This publication presents criteria and bases for the work organization in the safe practice of Hospital Radiopharmacy, in order to minimize the risk of viral transmission during the COVID-19 pandemic, in a reference facility of the National Energy Commission Atomic of Argentina, while continuing to perform essential services for the health system.
For this purpose, documents from the National Energy Commission Atomic, IAEA, WHO and other scientific publications were consulted as reference. These recommendations are under constant review and are permanently updated.
Within this framework, the present model of work organization for this essential activity is proposed, including general and specific recommendations and its epidemiological and immunological basis.
En esta publicación se presentan criterios y fundamentos para la organización del trabajo en la práctica segura de la Radiofarmacia Hospitalaria, a fin de minimizar el riesgo de transmisión viral durante la pandemia de COVID-19, en una instalación de referencia de la Comisión Nacional de Energía Atómica (CNEA) de Argentina, mientras se continúan desempeñando servicios esenciales para el sistema de salud.
Con este fin se consultaron como referencia documentos de la CNEA, OIEA, la OMS y otras publicaciones científicas. Estas recomendaciones se encuentran en proceso de revisión constante y son actualizadas de manera permanente.
En este marco se propone el presente modelo de organización laboral para esta actividad esencial incluyendo recomendaciones generales, particulares y su fundamento epidemiológico e inmunológico.
We are currently going through a pandemic in which all activities are modified in order to continue life as safely as possible. Hospital radiopharmacy is no exception and should have an adequate work plan which is objective of the present article.
Safe practice of the diagnostic and therapeutic procedures performed within the setting of nuclear medicine requires a permanent supply of high quality radiopharmaceuticals prepared according to good radiopharmaceutical practices.1 This is the objective of hospital radiopharmacy.
The head of hospital radiopharmacy should be a qualified professional who can guarantee the final product and the application of a quality system that ensures all the aspects of Good Radiopharmaceutical Practice. The need for acting with foresight and developing a culture of constant evaluation and supervision must be emphasized.2–4
The technological or organizational capacities and competences made available to Nuclear Medicine Departments by Hospital Radiopharmacy Services enables the adoption of measures to improve the services provided, being of added value to critical situations such as a pandemic.
In the current scenario, all of the most adequate health care measures are taken in patients who are positive or suspected of having COVID-19 and require nuclear medicine procedures. These measures are aimed at protecting the health care personnel performing the procedure and thereby ensuring the continuity of services.5
The documents consulted which include the institutional CNEA6 procedures as well as the IAEA7,8 and WHO9 recommendations and other scientific publications5,10–12 are the foundation of the safety protocol designed.
General recommendations for personnel for task developmentIn order to fulfill the specific objectives of hospital radiopharmacy, the general recommendations inherent to the Center of Nuclear Medicine (CNM) of the CNEA are listed taking into account the relationship between these two areas:
- •
Reinforce general hygiene measures.
- •
Respect hygiene norms of the admission of personnel and patients to avoid circulation of the virus from the exterior to the interior of the CNM.
- •
Train personnel by providing instructions indicated for the situation of a pandemic and define controls of compliance to ensure the functioning of radiopharmacy according to the present protocol.
According to the WHO, the following points must be considered in order to ensure the health of the personnel, the patient and related persons, family members, etc. With these considerations, the following work strategies are proposed:
1. Coordinate tasks.
Segregate the personnel in fixed equipment with different days, reducing the number of personnel to the essential minimum and promoting communication and interdisciplinary work.
Each group must have a professional who is responsible for the group and has autonomy for decision making, thereby avoiding delays in activities which could produce major exposure of both patients and personnel.
2. Establish relevant tasks
Achieve medical consensus to establish which studies are urgent and which can be reprogrammed in order to avoid unnecesarily expose of personnel in the hospital setting. For example, radiopharmaceuticals for studies such as the detection of sentinel lymph nodes, the localization of digestive hemorrhage or emergency cardiology studies should be labeled the same day.
3. Optimize platforms for maximum use of remote access possibilities
Promote internal telephone communication in the department, with periodical virtual meetings among professionals of different sectors. Fluid communication between the personnel and the heads of each area is essential.
4. Establish effective and safe flow of patients and personnel
Favor social distanicing between health personnel and patients and between the same health care providers, organizing a calendar of patients so that the personnel can perform their tasts calmly and carefully, as well as the corresponding disinfection tasks (of equipment, common areas, etc.)
5. Ensure rapid mechanisms of supply and replacement of personal protection equipment (PPE) according to the task.
Provide workers with the personal supplies necessary to ensure full protection. When some element is lacking, the head of the sector must be immediately informed in order to undertake the necessary measures to replenish the stock.Tasks must not be developed without the necessary elements of personal protection required.
6. Control the stock and record the delivery of products of disinfection and PPE
Each worker must have their own PPE and must establish that the PPE supplies were given in a closed package. Each person must be responsible for the care, correct use and discarding of their PPE.9,10
Special considerations related to hospital radiopharmacy personnel- 1
If any member of the hospital radiopharmaceutical staff is suspected of having become infected or presents any symptom of COVID-19 during or after their workday or has been in contact with a confirmed case in the last 14 days, the immediate correponding head and the Medical Services must be informed according to the department. Thus, this person must not come to the nuclear medicine department and must remain at home and follow the mandatory social isolation.
- 2
The personnel of Hospital Radiopharmacy are capacitated and trained in the aspetic management of injectables. Tasks are developed in a clean area under established protocols which allow safe delivery of radiopharmaceuticals according to the CNEA Hospital Radiopharmacy Quality Management System (Fig. 1).
- 3
In the clean area and hot lab the cleaning protocols are periodically reinforced and the registry of these tasks are verified.
- 4
Patients must be a safe distance before the entry of personnel into the hot lab.
Up to now there is no prophylaxis to fight this disease. Therefore, we must prevent contacts, seeking useful criteria to avoid dissemination of the virus in our essential shared work tasks. In addition to criteria for selecting diagnostic and prognostic tests and the detection of the development of protective immunity, the actions must minimize the factor of contagion, and this is not possible without mitigation measures.
Several articles report the care which health care personnel and other essential workers must have and describe useful criteria for the organization of work groups.5,9,10
The activity of hospital radiopharmacy should reflect the current situation of the pandemic, with simple management and clear biochemical and immunological concepts.
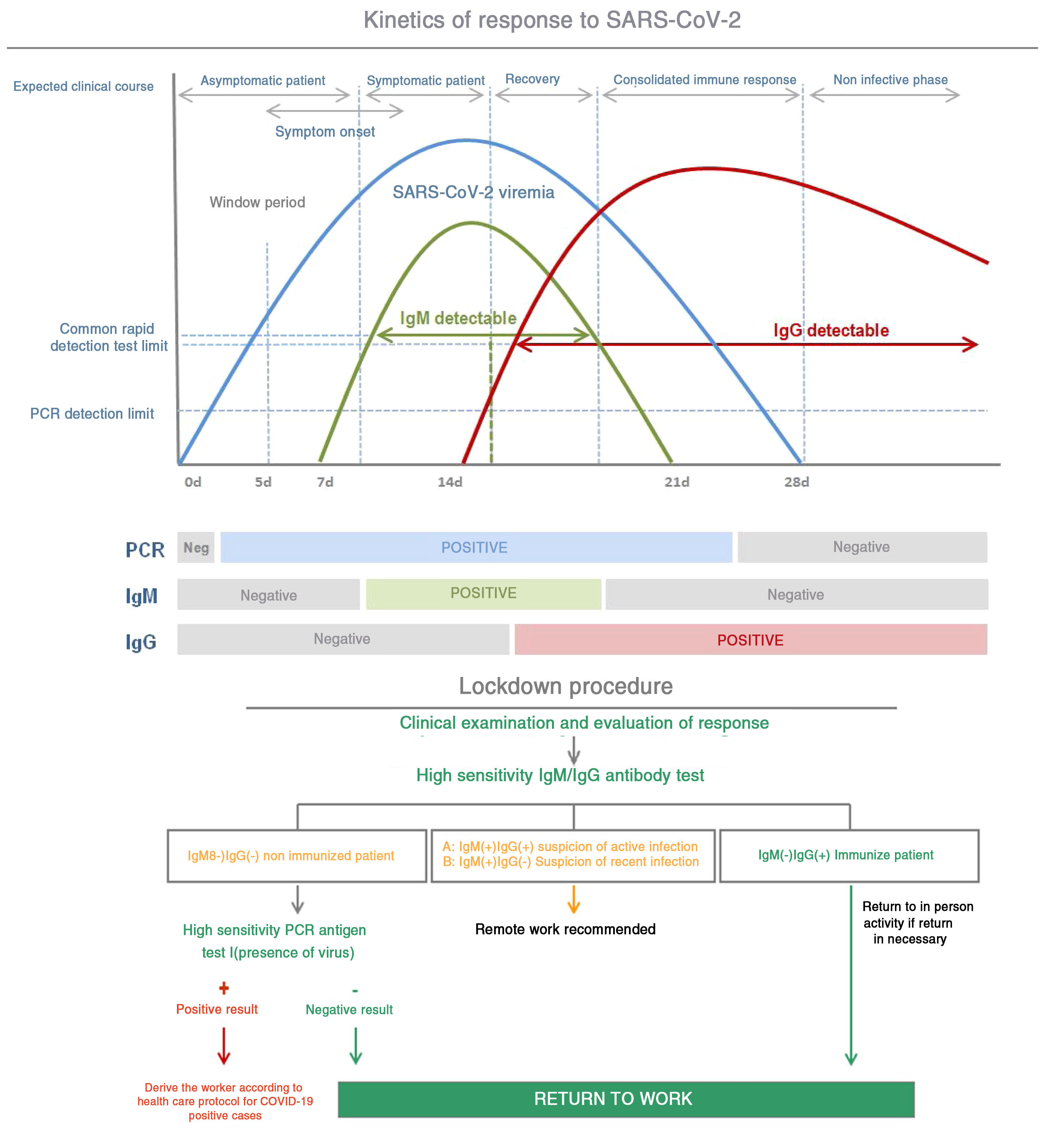
Following the decoding of the viral genoma in China, sequencing of the RNA of the virus was used to design different diagnostic tests around the world. The reverse transcriptase polymerase chain reaction (RT-PCR) test detects the presence of the virus. The theoretical specificity of these tests is 100%, because the design of the imprinting is specific of the SARS-CoV-2 genome sequence.
In regard to immunologial response, the recruitment mechanism of white blood cells or lymphocytes, which are the producers of antibodies (Ab), is of note.
There are two types of lymphocytes, the B and the T. B lymphocytes are responsible for the immune system mediated by antibodies; that is, they are differentiated into plasma cells which produce antibodies versus antigens which are proteins constituting the virus. T lymphocytes are responsible for the immune system mediated by cells. Some are cytotoxic T lymphocytes (cytolytic T lymphocytes) and natural cytolytic lymphocytes (natural killer [NK] cells) which establish physical contact and destroy cells which are foreign or altered by the infectious agent. Certain T lymphocytes are responsible for the onset and development (helper T lymphocytes) or suppression (Treg lymphocytes) of most immune responses mediated by antibodies or cells. This regulation occurs by the release of signaling molecules known as cytokines.
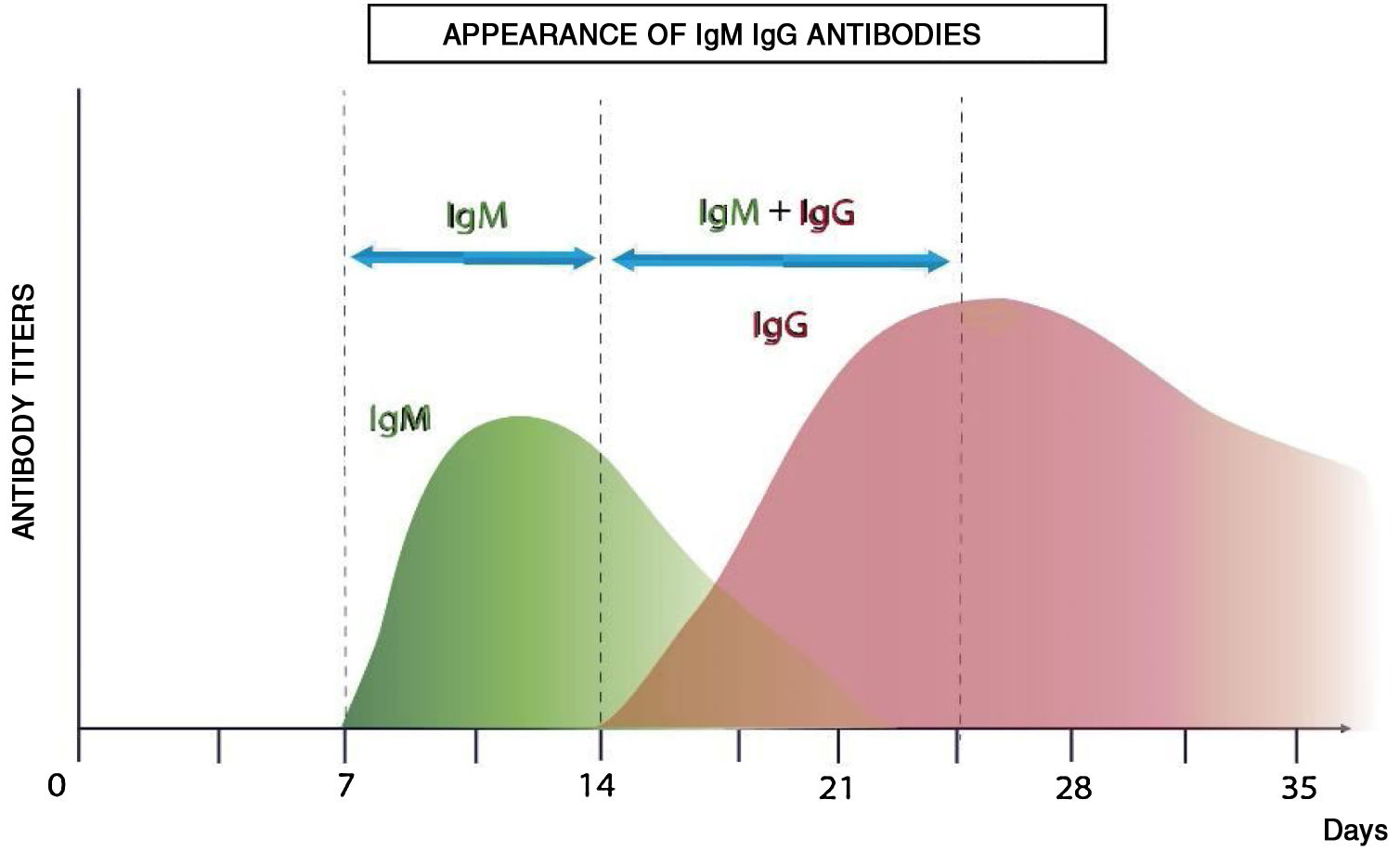
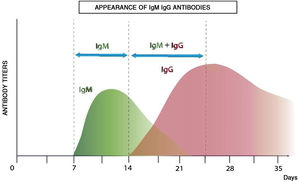
Antibody-mediated immunity is studied by serological analyses with enzyme-linked immunoassay (ELISA) tests which analyze and quantify immunoglobulins. The variety of antigens of the virus enables the design of different diagnostic kits. Fig. 2 shows the theoretical immunological profile of a virual infection.
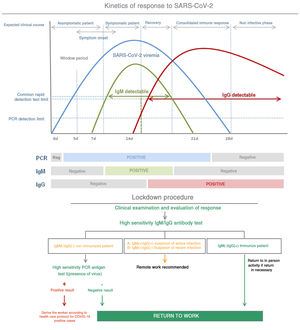
After infection, IgM antibodies are generated (detectable 7 days post infection) followed by IgG antibodies (detectable 14 days post infection).13Fig. 3 shows the kinetics of the immunological response of an infected person and the relationship with the evaluation of risk of contagion and propagation of the virus.
The viral load generally reaches its maximum peak early in the disease and then reduces as the antibodies develop and antibody titers increase during the following 2 to 3 weeks.
The utility of this criterion is that an immunocompetent person develops this immunological profile in the case of contagion, and it is therefore important to take this into account in the work groups.
The serological diagnosis is especially important for asymptomatic patients or those with mild to moderate disease. This is becoming an important tool for understanding the spread of COVID-19 in the community and to identify people who are immune and potentially “protected” against becoming infected.
The most sensitive and safe serological marker is the determination of IgM and IgG antibodies. The highest levels are produced in the second and third week of disease, although positive IgM and IgG have been found by ELISA even on the fourth day after symptom onset.11
This method has a specificity greater than 95% for the diagnosis of COVID-19. The diagnostic accuracy can be further increased by pairing the initial PCR and its repetition two weeks later. Nonetheless, the long-term persistence and duration of protection conferred by the neutralizing antibodies remains unknown.12
The rapid spread of SARS-CoV-2 has demonstrated that it is important to have specific diagnostic tests that can identify patients infected by the virus as well as those who have developed protection against the disease.
In patients with compatible clinical manifestations and negative PCR results, the presence of antibodies helps to confirm the diagnosis of COVID-19.11,12
The length of PCR positivity and seroconversion varies among children and other groups, including the large population of asymptomatic individuals who are not diagnosed without active surveillance.
Many questions remain to be answered, especially in regard to the potential length of immunity in both asymptomatic and symptomatic individuals infected by SARS-CoV-2.
Application to work organizationAt an occupational level, the bibliography indicates the following categorization within the work staff considering analysis of their immunological profile:
- •
Active personnel not presenting clinical manifestations: the presence of antibodies provides relevant data on possible current or previous infection allowing avoidance of dissemination and new infections.
- •
Personnel who have had the infection and return to work: information on immunocompetence status is of great importance for planning against new outbreaks of the pandemic. Protected personnel can be in contact with COVID-19 positive patients with minimum risk to both patients and colleagues.13
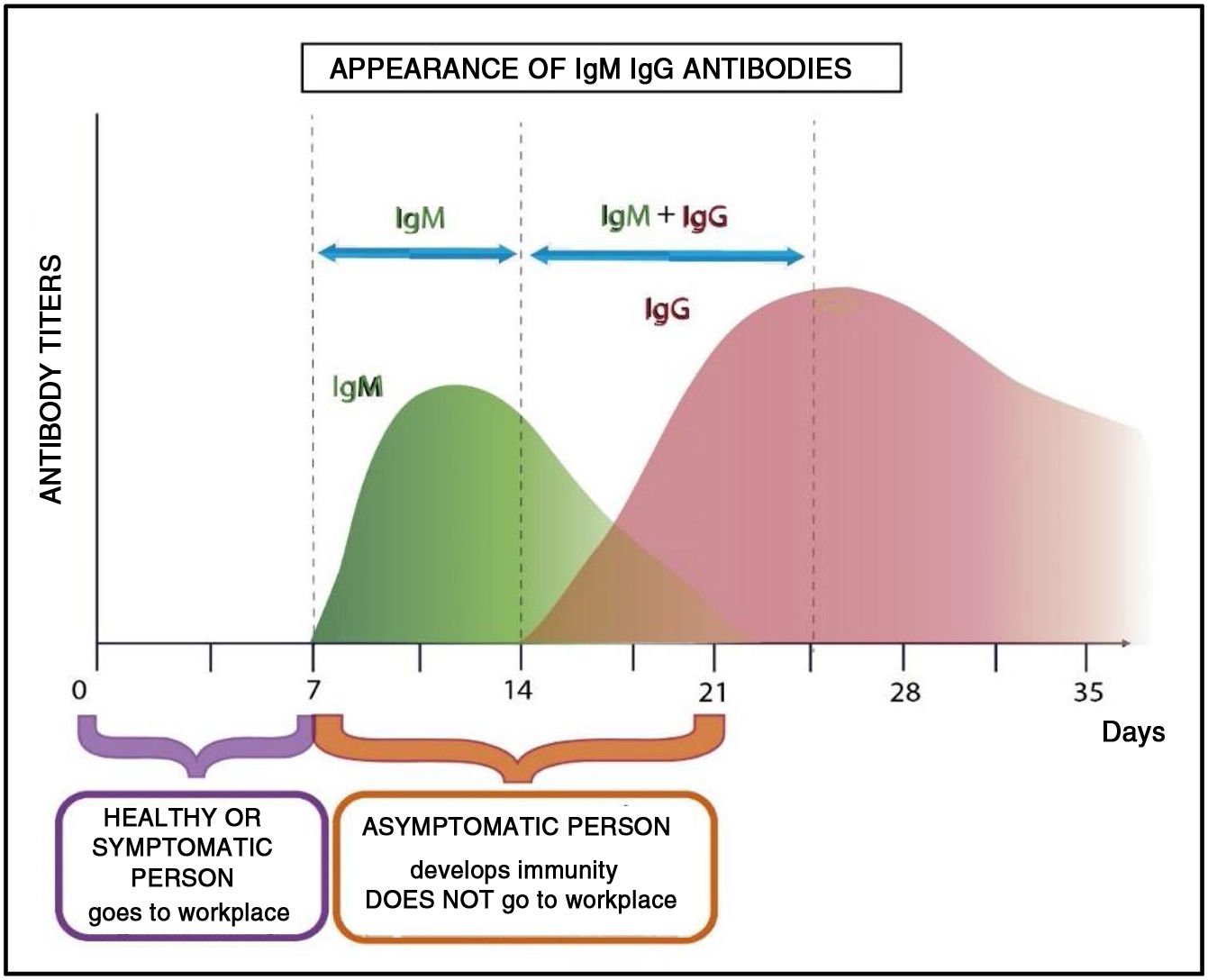
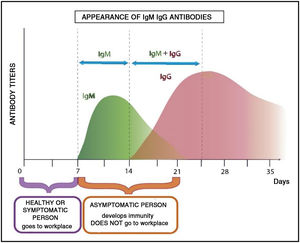
Considering the theoretical immunological profiles, the personnel affected must perform their work activities in person during one week and remotely for two weeks with the aim of minimizing the proability of contagion to their colleagues and hospital setting in general on their return.
Fig. 4 shows the development of the immunological profile of an asymptomatic worker who has contracted the disease during a week of in person work in a health institution.
If the infected person presents very mild or doubtful manifestations of infection, the immunological profile shown in the previous graphs would show that the disease followed its course. From the biochemical point of view, the variations in IgM and then IgG antibody levels would indicate that the worker had acquired immunity.14
As the meaning of the word “immunity” indicates, the invaded host resisted the invasive agent, and thus, transmission is not possible. This is fundamental to avoid massive contagion of personnel.
As with most virus, SARS-CoV-2 invades human cells with exclusive intracellular proliferation linked to its particular characteristics, many of which currently remain unknown. On the other hand, the particular reactions of the host do not vary greatly. These reactions mainly involve differences in the duration of immunty, with antivirus immunity generally being more prolonged than antibacterial immunity.15
ConclusionFor epidemiological studies and work planning it is important to consider the presence of specific antibodies in order to contain contagions as well as to determine the percentage of workers who have generated protective immunity.
The criteria described evaluate the state of immunocompetence to a virus and the return of personnel to the workplace to prevent contagion and viral dissemination and collaborate with the economic impact generated by the COVID-19 pandemic.
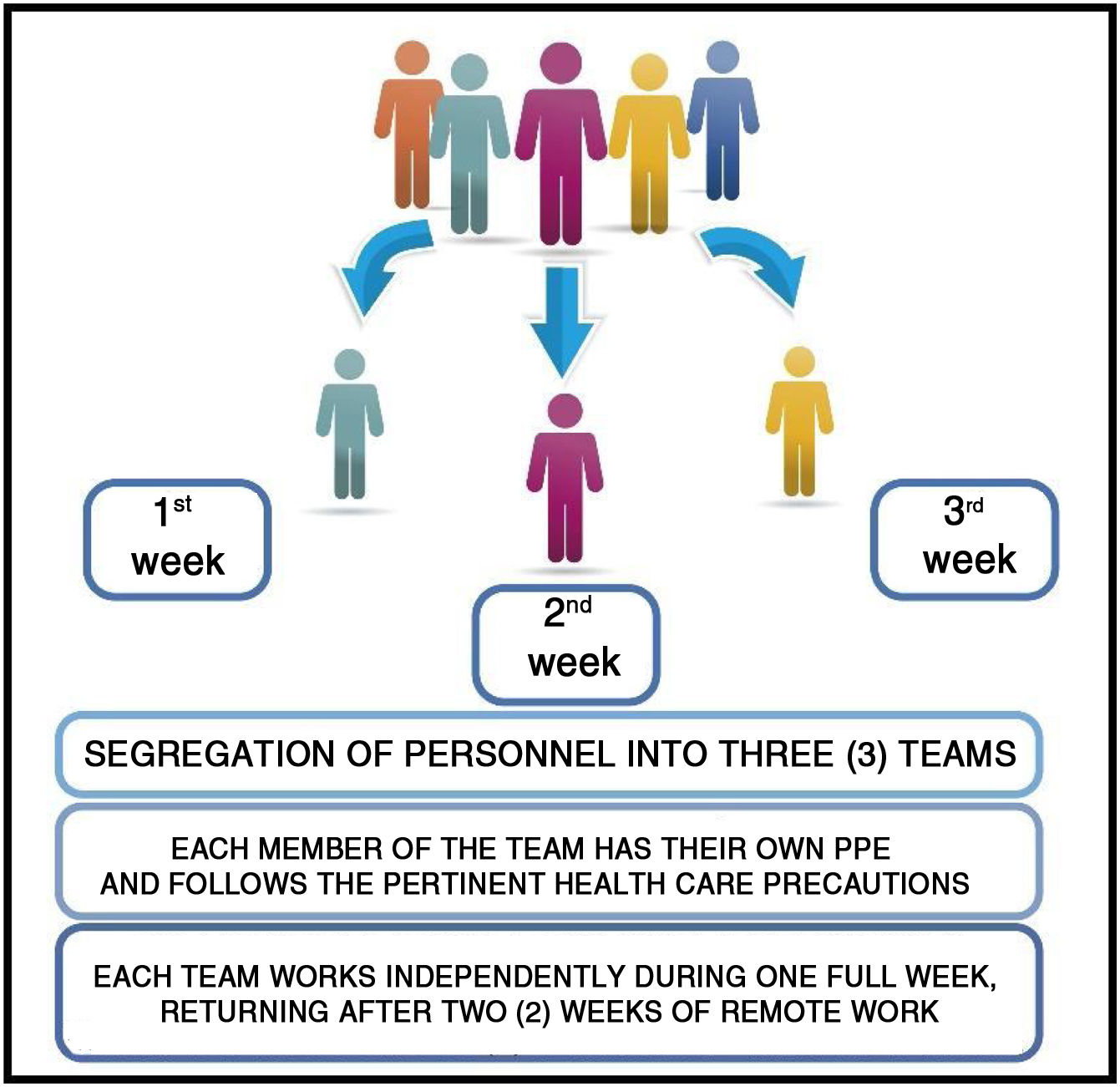
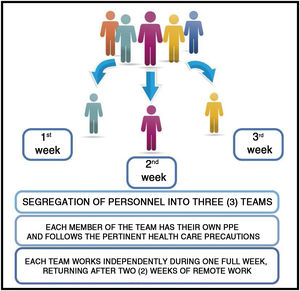
The safety of personnel replacement is fundamental for immunity and this supports the three team segregation model: one team that works in person for one week and is then replaced by two other teams during the following two weeks (Fig. 5).
In this way, if a member of the team acquires the disease, without presenting symptoms, this person will not be in contact with the other work teams because of the rotation system proposed. All follow a 14-day period during which they will develop the disease and/or acquire immunity, reducing the probability of dissemination and contagion.
The rotation scheme proposed guarantees the continuity of activities of Hospital Radiopharmacy which are essential for the diagnosis and treatment of patients taking into account the ensured quality of the labeled product delivered by this service.
In conclusion, considering the normal immunological profile of an individual in the face of a viral infection and supposing that an asymptomatic person goes to work, segregation into at least three work teams with weekly rotation ensures the provision of services in a Nuclear Medicine Center.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rabiller G, Cordoba N, Poch C, Silva Paulo P, Seijas M, Vazquez MF. Radiofarmacia Hospitalaria en Argentina durante la pandemia de COVID-19. Criterios y fundamentos para la organización del trabajo. Rev Esp Med Nucl Imagen Mol. 2020. https://doi.org/10.1016/j.remn.2020.08.015