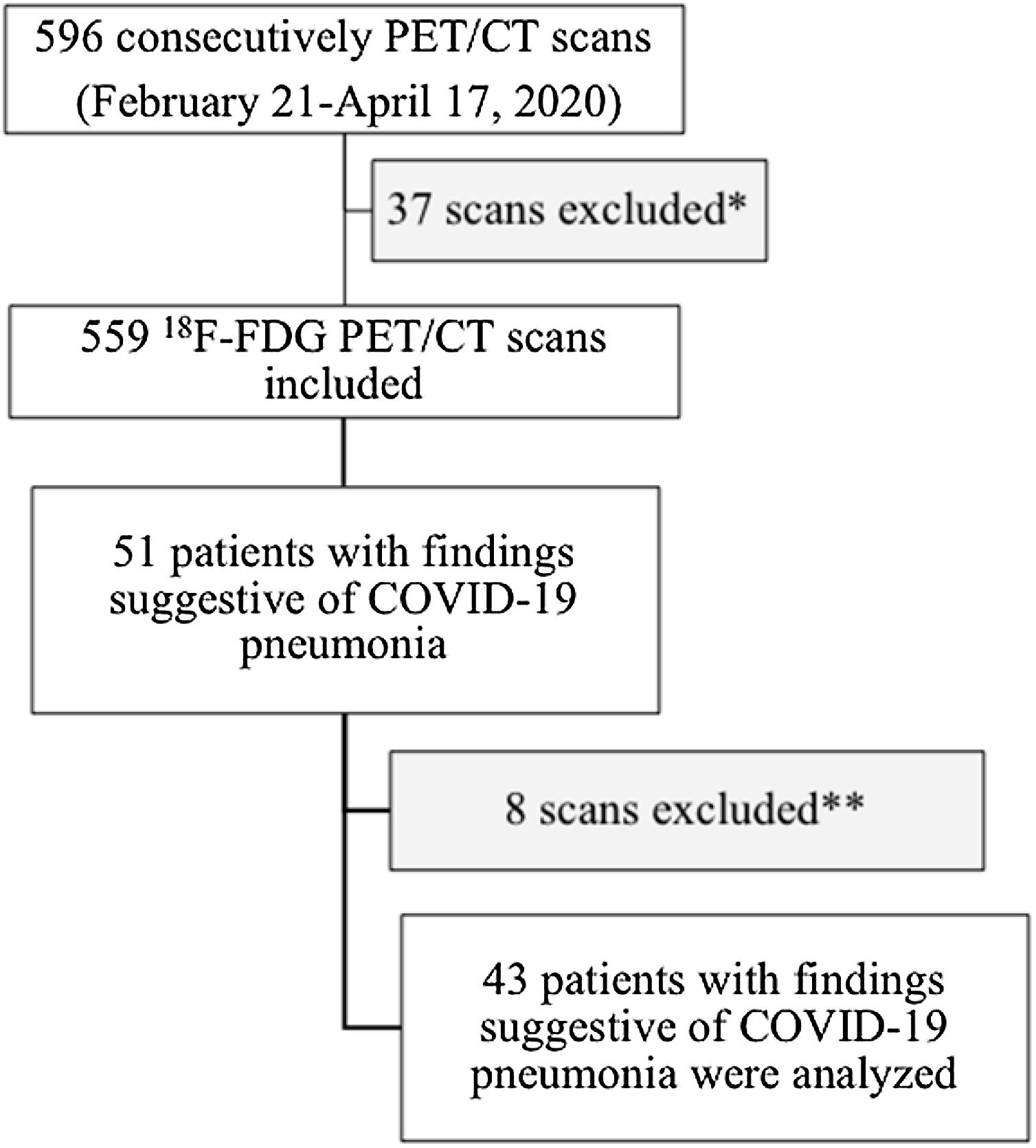
To evaluate the metabolic uptake of different tomographic signs observed in patients with incidental structural findings suggestive of COVID-19 pneumonia through 18F-FDG PET/CT.
Materials and Methods: We retrospectively analyzed 596 PET/CT studies performed from February 21, 2020 to April 17, 2020. After excluding 37 scans (non-18F-FDG PET tracers and brain studies), we analyzed the metabolic activity of several structural changes integrated in the CO-RADS score using the SUVmax of multimodal studies with 18F-FDG.
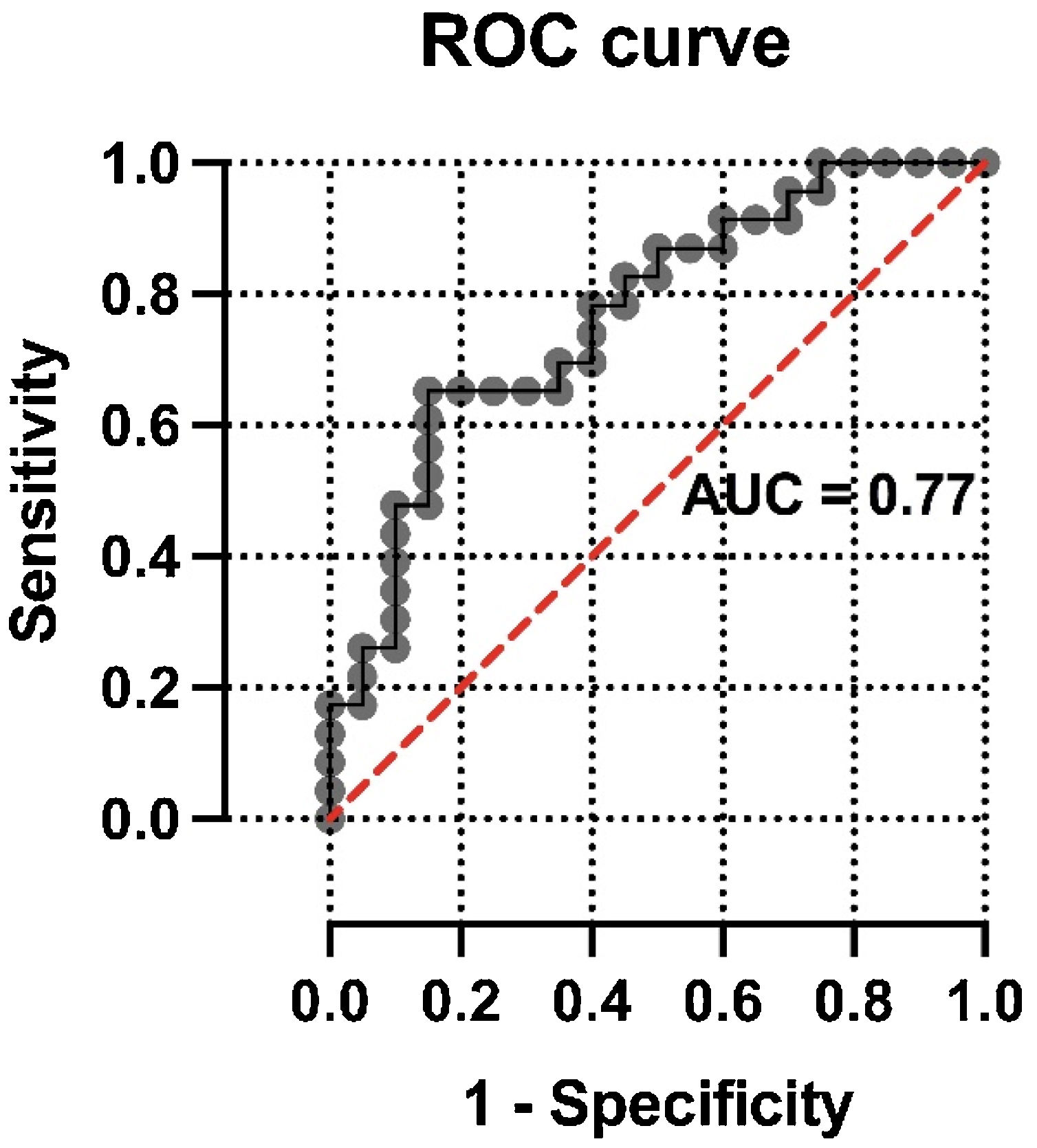
ResultsForty-three patients with 18F-FDG PET/CT findings suggestive of COVID-19 pneumonia were included (mean age: 68±12.3 years, 22 male). SUVmax values were higher in patients with CO-RADS categories 5−6 than in those with lower CO-RADS categories (6.1±3.0 vs. 3.6±2.1, p=0.004). In patients with CO-RADS 5−6, ground-glass opacities, bilaterality and consolidations exhibited higher SUVmax values (p-values of 0.01, 0.02 and 0.01, respectively). Patchy distribution and crazy paving pattern were also associated with higher SUVmax (p-values of 0.002 and 0.01). After multivariate analysis, SUVmax was significantly associated with a positive structural diagnosis of COVID-19 pneumonia (odds ratio=0.63, 95% confidence interval=0.41−0.90; p=0.02). The ROC curve of the regression model intended to confirm or rule out the structural diagnosis of COVID-19 pneumonia showed an AUC of 0.77 (standard error=0.072, p=0.003).
ConclusionsIn those patients referred for standard oncologic and non-oncologic indications (43/559; 7.7%) during pandemic, imaging with 18F-FDG PET/CT is a useful tool during incidental detection of COVID-19 pneumonia. Several CT findings characteristic of COVID-19 pneumonia, specifically those included in diagnostic CO-RADS scores (5−6), were associated with higher SUVmax values.
In December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) was isolated in Wuhan, China, and rapidly spread worldwide, confronting us with a global pandemic situation. Initially, Italy and Spain were the most affected countries after China.
The clinical spectrum of COVID-19 is highly variable. In most cases, it manifests as nonspecific symptoms or may remain asymptomatic. Most patients survive the primary infection without complications; however, a considerable proportion of them develop life-threatening complications, such as severe pneumonia with acute respiratory distress syndrome.
Although concern has been raised about the low sensitivity of reverse transcriptase-polymerase chain reaction (rRT-PCR) in samples obtained from the respiratory tract, to date it is the gold standard for the diagnosis of COVID-19.1 Some imaging techniques, like chest CT, can strongly suggest the infection since early stages. Moreover, some CT patterns observed in patients with COVID-19 pneumonia were even more sensitive than rRT-PCR results.
Patients with cancer, particularly those over 60 years of age, are a high-risk subpopulation during COVID-19 outbreaks.2 Multimodal PET/CT imaging with fluorine-18 fluorodeoxyglucose (18F-FDG) plays an important role during the evaluation, follow-up and monitoring of treatment response in various oncologic, infectious and inflammatory diseases. Although this imaging modality is not routinely used in the management of patients with COVID-19, in some cases it could provide complementary diagnostic value through simultaneous tomographic and metabolic information in same study. In addition, a deeper understanding of tissues affected by SARS-CoV-2 infection could provide new insights into the pathogenesis of viral infection and host response, and could even help to diagnose the pulmonary and distant involvement in selected cases. However, the potential diagnostic value of metabolic activity combined with tomographic lung findings suggestive of COVID-19 pneumonia on PET/CT studies has not yet been evaluated in detail. Applying 18F-FDG PET/CT, our study aimed to explore the pulmonary metabolic behavior of several incidental lung parenchyma changes integrated into the COVID-19 Reporting and Data System (CO-RADS) categories that have demonstrated diagnostic utility in COVID-19 pneumonia.3,4
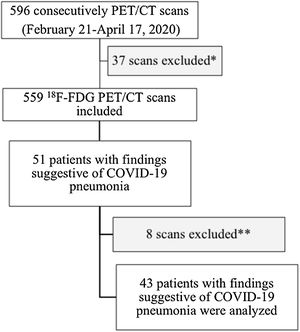
Materials and methodsPatient selectionA total of 596 PET/CT studies were performed between February 21, 2020 and April 17, 2020. After excluding PET studies with non-18F-FDG radiotracers and localized brain studies, a sample of 559 consecutive 18F-FDG PET/CT examinations were included. In accordance with the recommendations of the European Association of Nuclear Medicine at the time of the study, all patients with any symptoms suggestive of respiratory tract infection did not undergo PET/CT. Data obtained from these PET/CT studies were retrospectively reviewed to identify those with incidental findings suggestive of COVID-19 pneumonia. The presence of the different structural signs described in CO-RADS categories and the maximum standardized uptake value (SUVmax) on lung PET images were evaluated.3,4 Demographic variables, PET/CT indication (oncological vs. non-oncological), known autoimmune diseases/inflammatory states and rRT-PCR results were collected, when available. A flow diagram of the study is presented in Fig. 1.
PET/CT imaging protocolFollowing usual 18F-FDG PET/CT protocol, at least 6h fasting (4h for diabetic patients) and blood glucose level <200mg/dl prior to acquisition were required. All studies were performed after intravenous administration of 18F-FDG (5MBq/kg) assuming a physical resting state during 50min pre-scanner. All studies were acquired following the European Association of Nuclear Medicine guidelines in the same equipment (Biograph 6 True Point; Siemens) with a 6-ring detector CT, performing a diagnostic CT (Topogram Dose Modulation System, CARE Dose4D, slice thickness: 5mm, reconstruction interval: 3mm). In the absence of contraindications, an iodinated contrast agent was administered intravenously (130ml, 45s delay, rate: 2.5ml/s). A first inspirational chest CT study was reconstructed as 2.5mm slices, 60 mAs and 110 KV, with a tube rotation time of 0.6s and a pitch of 1.2. Then, free-breathing body CT was performed from the base of the skull to the proximal thighs, with modifications according to the pathology location. Finally, PET study was performed in the same locations as CT study, with an acquisition time of 3min per bed. PET data were iteratively reconstructed with and without attenuation correction (based on CT information) and reoriented in axial, sagittal and coronal slices.
Image interpretationAll PET/CT scans were reviewed by at least two certified physicians (a nuclear medicine physician and a radiologist). An expert radiologist recorded all individual signs suggestive of COVID-19 pneumonia and classified them according to the CO-RADS criteria.3,4 Volumes of interest were delimited by a nuclear medicine physician to obtain SUVmax of lung parenchyma. The diagnosis of COVID-19 pneumonia was established by the combination of structural lung changes on PET/CT with positive rRT-PCR or with the clinical judgment of the treating physician.
StatisticsDiscrete variables were presented as n (%) and continuous variables as mean±standard deviation or median (interquartile range). First, an univariate analysis was performed to describe the FDG SUVmax uptake of the different parenchymal lung changes detected on chest CT by a radiologist blinded to metabolic imaging in all patients. Various structural findings were calculated as absolute and relative frequencies (n, %), both in patients included in the diagnostic group (CO-RADS scores: 5−6) and in the group not suggestive of COVID-19 pneumonia (CO-RADS scores: 2−4).3,4 When changes were detected more frequently (at least in 50% of patients with pulmonary infiltrates), SUVmax was compared between patients with CO-RADS 5−6 vs. 2−4 by unpaired t-test or nonparametric Mann-Whitney tests. For less frequent structural changes (detected in less than 50% of patients), SUVmax values were compared between patients with or without each CT finding (yes/no). This first analytical approach was aimed to estimate the FDG uptake of various individual structural changes, signs and patterns contained in the CO-RADS categories. Next, a multivariate analysis was applied. A logistic regression model was fitted with the binary dependent variable considered as the tomographic diagnosis of COVID-19 pneumonia (CO-RADS score 5−6), according to its high diagnostic yield.3,4 Selected structural and metabolic findings obtained by multimodality imaging were then considered as independent variables to predict the tomographic diagnosis of COVID-19 pneumonia. Due to the small number of FDG scans with highly suspected or confirmed structural diagnosis, only those multimodal variables not considered or with lower relative weight in the CO-RADS score that demonstrated a p-value <0.05 after univariate analysis were included in the model. Goodness of fit was assessed using the log likelihood ratio (G-squared). Estimated beta coefficients, odds ratios with 95% confidence interval and Z values were calculated for each independent variable. A receiver operating characteristic (ROC) curve was constructed for the model, characterized by its area under the curve (AUC), standard error, and negative and positive predictive power. All statistical analyses were performed with GraphPad Prism 9.0, considering a value p<0.05 as significant (two-tailed).
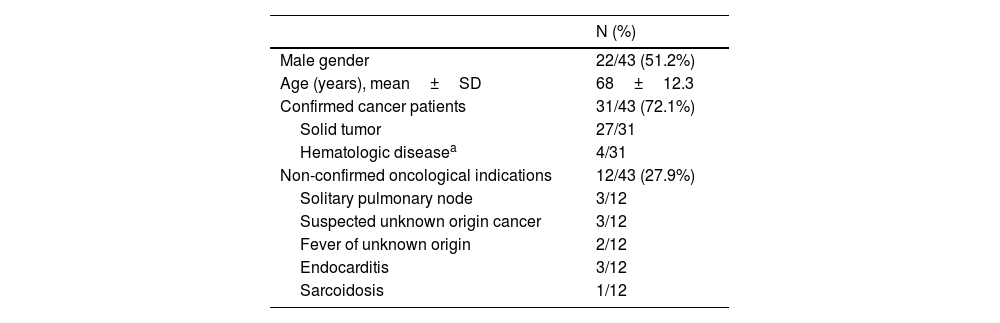
ResultsCharacterization of study populationForty-three of 559 consecutive patients referred for PET/CT scans with suggestive COVID-19 pneumonia were analyzed. About 51.2% of the population were male, aged from 42 to 93 years. Demographics variables and PET/CT indications of all subjects are summarized in Table 1. Patients included were referred primarily for PET/CT imaging during staging (n=8), radiotherapy planning (n=2), evaluation of treatment response/follow-up (n=19) or suspected recurrence (n=2) of oncological diseases (31/43, 72.1%), according to clinical judgement of the oncology team. None of the other patients (n=12, 27.9%) referred a previous history of confirmed oncological disease. They were derived under suspicious of inflammatory/infectious process or during usual diagnostic evaluation of solitary pulmonary node or tumor of unknown origin.
Characterization of study population. Demographic variables and PET/CT indications in patients with parenchymal lung findings suggestive of COVID-19 pneumonia (n=43).
| N (%) | |
|---|---|
| Male gender | 22/43 (51.2%) |
| Age (years), mean±SD | 68±12.3 |
| Confirmed cancer patients | 31/43 (72.1%) |
| Solid tumor | 27/31 |
| Hematologic diseasea | 4/31 |
| Non-confirmed oncological indications | 12/43 (27.9%) |
| Solitary pulmonary node | 3/12 |
| Suspected unknown origin cancer | 3/12 |
| Fever of unknown origin | 2/12 |
| Endocarditis | 3/12 |
| Sarcoidosis | 1/12 |
SD, standard deviation.
Eight of the 43 patients with pneumonia suggestive of COVID-19 had known autoimmune or inflammatory diseases at the time of PET/CT, 6 classified as CO-RADS 5−6 and 2 as CO-RADS 4.
The rRT-PCR was available in 14 patients: positive in 8 of them (resulting in CO-RADS 6) and negative in the remaining 6 patients (3 classified as CO-RADS 4 and 3 as CO-RADS 5).
CO-RADS and SUVmaxTwenty patients exhibited CO-RADS categories 5−6 and 23 patients were classified as CO-RADS 2−4. Patients included in both CO-RADS groups (scores: 5−6 vs. 2−4) were comparable in terms of gender (male: 50.0 vs. 52.2%, p>0.99) and age (68.9±12.2 vs. 67.0±13.4 years, p=0.65). SUVmax values were higher in those patients presenting CO-RADS categories 5−6 than in subjects with lower and non-specific CO-RADS categories (6.1±3.0 vs. 3.6±2.1, p=0.004). In contrast, SUVmax values did not differ from PET/CT indication (confirmed cancer: 4.6±2.9 vs. non-oncological indications: 5.1±2.8, p=0.62) nor according to the presence of solid tumors vs. hematological disorders (4.5±2.8 vs. 5.2±3.5, p=0.65).
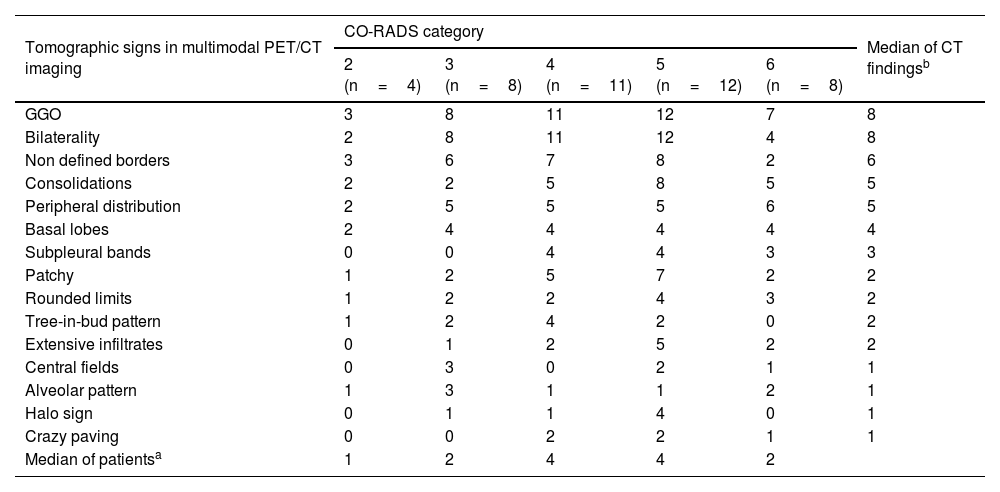
The frequencies of main tomographic changes are shown in Table 2. All patients showed at least two structural findings on chest CT, with a major overlap of patterns and signs in each patient. Ground-glass opacity (GGO) was found in almost all patients (41/43, 95.3%), predominantly bilateral (n=37). Eight concomitant CT changes of CO-RADS categories were found in 5 patients (3 of them CO-RADS 5−6), 7 changes in 10 patients (CO-RADS 5−6: 7), 6 changes in 16 patients (7 of them CO-RADS 5−6), 5 changes in 6 patients (CO-RADS 5−6: 1), 4 changes in 4 patients and 3 changes in 3 patients (one from each group classified as CO-RADS 5−6). All but 3 patients (17/20) classified as CO-RADS 5−6showed at least 6structural changes (Table 3). Less frequent findings were inverted halo sign (n=1, CO-RADS: 5) and hilar adenopathy that was interpreted as non-tumoral (n=1, CO-RADS: 6). Pleural effusion was not detected in any patient.
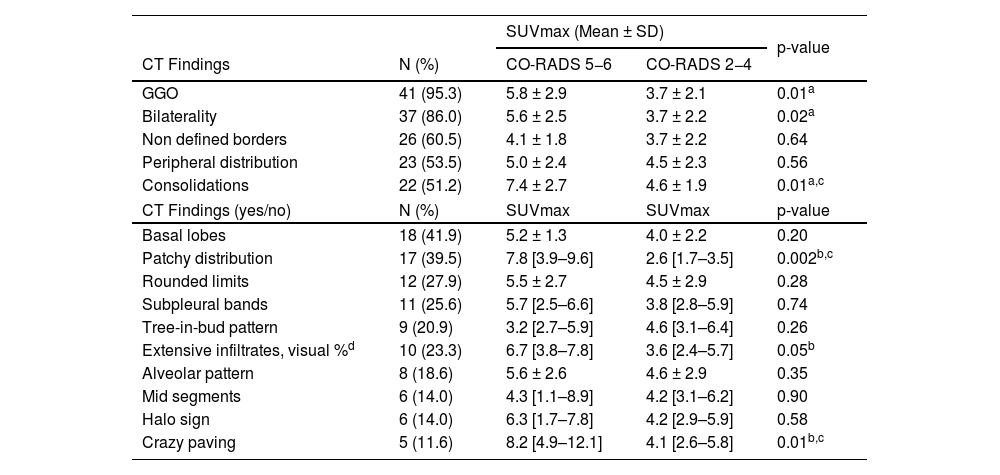
Univariate association of FDG uptake with individual structural findings. Data of parenchymal SUVmax measured in patients with different lung CT changes. Results are presented in relation with CO-RADS groups (scores: 5-6 vs. 2-4) or the presence of each structural change (yes/no). Each individual CT finding is expressed as mean+SD or median [IQR].
| SUVmax (Mean ± SD) | p-value | |||
|---|---|---|---|---|
| CT Findings | N (%) | CO-RADS 5−6 | CO-RADS 2−4 | |
| GGO | 41 (95.3) | 5.8 ± 2.9 | 3.7 ± 2.1 | 0.01a |
| Bilaterality | 37 (86.0) | 5.6 ± 2.5 | 3.7 ± 2.2 | 0.02a |
| Non defined borders | 26 (60.5) | 4.1 ± 1.8 | 3.7 ± 2.2 | 0.64 |
| Peripheral distribution | 23 (53.5) | 5.0 ± 2.4 | 4.5 ± 2.3 | 0.56 |
| Consolidations | 22 (51.2) | 7.4 ± 2.7 | 4.6 ± 1.9 | 0.01a,c |
| CT Findings (yes/no) | N (%) | SUVmax | SUVmax | p-value |
| Basal lobes | 18 (41.9) | 5.2 ± 1.3 | 4.0 ± 2.2 | 0.20 |
| Patchy distribution | 17 (39.5) | 7.8 [3.9–9.6] | 2.6 [1.7–3.5] | 0.002b,c |
| Rounded limits | 12 (27.9) | 5.5 ± 2.7 | 4.5 ± 2.9 | 0.28 |
| Subpleural bands | 11 (25.6) | 5.7 [2.5–6.6] | 3.8 [2.8–5.9] | 0.74 |
| Tree-in-bud pattern | 9 (20.9) | 3.2 [2.7–5.9] | 4.6 [3.1–6.4] | 0.26 |
| Extensive infiltrates, visual %d | 10 (23.3) | 6.7 [3.8–7.8] | 3.6 [2.4–5.7] | 0.05b |
| Alveolar pattern | 8 (18.6) | 5.6 ± 2.6 | 4.6 ± 2.9 | 0.35 |
| Mid segments | 6 (14.0) | 4.3 [1.1–8.9] | 4.2 [3.1–6.2] | 0.90 |
| Halo sign | 6 (14.0) | 6.3 [1.7–7.8] | 4.2 [2.9–5.9] | 0.58 |
| Crazy paving | 5 (11.6) | 8.2 [4.9–12.1] | 4.1 [2.6–5.8] | 0.01b,c |
FDG, fluorodeoxyglucose; CO-RADS, COVID-19 Reporting and Data System criteria; GGO, ground-glass opacities; SD, standard deviation; IQR, interquartile range.
CO-RADS categories with CT findings in each of them.
| Tomographic signs in multimodal PET/CT imaging | CO-RADS category | Median of CT findingsb | ||||
|---|---|---|---|---|---|---|
| 2 (n=4) | 3 (n=8) | 4 (n=11) | 5 (n=12) | 6 (n=8) | ||
| GGO | 3 | 8 | 11 | 12 | 7 | 8 |
| Bilaterality | 2 | 8 | 11 | 12 | 4 | 8 |
| Non defined borders | 3 | 6 | 7 | 8 | 2 | 6 |
| Consolidations | 2 | 2 | 5 | 8 | 5 | 5 |
| Peripheral distribution | 2 | 5 | 5 | 5 | 6 | 5 |
| Basal lobes | 2 | 4 | 4 | 4 | 4 | 4 |
| Subpleural bands | 0 | 0 | 4 | 4 | 3 | 3 |
| Patchy | 1 | 2 | 5 | 7 | 2 | 2 |
| Rounded limits | 1 | 2 | 2 | 4 | 3 | 2 |
| Tree-in-bud pattern | 1 | 2 | 4 | 2 | 0 | 2 |
| Extensive infiltrates | 0 | 1 | 2 | 5 | 2 | 2 |
| Central fields | 0 | 3 | 0 | 2 | 1 | 1 |
| Alveolar pattern | 1 | 3 | 1 | 1 | 2 | 1 |
| Halo sign | 0 | 1 | 1 | 4 | 0 | 1 |
| Crazy paving | 0 | 0 | 2 | 2 | 1 | 1 |
| Median of patientsa | 1 | 2 | 4 | 4 | 2 | |
As shown in Table 2, ground-glass opacities, bilaterality and consolidations exhibited higher SUVmax values (p-values of 0.01, 0.02, 0.01, respectively) in those patients with CO-RADS scores 5−6. Patchy distribution and crazy paving pattern were also associated with higher SUVmax (p-values of 0.002 and 0.01). Pulmonary SUVmax was higher in patients with more widespread infiltrates, but did not reach statistical significance. There were no differences in FDG uptake associated with other individual structural findings.
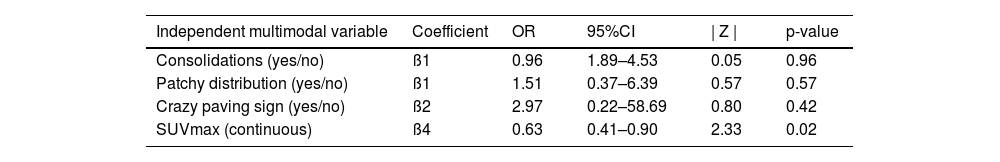
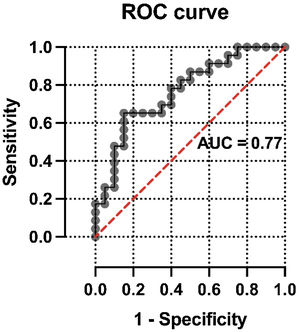
Multivariate analysis of the association of CO-RADS diagnosis with variables included in the modelThe results of logistic regression analysis including selected multimodal findings as independent variables are summarized in Table 4. No statistical association was found between CO-RADS diagnosis and other structural variables included in the model, such as consolidations (odds ratio=0.96, 95% confidence interval=1.89–4.53; p=0.96), patchy distribution of infiltrates (odds ratio=1.51, 95% confidence interval=0.37–6.39; p=0.57) and crazy paving pattern (odds ratio=2.97, 95% confidence interval=0.22–58.69; p=0.42). In contrast, lung SUVmax was significantly associated with a positive structural diagnosis of COVID-19 pneumonia (odds ratio=0.64, 95% confidence interval=0.41–0.90; p=0.02). The ROC curve of the regression model intended to confirm or discard the structural diagnosis of COVID-19 pneumonia showed an AUC of 0.77 (standard error=0.072, p=0.003) (Fig. 2).
Results of the multivariate analysis. Selected multimodal imaging data was included as independent variables to predict the structural diagnosis of COVID-19 pneumonia.
| Independent multimodal variable | Coefficient | OR | 95%CI | | Z | | p-value |
|---|---|---|---|---|---|
| Consolidations (yes/no) | ß1 | 0.96 | 1.89–4.53 | 0.05 | 0.96 |
| Patchy distribution (yes/no) | ß1 | 1.51 | 0.37–6.39 | 0.57 | 0.57 |
| Crazy paving sign (yes/no) | ß2 | 2.97 | 0.22–58.69 | 0.80 | 0.42 |
| SUVmax (continuous) | ß4 | 0.63 | 0.41–0.90 | 2.33 | 0.02 |
OR, odds ratio; 95%CI, 95% confidence interval; ß, beta coefficient.
A sample of selected cases, showing a combination of different structural changes and parenchymal lung FDG uptake are illustrated in Figs. 3 and 4.
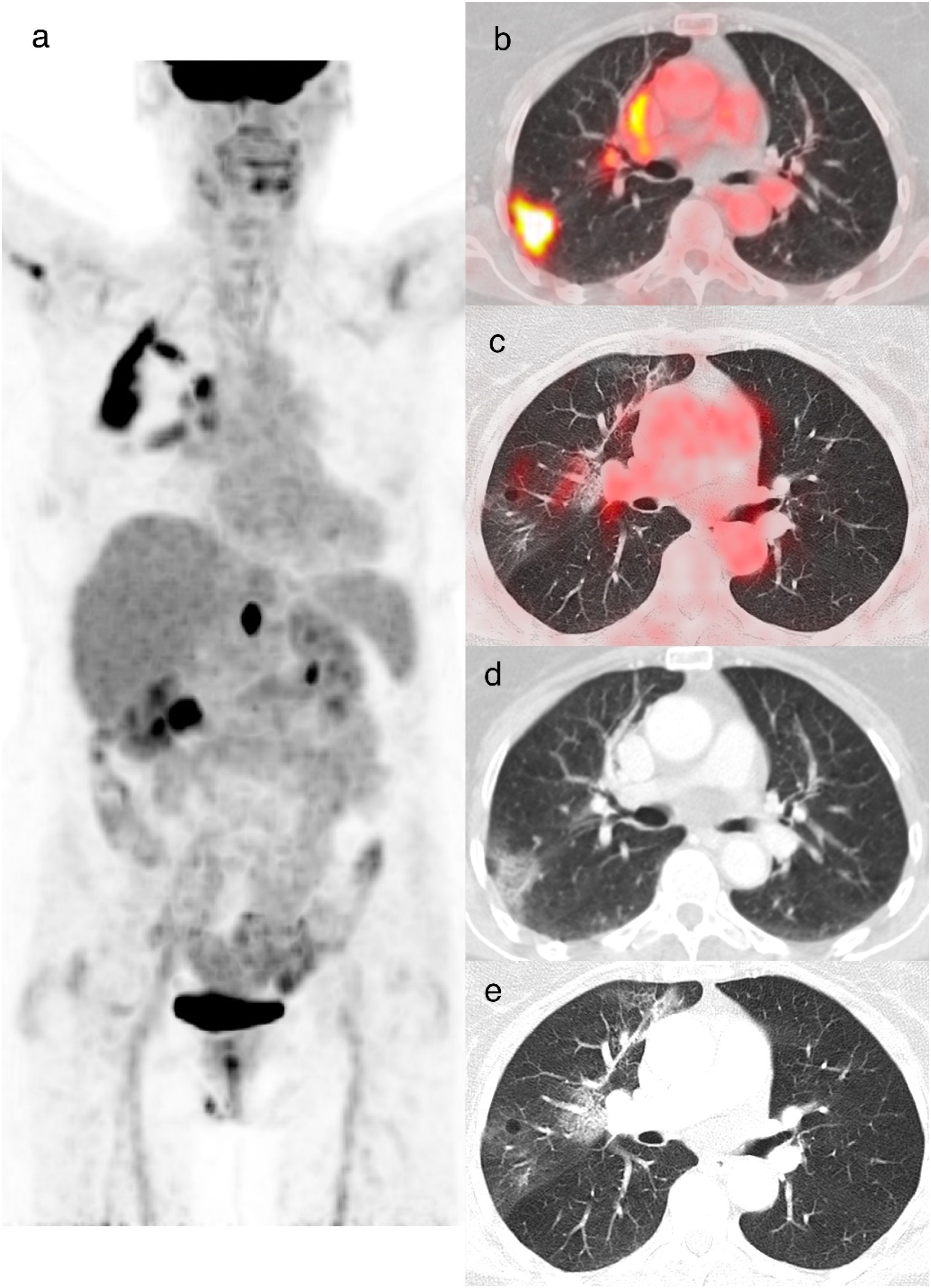
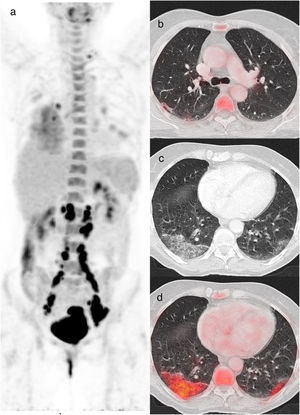
A 60-year-old woman diagnosed with cervical cancer, PET/CT to evaluate lymph node involvement. The maximum intensity projection image (a) shows increased FDG uptake in the cervix and FDG-avid lymph nodes in the left supraclavicular and infradiaphragmatic locations related to her oncologic pathology. FDG uptake is also visualized in lungs and in hilar and subcarinal lymph nodes, related to infectious/inflammatory pathology. Axial and CT images showed a subpleural band in the right upper lobe (b), peripheral crazy paving pattern in the right lower lobe and bilateral GGO (c) with increased 18F-FDG uptake with SUVmax value of 5.5 (d). The rRT-PCR was positive for COVID-19 (CO-RADS 6).
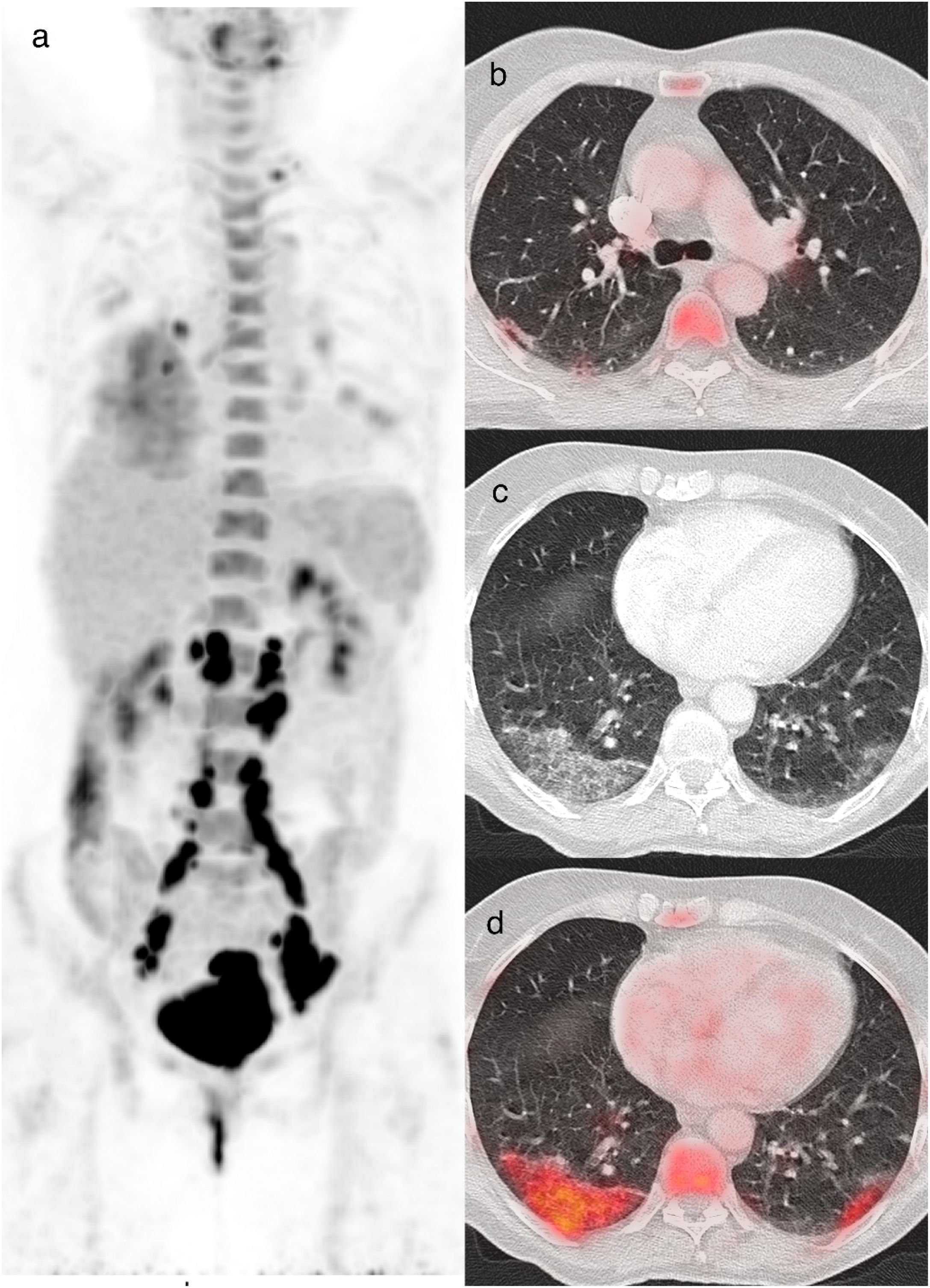
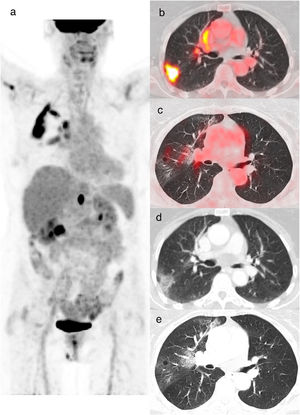
An 85-year-old woman with colon cancer, PET/CT to evaluate treatment response. The maximum intensity projection image (a) demonstrates an FDG-avid abdominal focus related to a retrocrural tumoral lymphadenopathy. Also shows increased FDG uptake in the right lung. Axial fusion (b, c) and CT images (d, e) showed increased FDG uptake (SUVmax 13.2) in the peripheral GGO opacities of the right upper lobe. Axial CT image (e) shows peripheral crazy paving pattern in the right upper lobe. rRT-PCR for COVID-19 was positive (CO-RADS 6).
We found incidental findings suggestive of COVID-19 pneumonia in 7.7% of patients derived to PET/CT. This proportion is similar to the rate described by a recent meta-analysis of 18F-FDG PET/CT findings (2.1–16.2%).5 Although some investigations have been focused on the possible diagnostic value of pulmonary SUVmax in COVID-19 pneumonia, the metabolic behavior of several lung parenchyma changes integrated and beyond CO-RADS categories has not been evaluated in detail so far.
As expected, we found a major overlap of CT signs in each patient, that were most evident in those patients with typical CT findings or confirmed COVID-19 pneumonia (85% of our patients classified as CO-RADS 5−6showed at least 6structural changes on CT). In addition, higher lung SUVmax was associated with some CT sings in patients with higher CO-RADS scores, especially GGO, bilaterality and consolidations. Focusing on COVID-19 pneumonia, the most frequently described radiological manifestation is bilateral GGO.6
Metabolic shift toward anaerobic glycolysis of activated inflammatory cells overexpressing glucose transporters results in increased FDG uptake.7 Some previous studies have revealed that the number of CD45+ cells and CD8+T cells in lung parenchymal findings correlates positively with SUVmax.8 Perivascular lymphocytic inflammation with sparing of distal airway lumen is the main histological feature observed during both early and late stages of SARS-CoV-2 pneumonia.9–12
Pathophysiological and structural evolution of COVID-19 pneumonia can be subdivided into 4 main morphological stage. First, early stage (day 0–1) is characterized by focal edema, incipient epithelial damage and neutrophilic capillaritis/endothelitis, often combined with microthrombosis. A second stage of exudative diffuse alveolar damage (DAD) is developed in days 1−7. At this moment, patients with overt respiratory failure present DAD with hyaline membrane formation and type 2 pneumocyte hyperplasia, variably complicated by superinfection. GGO with interstitial reticular thickening on CT is associated with this mid-stage DAD. As disease progresses, GGO may disappear or become more confluent and generalized, even reaching frank parenchymal consolidation. In this situation, the consolidation pattern is mainly associated with the later stages of DAD, consisting of the third organizing phase (≥1 week) and the late, fibrotic phase (weeks-months). Although this chronology scheme indicates an orderly progression through different stages, and even when the fibrotic phase is usually associated with long-standing severe disease, it is striking that different manifestations of DAD often coexists, reflecting the marked spatial and temporal heterogeneity of COVID-19 pneumonia.13–16
Since first morphological stage of COVID-19 pneumonia is characterized by fewer cellular components (especially in the fluid-filled intraalveolar regions), it is coherent to find lower SUVmax values on PET/CT in patients suffering an early pulmonary infection. During morphological stages 2 and 3, which in turn would correspond to GGO and consolidation on CT (especially observed in CO-RADS categories 3–5), the cellular and inflammatory component becomes more prominent, resulting in higher SUVmax values, as observed in our patients. Finally, during morphological stage 4, the destruction of the lung parenchyma and the development of pulmonary fibrosis of varying degrees could explain the lower SUVmax values on PET/CT (CO-RADS 1).3,4,14–16 Because our experience included patients referred to PET/CT during the first local outbreak of the pandemic, it was difficult to detect and analyze this late metabolic stage of COVID-19 pneumonia.
As for the crazy paving pattern, which presents as thickened interlobular septa and/or superimposed intralobular lines on a background of GGO, histopathologically corresponds to exudative DAD (stage 2) characterized by thickened alveolar septa with perivascular lymphocytic-plasmacytic infiltration. On the other hand, the patchy distribution corresponds histologically to the presence of vascular damage and thrombosis (endothelitis/microthrombi/microhemorrhages), as well as exudative DAD (stage 1 and 2).17,18 Therefore, the high inflammatory cellular component translates into a high FDG uptake observed in patients in our study who exhibited these two patterns (crazy paving and patchy distribution).
Some investigators have postulated that FDG uptake in COVID-19 pneumonia could be mainly related to the presence of neutrophil infiltration found at autopsy in patients with bacterial superinfection.19 It has also been suggested that these changes could be fundamentally related to the frequent development of ventilator-associated bacterial pneumonia. We think that the hypothesis of a histological scenario differing markedly from the typical lymphocytic inflammation around pulmonary vessels, bronchioles, and within interstitial spaces (predominantly CD8+ and CD4+T cells) of SARS-CoV-2 pneumonia10–12,20 should be evaluated in future prospective investigations.
The histopathological complexity of COVID-19 pneumonia is not limited to a simple infiltration of infectious and inflammatory cells, but can be accompanied by a variable vascular damage such as capillary leak and thrombosis. In addition, the variation of lung parenchyma SUVmax could also be influenced not only by the time from infection, viral strain and comorbidities, but also by several factors such as patient weight, motion artifacts, systemic and regional blood glucose levels, dose extravasation, accuracy of dose calibration and time between injection and imaging. The broad variation and combination of structural lung changes observed in our patients, usually associated with a systemic inflammatory state of diverse degrees, could justify the heterogeneous results obtained by different researchers. Bahloul et al., for example, found that patients with confirmed COVID-19 pneumonia showed larger CT abnormalities but lower consolidation rates and SUVmax compared to non-COVID-19 subjects,21 whereas Dietz et al. found no correlation between the inflammatory status with CT chest evolution or adverse clinical outcomes after comparing patients with COVID-19 showing a SUVmax ≥7 vs. <7, respectively, for assessing “inflammatory” vs. “low inflammatory” findings. In this last research, the authors found that COVID-19 patients presenting GGO or consolidation on initial chest CT exhibited several lung inflammation signs at the presumed peak of the inflammatory phase that varies widely from minor to extensive involvement.22
It is important to clarify that the presence of lymphadenopathies (defined as those with a short-axis dimension ≥10mm) has been analyzed exclusively on CT, as the CO-RADS categories are based on CT findings and was found in only one patient.3 This number would be higher if we consider the FDG-avid mediastinal lymph nodes described in COVID-19 patients undergoing PET/CT, generally without corresponding enlarged nodes on CT.22–27
In relation to oncological patients, it is relevant to perform differential diagnosis of COVID-19 pneumonia with several inflammatory/infectious lung diseases such as other viral, bacterial or fungal agents and co-infectious or other infrequent pneumonias, non-infectious diseases like pulmonary vasculitis, dermatomyositis, organizing pneumonia and post-therapeutic changes related to cancer, such as radiant or cytostatic pneumonitis, carcinomatous lymphangitis or secondary or new-onset tumor lesions, should be taken into account. In non-cancer patients, this differentiation is also extremely important to define an adequate therapeutic management. In both cases, it is clinically relevant for nuclear medicine and radiology specialists to pay special attention to incidental findings suggestive of COVID-19 pneumonia on 18F-FDG PET/CT studies, especially in high-risk populations.2
We assume that our study has some limitations. The first is intrinsically due to the small sample of patients studied in a single referral center. Unfortunately, clinical practice during the public health emergency made rRT-PCR unavailable in many patients with PET/CT findings suggestive of COVID-19 pneumonia. Although the diagnosis of COVID-19 pneumonia was based on the results of previous researches demonstrating a high diagnosis yield of a combination of CT findings with or without molecular confirmation (CO-RADS categories 6 or 5, respectively), our results require further confirmation in larger-scale investigations. A larger sample could provide more power to the multivariate analysis and define the potential predisposition of patients with autoimmune or inflammatory diseases. Second, as time elapsed between infection and 18F-FDG PET/CT study was unknown, the real impact of an early diagnosis during the course of infection and a more accurate interpretation of metabolic activity related to some individual structural lung changes was not possible. Third, only one examiner interpreted CT images, and interobserver variability in each CO-RADS category was not assessed. Finally, the magnitude of a referral bias could not be estimated.
Conclusions18F-FDG PET/CT is a useful tool during incidental detection of findings suggestive of COVID-19 pneumonia in selected patients undergoing this imaging technique. The finding of a high lung SUVmax was associated with several combined CT findings of COVID-19 pneumonia, especially in those exhibiting diagnostic CO-RADS scores. Further studies are required to assess the potential benefits of these multimodal findings in predicting the prognosis and monitoring the response to emerging new therapeutic interventions, especially in high-risk subpopulations such as cancer patients.
FundingThis study was supported by Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IdISSC).
Ethical approvalThis research was approved by the institutional Ethical Committee of Clinical Research (IRB no. 20/524-E) and followed the 1964 Helsinki Declaration and its later amendments. All patients included in the study signed a generic consent before PET/CT. Due to the retrospective and real-life design during the first outbreak of COVID-19 pandemic in Spain; the need for individual informed consent was waived.
Conflict of interestThe authors have no relevant financial or non-financial interests to disclose.