To study the efficacy of tranexamic acid to decrease perioperative bleeding in patients who have undergone a total hip arthroplasty operation and to evaluate drug safety.
Materials and methodsObservational, prospective, controlled and randomised study on the efficacy of tranexamic acid as a method to reduce bleeding in primary hip replacement surgery. We included 134 patients operated during 2014 in our centre, who were divided into 2 groups according to whether or not they had received tranexamic acid. The main study variables were haemoglobin and haematocrit levels, the amount of blood collected from the post-operative drain in the first 12, 24 and 48h and transfusion requirements.
ResultsPost-operative haemoglobin and haematocrit levels were statistically higher (p<0.001) in the group with treatment. During the first 48h bleeding values from the group that did not receive TAX were higher compared to patients treated with tranexamic acid.
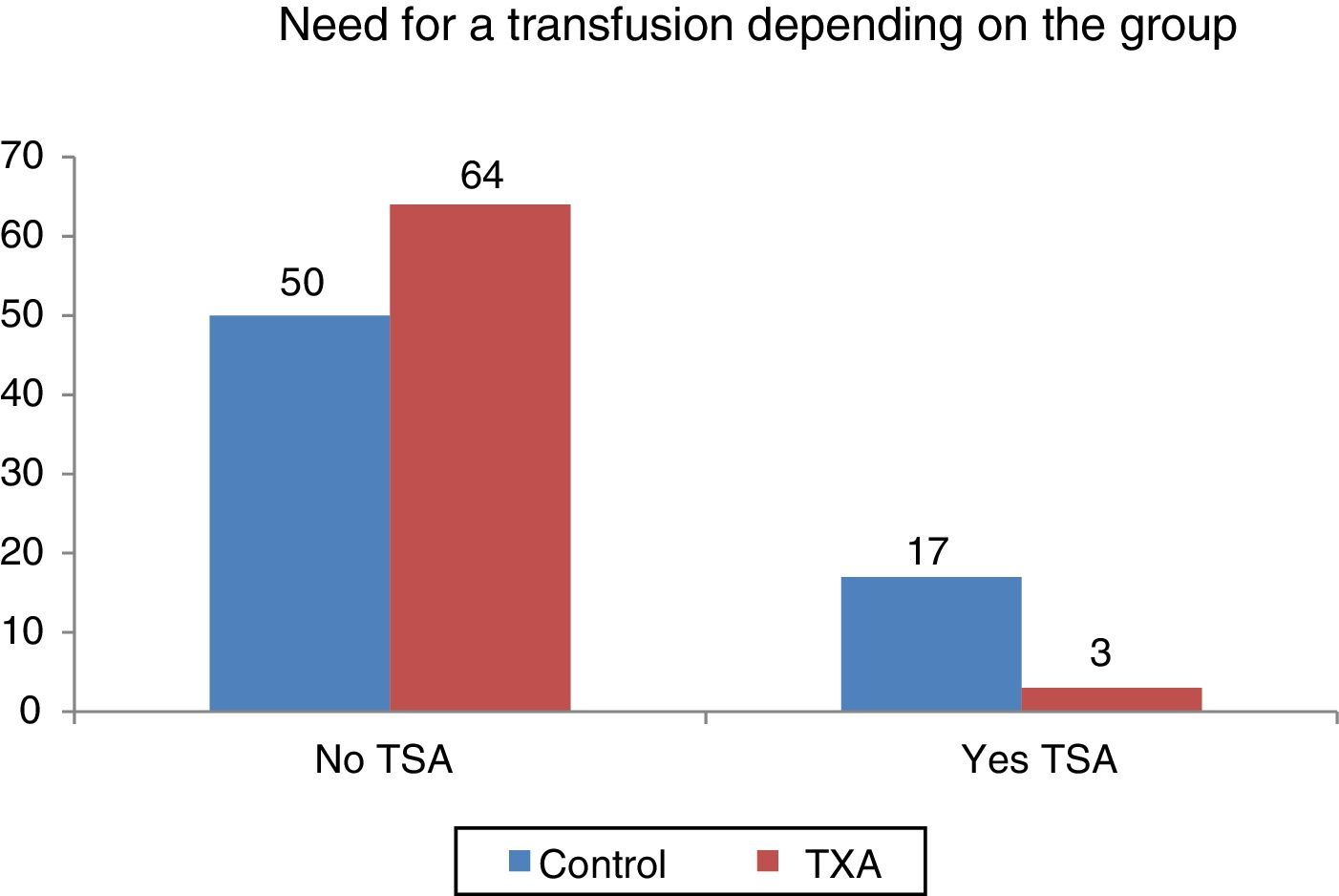
Statistically significant differences (p=0.001) were found as to the need for transfusion according to group, more transfusions were performed in the cohort that had not received tranexamic acid: 25.37% compared to 4.48% for the group with tranexamic acid.
No adverse events related to administration of tranexamic acid were recorded.
ConclusionAdministration of tranexamic acid has proved to be an effective and safe method to reduce peri-operative bleeding in patients who underwent total hip arthroplasty and avoids allogenic blood transfusion. Therefore, tranexamic acid treatment could entail a financial saving for the healthcare system and expose the patient to less risk.
Estudiar la efectividad del ácido tranexámico como método para disminuir el sangrado perioperatorio en pacientes intervenidos de artroplastia total primaria de cadera, así como su seguridad.
Material y métodosEstudio prospectivo, aleatorizado y controlado sobre la eficacia del ácido tranexámico como método para disminuir el sangrado en cirugía protésica primaria de cadera. Se han incluido 134 pacientes intervenidos durante el año 2014 en nuestro centro, los cuales se han dividido en 2 grupos según se le ha administrado o no ácido tranexámico. Las variables principales del estudio fueron los niveles de hemoglobina y hematocrito posquirúrgicos a las 24horas, la cantidad de sangre recogida en el drenaje postoperatorio a las 12, 24 y 48horas, así como las necesidades transfusionales.
ResultadosLos niveles de hemoglobina y hematocrito posquirúrgicos fueron estadísticamente superiores (p<0,001) en el grupo al que se le administró ácido tranexámico. En las primeras 48horas los valores de sangrado del grupo control fueron mayores con respecto a los pacientes tratados con ácido tranexámico.
Se encontraron diferencias estadísticamente significativas (p=0,001) en cuanto a la necesidad de transfusión en función del grupo, siendo superior en el grupo control (25,37% frente a 4,48% del grupo tratado).
No se registraron eventos adversos relacionados con la administración de ácido tranexámico.
ConclusionesLa administración de ácido tranexámico ha demostrado ser un método efectivo y seguro para disminuir el sangrado perioperatorio en pacientes intervenidos de artroplastia total primaria de cadera, y así disminuir las necesidades transfusionales.
The demand for blood transfusion in orthopaedic surgery has increased during the last few decades. Despite exhaustive controls, allogenic transfusion is not risk-free, with regards to both the transmission of infectious and contagious diseases and reactions to transfusions, or the immunomodulator effect which may increase the risk of post-operative infection. Added to this is the fact that transfusion increases hospital stay and healthcare costs. As a result, a series of measures have been developed, aimed at reducing to a maximum the need for blood transfusions. Pharmacological measures, such as tranexamic acid (TXA), marketed as Amchafibrin®, which is a synthetic derivative of lysine with a pure antifibrinolytic action,1–7 are among these.
There is scientific evidence reported in the literature on the use of tranexamic acid as a means of saving on transfusions in total hip arthroplasty (THA), due to its efficacy and safety in randomised studies and meta-analysis, but its use to this end remains controversial in Spain.8–10 For this reason we have designed this study, with which we have tried to achieve a higher level of evidence for the use of this drug in our specialty.
The main aim of this study was to examine the effectiveness of tranexamic acid as a method of reducing perioperative bleeding in patients who had undergone primary THA. Our secondary objective was to evaluate how this drug affected homologous blood transfusion needs, and to assess its safety.
Materials and methodsA prospective, randomised study was conducted which included 134 consecutive patients, diagnosed with coxarthrosis and who had been treated with THA by 5 surgeons experienced in this surgery, under spinal anaesthesia, in our hospital in 2014. To ensure randomisation, the hospital clinical file number was used, assigned in order of patient entry, with the result that even numbers were assigned to group A and odd numbers to group B. Both groups were paired regarding variables which could affect the study, such as the comorbidity measured by the Charlson index, grade ASA, IMC, age, gender and pre-surgical analytical parameters, including Hb and Hct values, the 2 groups thus being homogenous prior to intervention. The administration of tranexamic acid was performed by the anaesthesia team prior to surgery. No pre-surgical preparation or anaesthesia procedure was performed to try to reduce intraoperative bleeding. Careful surgical technique and haemostasis were employed as was a direct measurement of intraoperative bleeding. The implant model used was the same in all cases (Furlong® uncemented prosthesis). Minimum patient follow-up was 12 months.
Exclusion criteriaThe following patients were eliminated from the study: those allergic to TXA, those with liver failure, haematological diseases, retinopathy, cerebrovascular disease, severe ischaemic cardiopathy, severe kidney failure, severe lung failure, INR>1.4, coagulopathies, and a background of arterial or venous thromboembolic disease.
Study variablesThe sociodemographic variables of the patients were collected (age at the time of surgery, gender), clinical data (pre and post intervention haemoglobin and haemocrit levels, and 24h later, and number of transfusions used) plus variables inherent to surgery (time in surgery, number of days stay in hospital, amount of blood collected from the post-operative drain in the first 12, 24, and 48h).
Hospital surgical protocolA modified Hardinge lateral or posterior hip approach was performed depending on the surgeon's preference. In all cases, on completion of surgery, a drain was connected under sterile conditions with which the amount of blood collected in the first 12, 24, and 48h was measured, and the drain was then removed. After the intervention, the patients were moved to the recovery room, and were taken to the ward 3–4h later.
Surgery was performed under antibiotic prophylaxis with intravenous cefazolin with a pre-surgical dose of 2g, administered 30min prior to intervention and the same administered after surgery with a 1g dose every 8h (3 post-surgical doses). When the patient was allergic to penicillin they were administered intravenous clindamycin at a pre-surgical dose of 600mg (30min before intervention) a post-surgical dose of 600mg/every 8h (3 post-surgical doses).
All patients received thromboprophylaxis with a dose of low molecular weight (sodium enoxaparin [40mg; 0.4ml or 4000UI of Clexane®]) 12h before surgery and another dose 6h after surgery. It was then administered in a single dose every 24h up to one month after surgery.
The patient cohort with TXA were administered an intravenous dose of 15mg/kg of TXA (Anchafibrin®, Rottapharm) in 100ml of saline solution 15min before surgery, whilst the control group received 100ml of saline solution 15min prior to surgery.
Minimum pre-surgical Hb levels were established at 10g/dl.
Transfusion criteria were based on the clinical practice guidelines recommended by the American Association of Blood Banks.11–14 The patients were transfused when postoperative Hb levels, measured 24h after surgery, were below 8g/dl in patients with no previous heart disease and who were asymptomatic or below 9g/dl if the patient presented with a background of cardio respiratory disease or clinical signs during the immediate postoperative period.
Control of possible adverse effects such as deep vein thrombosis (DVT) was carried out with clinical follow-up, and a Doppler ultrasound was only used in cases where clinical suspicion was present.
Ethical aspectsThis study was approved by the regional ethical committee and informed consent from all patients in the study was obtained.
Statistical analysisInitially a descriptive statistical analysis was conducted where qualitative variables were expressed as frequency and percentage. Gaussian continuous variables were expressed as mean±standard deviation and non-Guassian variables as median (minimum–maximum). Kolmogorov–Smirnov tests were used to find out the normality of variables.
Parametric/non parametric tests were carried out to determine the potential association between study variables (Chi-square, Student's t-test, Mann–Whitney U test).
Differences of p<0.05 was considered statistically significant in all analysis. The statistical programmes SPSS 22.0 and Epidat 4.1 were used for analysis.
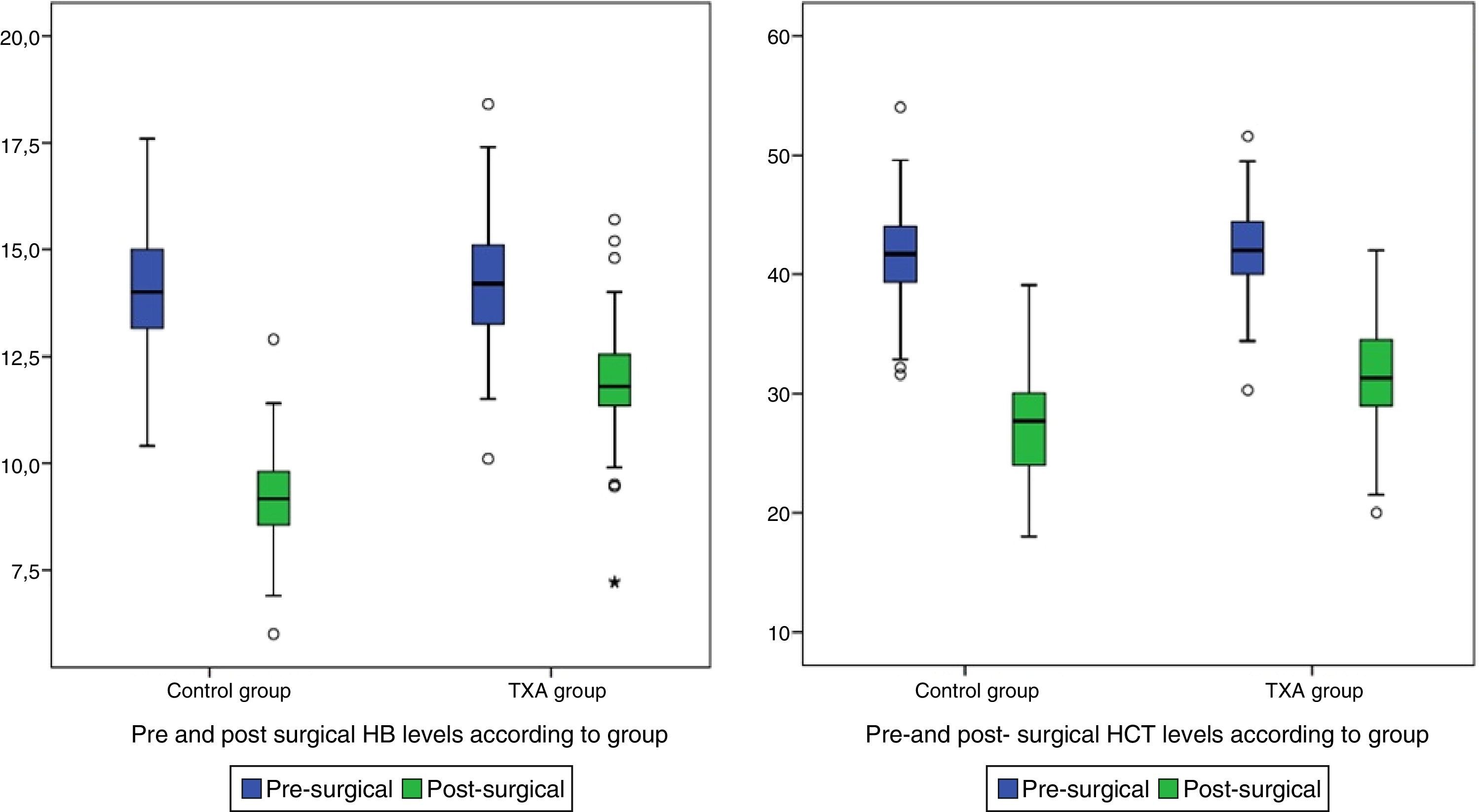
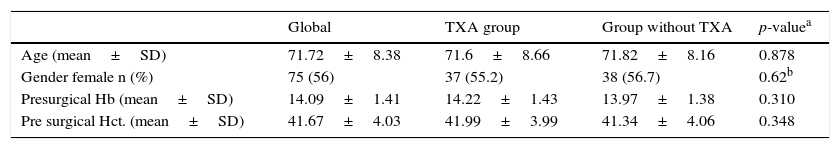
ResultsOut of the 134 patients who underwent surgery, 67 received treatment with TXA. Of the total patients included in the study 75 were women, which accounted for 66%, with a mean age of 3.26 years older than the men. Presurgical Hb and Hct levels were similar in both groups. In the group which received TXA an Hb level of 1422 and a Hct level of 41.99 was present whilst in the control group the levels were Hb 13.97 and Hct 41.34 (Table 1).
Description of the sample regarding administration of TXA.
| Global | TXA group | Group without TXA | p-valuea | |
|---|---|---|---|---|
| Age (mean±SD) | 71.72±8.38 | 71.6±8.66 | 71.82±8.16 | 0.878 |
| Gender female n (%) | 75 (56) | 37 (55.2) | 38 (56.7) | 0.62b |
| Presurgical Hb (mean±SD) | 14.09±1.41 | 14.22±1.43 | 13.97±1.38 | 0.310 |
| Pre surgical Hct. (mean±SD) | 41.67±4.03 | 41.99±3.99 | 41.34±4.06 | 0.348 |
%, percentage; SD, standard deviation; n, number.
Time in surgery and hospital stay days were also similar in both groups (Table 2).
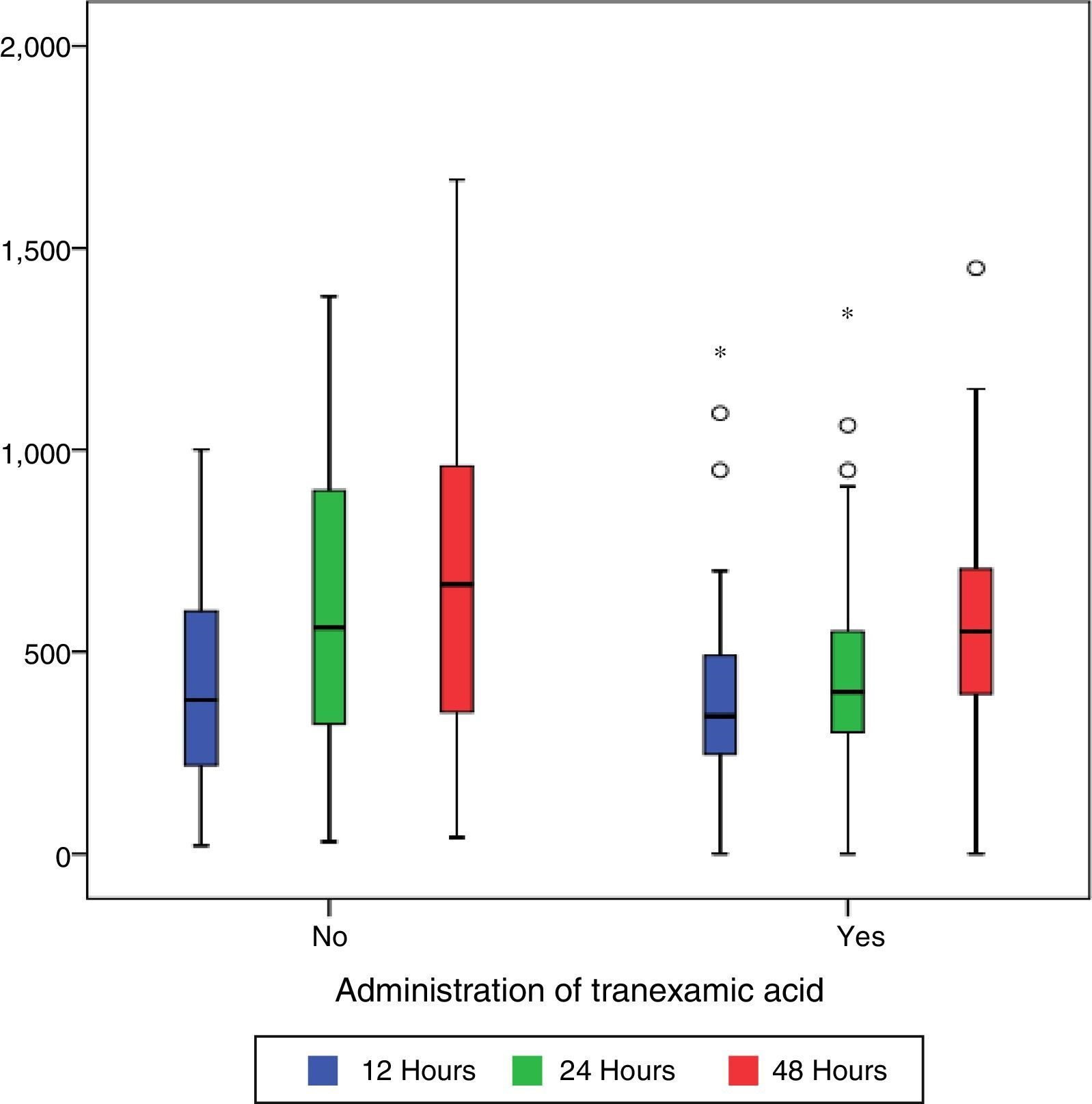
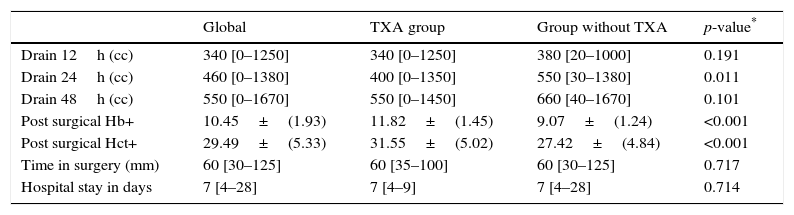
Post-surgical drainage levels and Hb (haemoglobin) and Hct (haematocrit). Median [minimum and maximum].
| Global | TXA group | Group without TXA | p-value* | |
|---|---|---|---|---|
| Drain 12h (cc) | 340 [0–1250] | 340 [0–1250] | 380 [20–1000] | 0.191 |
| Drain 24h (cc) | 460 [0–1380] | 400 [0–1350] | 550 [30–1380] | 0.011 |
| Drain 48h (cc) | 550 [0–1670] | 550 [0–1450] | 660 [40–1670] | 0.101 |
| Post surgical Hb+ | 10.45±(1.93) | 11.82±(1.45) | 9.07±(1.24) | <0.001 |
| Post surgical Hct+ | 29.49±(5.33) | 31.55±(5.02) | 27.42±(4.84) | <0.001 |
| Time in surgery (mm) | 60 [30–125] | 60 [35–100] | 60 [30–125] | 0.717 |
| Hospital stay in days | 7 [4–28] | 7 [4–9] | 7 [4–28] | 0.714 |
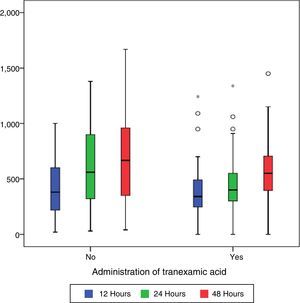
During the first 48h after surgery, levels of bleeding from drains in the control group were higher (660cc) than those in patients treated with TXA (550cc) (p=0.101), although the differences were only statistically significant (p=0.01) compared with levels collected by draining after 24h (400cc in the group treated with TXA compared with 550 of the control group) (Table 2 and Fig. 1).
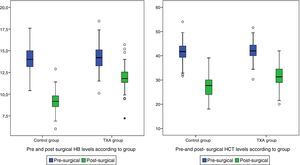
Post-surgical Hb and Hct levels were statistically lower (p<0.001) in the control group (Hb [9.07±1.24] and Hct [27.42±4.84]) compared with the group treated with TXA (Hb [11.82±1.45] and Hct [31.55±5.02]) (Fig. 2).
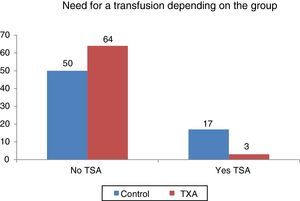
Statistically significant differences were found (p<0.001) regarding the need for transfusion and the group to which the patients belonged. Out of 134 patients who required transfusion 20 (14.9%), 17 of them (85%) were patients belonging to the control group. The transfusion percentage per group was 7% in the group treated with TXA and 25.37% in the control group, which constitutes a difference of 20.9 percentage points (Fig. 3).
There were no thromboembolic events in any of the groups.
DiscussionThe essential findings from this study were that the administration of TXA reduces postoperative bleeding, especially in non-visible losses, and transfusion requirements of patients who have undergone total hip arthroplasty. It equally proves to be a safe procedure, as there is no complication regarding its administration. This is the first article published in Spain in which the use and usage complications of TXA in THA have been studied prospectively.
Our findings concur with those reported in the literature to date. In the last 5 years 5 meta-analyses have been published on the use of TXA in THA,15–19 2 of them include joint procedures of THA and ATR17,18 and the other 3 focus on THA.15,16,19 Of the meta-analyses that of Moskal et al.15 is of note, published in 2016, and which analyses the effects of intravenous administration of TXA in patients who had undergone THA in 16 studies, all of them prospective, randomised and controlled. An analysis of 993 surgical interventions was therefore made. This work concluded that the patients treated with TXA bled less and received fewer transfusions than the control group. Other publications such as the Seville Document and the Guidelines from the European Society of Anaesthesiology20–22 suggested the use of TXA in COT, with a weak recommendation supported by high quality proofs. Furthermore, with regards to ATR about which more publications exist, the American Academy of Orthopaedic Surgeons publishes a practical guide on knee arthrosis with recommendation of TXA use in patients with no known contraindications.22 Use of TXA in orthopaedic surgery in Spain is increasing, and several articles have been published on its use in the country.23 The article published by Sanz-Reig et al. in 201424 is outstanding in this regard. This was a prospective and randomised study in which the use of TXA in knee replacement surgery was proven to reduce transfusion needs. The latest article published in Spain by Castro et al. (2016) is a prospective and randomised study, although the control group is retrospective, in which the efficacy of the intravenous administration of TXA was studied (on a group with a dose of 1g intraoperatively and 1g 3h after surgery. The other group was administered with 2g intravenously 30min before), in patients who underwent both ATR and THA.9
We found no significant increase in complications in the first group treated with TXA compared with the control group. These data coincide with those published in the literature, except with regards to TVP, where there is no consensus due the fact that in several studies, such as the meta-analysis of Moskal et al.,15 it is clear that there is a greater increase in the TVP rate in patients who received TXA, but in others, however, no differences were found in the cases of TVP.9,24 For this reason its use in orthopaedic surgery is still controversial. This discordance may be due to the different study sample sizes.
Controversy continues to arise with regards to dose and administration route. Several studies were published last year which compared different guidelines, dose and administration routes, concluding that they were all effective,9,25–27 and we thus continue without any conclusive scientific evidence today as to what is the ideal for obtaining maximum benefit with the lowest risk to the patient. With regards to time in surgery, it is notable that in the majority of publications9,28 this is not specified, not even if the differences between both groups had been studied according to this parameter. This aspect appears to have an effect on greater intraoperative blood loss. However, although in the present study no direct relationship was found between time in surgery and the need for transfusion, nor in the lowering of post-surgical Hb levels, but there is a trend which was not statistically significant. We must, however, not forget that correct perioperative management includes not only the use of antifibrinolytic drugs but many other factors, including pre-operative calculation of transfusion requirements, individualisation of patient needs, the use of self-recovery drains and correct anaesthetic and surgical techniques and they should be included in a programme to prevent allogenic blood transfusion.20,22,29
There are several limitations to this study. One of them is the sample size (limited to 134 patients). Secondly, TVP diagnosis was made with clinical follow-up, indicating only Doppler ultrasound in cases in which there was clinical suspicion, which is an attitude that is more similar to daily clinical practice, but no Doppler ultrasound was systematically performed to detect the cases of subclinical TVP. The strong points of this study are that this is a prospective, randomised and controlled study and that the surgical team were stable, as was the nursing team and the laboratory team. Strict written compliance of perioperative protocol and measurements were thus achieved, with no bias of untrained staff present. Surgeries were also consecutive and with very similar demographics, thus constituting homogenous groups.
ConclusionThe administration of TXA has proven to be a safe, effective method for reducing non-visible losses and through drains 24h after patients underwent THA. Transfusion requirements were also lower in the group treated with TXA compared with the control group.
Level of evidenceLevel of evidence II.
Ethical disclosuresProtection of humans and animal subjectThe authors declare that no experiments on humans or animals were involved in this research.
Confidentiality of dataThe authors declare they have adhered to the protocols of their centre of work on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
We wish to thank the collaboration of our hospital management bodies, who from the outset supported this study, and all the anaesthesia service colleagues and nursing team, who help and support our work daily. This project could not have been completed without the statistical work of the statistician María Teresa Alves Pérez.
Please cite this article as: Fernández-Cortiñas AB, Quintáns-Vázquez JM, Gómez-Suárez F, Simón Murillo O, Sánchez-López BR, Pena-Gracía JM. Efecto de dosis única intravenosa de ácido tranexámico sobre el sangrado en artroplastia total de cadera. Estudio prospectivo, controlado y aleatorizado. Rev Esp Cir Ortop Traumatol. 2017;61:289–295.