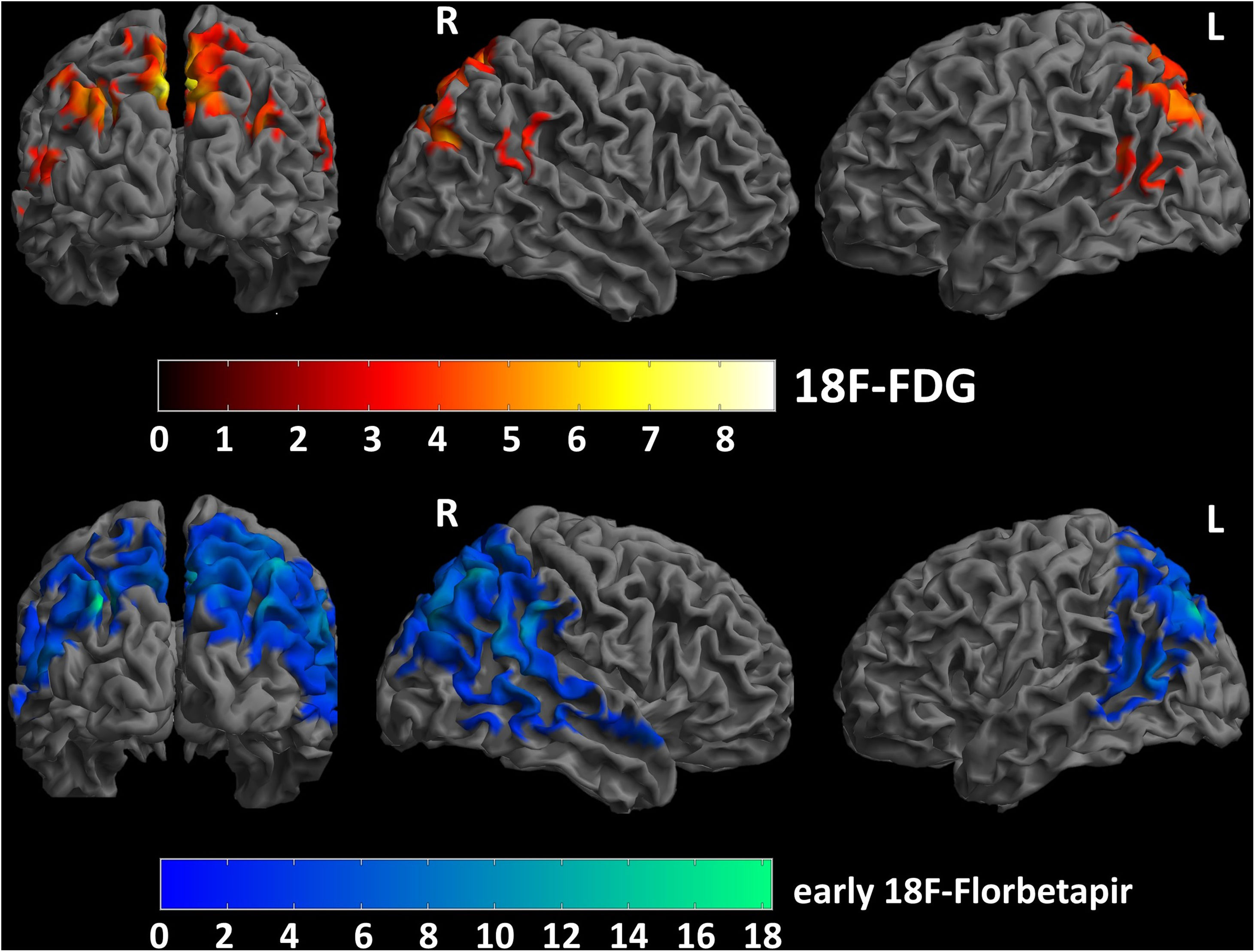
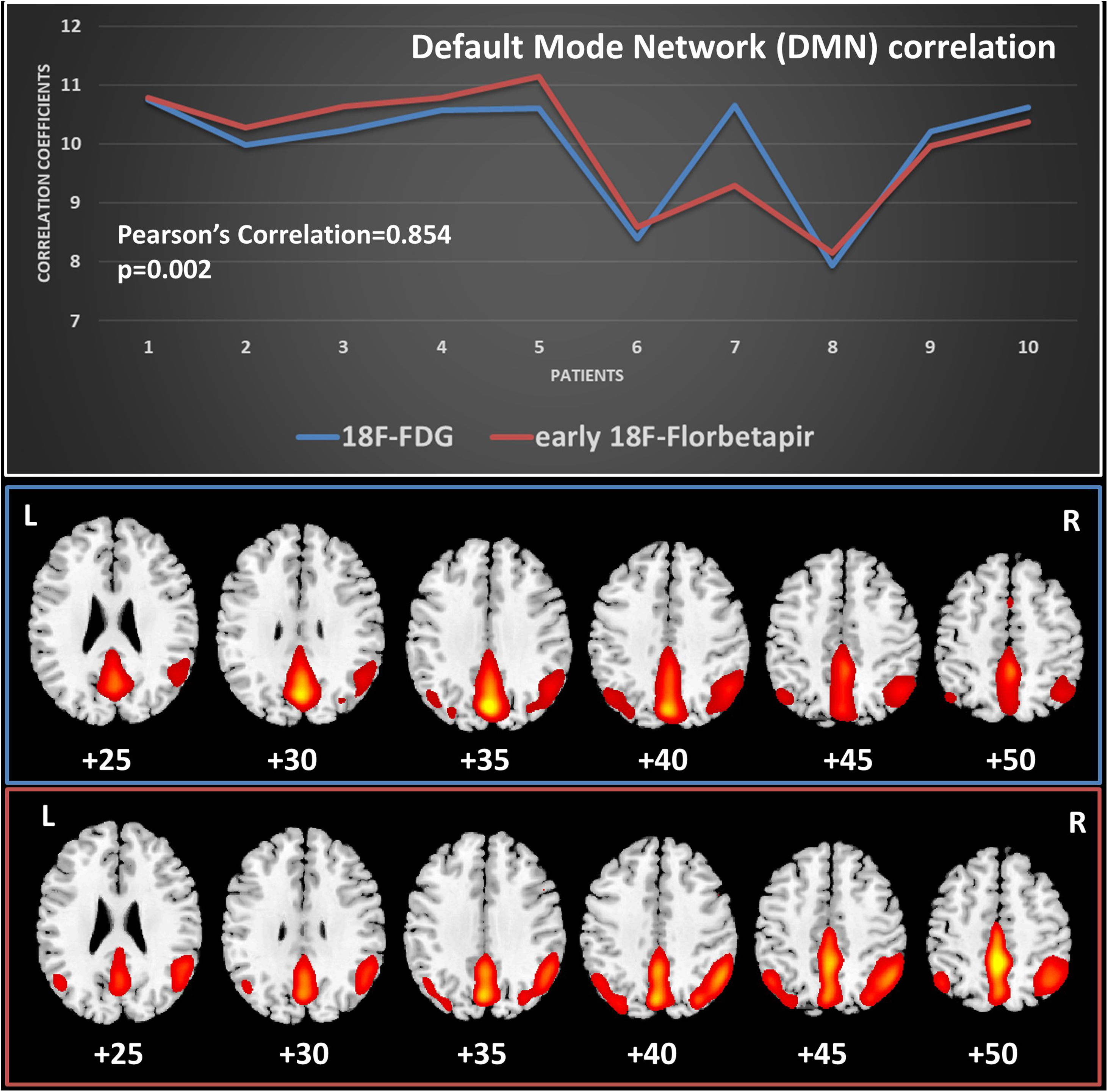
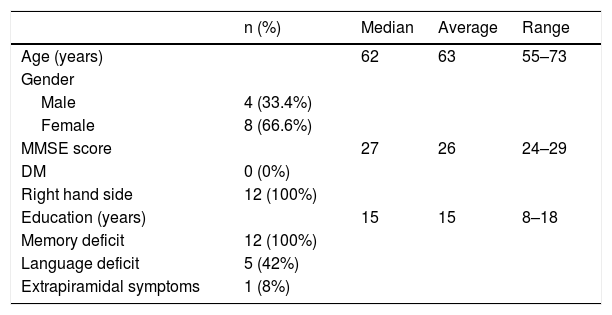
MethodsWe included 12 patients who underwent 18F-FDG PET/CT and a dual-time 18F-Florbetapir PET/CT (1–6 min early-scan and 50 min late-scan). PET/CT were analyzed visually by three nuclear medicine physicians with different experience using a four-point scale (0 = no reduction, 1 = slight, 2 = moderate, 3 = severe reduction) for 18F-Florbetapir early-phase and 18F-FDG images in 10 cortical regions (bilateral frontal, temporal, parietal, occipital, posterior cingulate/precuneus), and 18F-Florbetapir late-phase in the same cortical regions using a three-point scale (0 = normal, 1 = abnormal with minor plaques, 2 = abnormal with major plaques). We used SPM12 for semiquantitative analysis applying a ROI-based correlation analysis (considering precuneus as target region and normalized for the mean global binding), a covariance-analysis taking precuneus as target and a comparison of global DMN (default mode network).
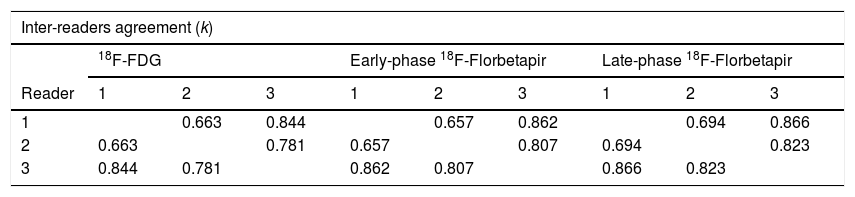
ResultsInter-reader agreement was high (Cohen’s kappa 0.762 for 18F-FDG, 0.775 for 18F-Florbetapir early-phase and 0.794 for late-phase). Regional visual scores of early-phase and 18F-FDG were significantly correlated (ρ = 0.867). Also ROI-based analysis, global brain visual analysis and DMN comparison revealed concordant results, especially at parietal and precuneus (p < 0.001).
Conclusions18F-Florbetapir early-phase scans significantly correlate on quantitative and visual images with 18F-FDG-PET/CT scans, suggesting that amyloid tracer could be instead of 18F-FDG.
Este estudio comparó el rendimiento de las adquisiciones precoces de 18F-Florbetapir PET/TC con la de 18F-FDG PET/TC.
MétodosSe incluyeron 12 pacientes que se realizaron una a PET/TC con 18F-FDG y una PET/TC con 18F-Florbetapir en dos tiempos (exploración precoz de 1 a 6 minutos y exploración tardía a los 50 minutos). La PET/TC fue analizada visualmente por tres médicos de medicina nuclear con diferente experiencia utilizando una escala de cuatro puntos (0 = sin disminución, 1 = leve, 2 = moderada, 3 = disminución severa) para 18F-Florbetapir en fase precoz y 18F-FDG imágenes en 10 regiones corticales (frontal bilateral, temporal, parietal, occipital, cingulado/precuneus posterior) y fase tardía de 18F-Florbetapir en las mismas regiones corticales utilizando una escala de tres puntos (0 = normal, 1 = anormal con placas menores, 2 = anormal con elevada carga de placas). Usamos SPM12 para el análisis semicuantitativo aplicando un análisis de correlación basado en ROI (considerando precuneus como región objetivo y normalizado para la unión global media), un análisis de covarianza tomando precuneus como objetivo y una comparación de DMN global (red de modo predeterminado).
ResultadosLa concordancia entre lectores fue alta (kappa de Cohen 0,762 para 18F-FDG, 0,775 para 18F-Florbetapir en la fase temprana y 0,794 para la fase tardía). Las puntuaciones visuales regionales de la fase temprana y la 18F-FDG se correlacionaron significativamente (ρ = 0,867). También el análisis basado en el ROI, el análisis visual cerebral global y la comparación de DMN revelaron resultados concordantes, especialmente en parietal y precuneus (p < 0,001).
ConclusionesLas exploraciones de fase temprana de 18F-Florbetapir se correlacionan significativamente en imágenes cuantitativas y visuales con las exploraciones de 18F-FDG-PET/TC, lo que sugiere que se podría usar un marcadores de amiloide en lugar de 18F-FDG.
Article

Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)