To determine the use of healthcare resources and costs associated with the diagnosis and treatment of thrombosis and bleeding patients who have undergone elective hip or knee replacement surgery, in routine clinical practice conditions.
Patients and methodsThis multicentre observational and retrospective study extracted data from the medical records of three Spanish public hospitals (2010). Patients ≥40 years who had received prophylaxis-anticoagulation were included. They were randomised into three groups: (a) control (no hospital complications), (b) bleeding, and (c) thrombosis. General variables, use of resources and costs were analysed. Statistical analysis: logistic regression and ANCOVA for model correction (P<.05) was included.
ResultsA total of 141 patients (control: 60; bleeding: 60; and thrombosis: 21), with a mean age 68.7 (SD: 10.4) years, and 68.1% females were identified. Hip arthroplasty was more frequent (71.6%). The bleeding risk was associated with age (OR=1.1) and thrombosis with COPD (OR=1.8); P<.05). The average length of stay for the thrombosis, bleeding and control groups was 13.9, 11.5 and 7.4 days, respectively; P<.001). The total costs for each group were €10,484.3; €8766.4 and €6496.1 respectively; P<.05. All grouped results were comparable between them according to the hospital analysed and the type of replacement.
ConclusionsCosts were higher for thrombosis and bleeding patients, respectively. Costs were associated with length of stay and hospital-acquired infections.
Conocer la utilización de recursos sanitarios y los costes asociados al diagnóstico y tratamiento de la trombosis y sangrado en pacientes intervenidos de artroplastia primaria total de cadera (ATC) o rodilla (ATR), durante 3 meses de seguimiento.
Pacientes y métodoEstudio observacional de carácter multicéntrico y retrospectivo, realizado a partir de los registros médicos de pacientes pertenecientes a 3 centros hospitalarios-públicos españoles (año 2010). Se consideraron aleatoriamente 3 grupos de pacientes: a) control (sin complicaciones hospitalarias); b) sangrado, y c) trombosis. Se incluyeron variables generales, de utilización de recursos y sus costes. Análisis estadístico: regresión logística y ANCOVA, p<0,05.
ResultadosSe incluyeron pacientes ≥ 40 años y que hubieran recibido profilaxis anticoagulante. Se incluyó un total de 141 pacientes (control: 60; sangrado: 60; y trombosis: 21). La edad media fue de 68,7 (DE: 10,4) años y el 68,1% fueron mujeres. La ATR fue la técnica más frecuente (71,6%). El riesgo de sangrado se relacionó con la edad (OR=1,1) y el de trombosis con la EPOC (OR=1,8), p<0,05. El promedio de días de estancia de los grupos de trombosis, sangrado y control fue de 13,9; 11,5 y 7,4 días, respectivamente, p<0,001). Los costes totales fueron: 10.484,3 €; 8.766,4 €, y 6.496,1 €, respectivamente, p<0,05. Todos los resultados agrupados fueron comparables entre ellos según el hospital analizado y el tipo de artroplastia.
ConclusionesLos costes más elevados se producen en los pacientes que habían desarrollado una trombosis y sangrado, respectivamente. Los costes se relacionaron con la prolongación de los días de estancia y las infecciones intrahospitalarias.
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are generically referred to by the term venous thromboembolism (VTE) and constitute a major public healthcare problem, since they affect several million people worldwide every year.1,2 Both diseases are relatively common in hospitalised patients, both medical and surgical, as well as in recently discharged patients.3,4 The data available in our country, based on the records of the Study Group for Venous Thromboembolism and Computerised Registry of Patients with Thromboembolic Disease, indicate that the incidence of DVT is about 116 cases per 100,000 inhabitants.5 Other studies indicate that the mortality of patients with DVT is 2.2% at 3 months, and 7.8% in the case of PE.6 The mean hospital stay of patients who develop an episode of PE is over 11 days, and for DVT it is 8 days.7One of the groups most at risk of developing an episode of DVT/PE is a group of patients undergoing orthopaedic or pelvic surgery (orthopaedic, urological and gynaecological).8 Orthopaedic surgery includes total hip arthroplasty (THA), total knee arthroplasty (TKA) and hip fracture surgery. The incidence of DVT without prophylaxis ranges between 40 and 60% (10–30% corresponds to proximal DVT). Regarding the incidence of PE, this has been reported in 3–28% of patients undergoing scintigraphy scans during the 2 weeks following surgery.9,10 Therefore, prophylaxis is systematically established in most patients in this type of surgery.11,12 In general, for patients older than 21 years, the use of any type of heparin (unfractionated heparin [UFH] or low molecular weight heparin [LMWH]) is considered as an appropriate prophylaxis as long as adequate doses are used for high-risk patients and treatment is maintained for at least 21 days, beginning on the day of surgery.13,14 However, the search for an ideal anticoagulant has been one of the most active research fields in recent years in different clinical environments. Despite the use of prophylaxis, episodes of DVT still take place and must be addressed in order to avoid major complications.4,9,15–17
There is little information on the costs caused by DVT/PE. The total approximate expenditure estimated for the prevention or VTE treatment is around 1300 million dollars in Western countries. In Spain, hospitalisation due to PE involves an expenditure of 20 million euros per year.5 In addition, this pathology reduces the work capacity of many individuals, thus generating significant indirect costs for society as a whole. Given this scenario, it is necessary to develop strategies aimed at prevention among high-risk populations, as well as active local and national policies to promote awareness of the problem and adherence to international consensus clinical guidelines.18
The available evidence regarding the impact, management and monitoring of patients with DVT in routine clinical practice in our country is uncertain. Moreover, available information on clinical variables and resource utilisation as a whole is very limited, so this study may be very relevant. In this regard, there is no evidence in our country on resource utilisation and costs associated with treatment of each episode of DVT and bleeding among patients undergoing orthopaedic surgery and receiving prophylaxis with anticoagulants. The main objective of this study was to determine the utilisation of health resources and associated costs arising from the diagnosis and treatment of each episode of DVT and bleeding among patients undergoing hip or knee orthopaedic surgery, with 3 months follow-up, in a Spanish environment and under routine clinical practice conditions. As a secondary objective we examined the determinants and/or explanatory variables of this resource utilisation and associated costs in both complications (DVT and bleeding).
Patients and methodsDesign and study populationWe conducted an observational, multicentre, non-interventional study, based on a retrospective review of medical records (computerised databases and medical records) of outpatients and hospitalised patients at 3 Spanish hospitals. The records were obtained from the dissociated data of medical histories, discharge reports and/or the databases from the centres. The study differentiated 3 groups of patients: (a) patients undergoing THA or TKA who had received prophylaxis with anticoagulants and who had not suffered any episodes of DVT or bleeding during hospitalisation (control group); (b) patients undergoing THA or TKA who had received prophylaxis with anticoagulants and who had suffered an episode of bleeding of any severity, and (c) patients undergoing THA or TKA who had received prophylaxis with anticoagulants and who had suffered an episode of DVT.
The study population consisted of patients from Hospital Municipal de Badalona, Hospital Universitario Puerta de Hierro Majadahonda and Hospital de Alzira. Each investigator randomly selected 60 patients (20 in each of the groups mentioned above) who had undergone THA or TKA at their hospital. The selection of patients took place through the discharge records of the Admissions, Medical Documentation and/or Traumatology Services, and always from major surgical procedures coded according to the International Classification of Diseases (9th review), Clinical Modification (ICD-9-CM).
Inclusion and exclusion criteriaWe included all patients undergoing primary THA or TKA (programmed total replacement; ICD-9-CM codes 81.51 and 81.54, respectively) between January 1, 2010 and December 31, 2010 and who met the following criteria: (a) age over 40 years; (b) having received anticoagulant prophylaxis for at least 21 days; (c) attending scheduled appointments to monitor the evolution of the intervention; (d) having available quality records from medical records; (e) primary replacement of uncemented prosthesis for a diagnosis of osteoarthritis, and (f) scheduled surgical interventions (not urgent). We excluded those patients with: (a) absolute contraindication of anticoagulation (severe haemorrhagic diathesis, active haemorrhagic processes, uncontrollable severe arterial hypertension, recent intracranial haemorrhage, dissecting cerebral or aortic aneurysm); (b) patients with contraindications due to anticoagulation (currently inactive bleeding tendency, pericarditis or pericardial effusion, recent surgery, especially ophthalmic or neurosurgery, recent childbirth, major trauma); (c) patients following anticoagulation therapy with prophylactic or therapeutic dosages upon admission or those requiring therapeutic doses during hospitalisation; (d) primary partial THA (ICD-9-CM: 81.52) as we considered that this was a different population, since this type of intervention takes place mostly in cases of fracture and in older patients, and (e) patients who had undergone reintervention due to intrahospital complications arising from the placement of the prosthesis. The evolutionary monitoring of patients from the date of the intervention was 3 months for the main variables of the study (DVT, bleeding and use of resources).
In addition, due to the low number of patients found for the DVT/PE group, we decided to extend the recruitment period for an additional 4 years. We did not consider extending this time period any further, because cases beyond these 4 years could be difficult to compare with the current style of clinical practice.
Calculation of sample sizeThe calculation of sample size provided some methodological difficulties, since there were no data on the costs of diagnosis and treatment of DVT and bleeding in our country. However, based on a maximum expected prevalence of complications of 8.5% (DVT and bleeding) and assuming a random error of 5% and an estimated accuracy of less than 4%, we aimed to select a minimum of 180 patients (60 in each study group). The statistical power of the model was 80%. The selection of patients in each group, especially in the control group (patients undergoing THA or TKA without evidence of DVT or bleeding), was carried out by a simple random probability sampling of all available cases.
Sociodemographic and morbidity variablesThe main variables studied were: (a) patient identifier (dissociated); (b) hospital identifier; (c) sociodemographic data, and (d) comorbidity. These included age (continuous and by ranges [18–44, 45–64, 65–74 and ≥75 years]), gender and employment status (active, retired and others). We also noted the medical history: arterial hypertension, diabetes mellitus, dyslipidemia, obesity, smoking, alcoholism, heart, kidney or liver failure, ischemic heart disease, stroke, chronic obstructive pulmonary disease (COPD), asthma, dementia or memory disorders, neurological diseases (Parkinson's disease, epilepsy, multiple sclerosis), affective psychosis, depressive syndrome, malignancies, varicosities of lower limbs and osteoporosis (according to ICD-9-CM criteria). We used the Charlson comorbidity index as a summary variable of general comorbidity for surgical patients.19
Study groups and other variables analysedThe thrombosis and bleeding groups were obtained during hospitalisation of patients. The information was obtained from the following codes: DVT (ICD-9-CM: 451x–453x), and postoperative haemorrhage or bleeding (ICD-9-CM: 998x). All discharged patients were reviewed by a reporting physician, who validated the information (especially clinical courses, reoperations and diagnostic/therapeutic tests), following the judgment of medical staff. Furthermore, all patients included in the study were followed exhaustively through their medical histories so as to avoid introducing a selection bias. The presence of DVT was defined based on clinical judgment and according to an established diagnosis,20 while bleeding was defined as an exudate exceeding 500ml collected in redon drains during the first 12h.21,22 During the 3 months after surgery (outpatient monitoring), the presence of DVT (Wells criteria)20 or bleeding (criteria of Nelson et al. and the Spanish Study Group for the Use of Aprotinin in Hip Arthroplasty [GEEEAAC]) was reported at the discretion of the traumatologist.21,22
In addition, information was also obtained on the place of origin (home, nursing home), date of surgery, type of anticoagulant (active principle) used in prophylaxis, days of inpatient and outpatient treatment (LMWH; follow-up from clinical course), days of hospitalisation (difference between the discharge date and date of admission), reason for discharge (recovery, transfer, death, other causes), hospital mortality (up to 30 days after discharge) hospital readmission (up to 30 days after discharge), nosocomial infection (according to SENIC criteria)23 and other complications (respiratory, cardiac, motility, etc.).
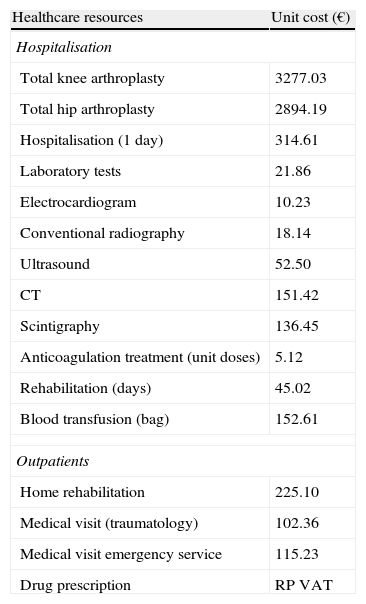
Use of resources and cost modelDirect healthcare costs were defined as those related to healthcare activities carried out by professionals. Costs were expressed as the mean cost (retail price-VAT) per patient attended, from admission until 3 months follow-up in the 3 study groups (control, bleeding and thrombosis). The unit costs for the year 2009 of the different study variables are shown in Table 1. The various rates were obtained from the analytical accounts of Hospital Municipal de Badalona. Outpatient prescriptions were quantified according to the retail price per pack at the time of prescription.
Detail of unit costs employed.
| Healthcare resources | Unit cost (€) |
| Hospitalisation | |
| Total knee arthroplasty | 3277.03 |
| Total hip arthroplasty | 2894.19 |
| Hospitalisation (1 day) | 314.61 |
| Laboratory tests | 21.86 |
| Electrocardiogram | 10.23 |
| Conventional radiography | 18.14 |
| Ultrasound | 52.50 |
| CT | 151.42 |
| Scintigraphy | 136.45 |
| Anticoagulation treatment (unit doses) | 5.12 |
| Rehabilitation (days) | 45.02 |
| Blood transfusion (bag) | 152.61 |
| Outpatients | |
| Home rehabilitation | 225.10 |
| Medical visit (traumatology) | 102.36 |
| Medical visit emergency service | 115.23 |
| Drug prescription | RP VAT |
CT: computed tomography; RP VAT: retail price – value added tax.
Source of healthcare resources: analytical accounts from Hospital Municipal de Badalona. Values expressed in Euros (2011).
We respected the confidentiality of records as expressed by the Law on Data Protection (15/1999 of December 13), through the dissociation of data. The study was classified by the Spanish Agency for Drugs and Healthcare Products (as non-PAS [post-authorisation study]) and subsequently approved by the Clinical Research Ethics Committee of each hospital, with Hospital Universitario Germans Trias i Pujol of Badalona acting as the coordinating centre.
Statistical analysisWe performed a univariate-descriptive statistical analysis with 95% confidence intervals (CI), and assessed normal distribution through the Kolmogorov–Smirnov test. We used Student's t test, ANOVA test, chi-square test and Pearson's linear correlation for the bivariate analysis. We performed a logistic regression analysis for categorical variables (DVT and bleeding), to determine associated variables (dependent variables) with the enter procedure (Wald statistic). The comparison of outpatient and hospital costs was performed, as recommended by Thompson and Barber,24 by analysis of covariance (ANCOVA), with gender, age and the Charlson index as covariates (method: estimated marginal means, with Bonferroni adjustment and significance of P<.0125). We calculated a 95% CI of mean costs generated by the re-sampling (bootstrapping) technique. In order to predict the factors associated with total healthcare costs (dependent variable), we used a multiple linear regression model (stepwise method; β coefficients were obtained as a measure of association). We used the statistical program SPSSWIN version 17 (IBM, USA), and established a statistical significance value of P<.05.
ResultsWe included 141 patients (control: 60; bleeding: 60; thrombosis: 21) out of the 680 subjects ≥40 years who underwent THA or TKA in the 3 hospitals. No patient suffered a PE. Of the 141 patients analysed from the day of admission until 3 months postoperatively, no patient died and there were no follow-up losses.
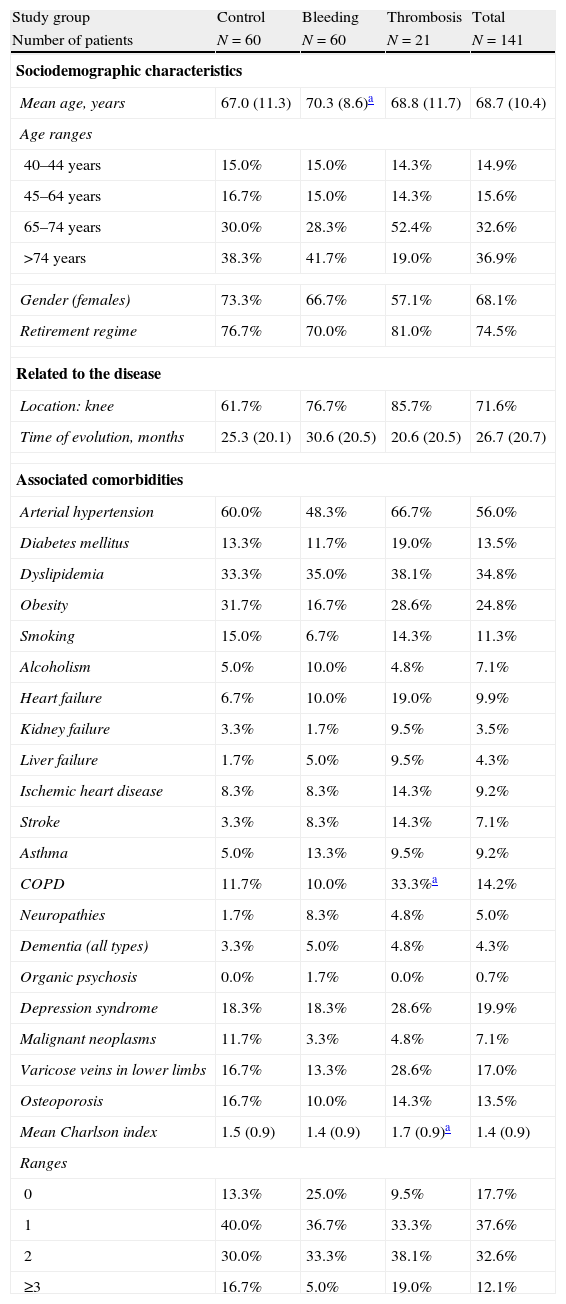
Table 2 describes the general characteristics and comorbidities of patients according to the studied series. The mean age was 68.7±10.4 years and 68.1% of patients were female. The most common arthroplasty was in the knee (71.6%). Compared with the control group, the thrombosis group showed higher percentages of COPD (33.3 vs 11.7%; P=.023) and overall comorbidity (1.7 vs 1.5 points in the Charlson scale; P=.036). Compared with the control group, the bleeding group showed no statistically significant differences in the variables analysed. In the logistic model, age showed an increased risk of bleeding (OR=1.1; CI: 1.0–1.2; P<.05), whereas COPD showed it for thrombosis (OR=1.8; CI: 1.6–2.1; P<.05).
General characteristics of the studied series.
| Study group | Control | Bleeding | Thrombosis | Total |
| Number of patients | N=60 | N=60 | N=21 | N=141 |
| Sociodemographic characteristics | ||||
| Mean age, years | 67.0 (11.3) | 70.3 (8.6)a | 68.8 (11.7) | 68.7 (10.4) |
| Age ranges | ||||
| 40–44 years | 15.0% | 15.0% | 14.3% | 14.9% |
| 45–64 years | 16.7% | 15.0% | 14.3% | 15.6% |
| 65–74 years | 30.0% | 28.3% | 52.4% | 32.6% |
| >74 years | 38.3% | 41.7% | 19.0% | 36.9% |
| Gender (females) | 73.3% | 66.7% | 57.1% | 68.1% |
| Retirement regime | 76.7% | 70.0% | 81.0% | 74.5% |
| Related to the disease | ||||
| Location: knee | 61.7% | 76.7% | 85.7% | 71.6% |
| Time of evolution, months | 25.3 (20.1) | 30.6 (20.5) | 20.6 (20.5) | 26.7 (20.7) |
| Associated comorbidities | ||||
| Arterial hypertension | 60.0% | 48.3% | 66.7% | 56.0% |
| Diabetes mellitus | 13.3% | 11.7% | 19.0% | 13.5% |
| Dyslipidemia | 33.3% | 35.0% | 38.1% | 34.8% |
| Obesity | 31.7% | 16.7% | 28.6% | 24.8% |
| Smoking | 15.0% | 6.7% | 14.3% | 11.3% |
| Alcoholism | 5.0% | 10.0% | 4.8% | 7.1% |
| Heart failure | 6.7% | 10.0% | 19.0% | 9.9% |
| Kidney failure | 3.3% | 1.7% | 9.5% | 3.5% |
| Liver failure | 1.7% | 5.0% | 9.5% | 4.3% |
| Ischemic heart disease | 8.3% | 8.3% | 14.3% | 9.2% |
| Stroke | 3.3% | 8.3% | 14.3% | 7.1% |
| Asthma | 5.0% | 13.3% | 9.5% | 9.2% |
| COPD | 11.7% | 10.0% | 33.3%a | 14.2% |
| Neuropathies | 1.7% | 8.3% | 4.8% | 5.0% |
| Dementia (all types) | 3.3% | 5.0% | 4.8% | 4.3% |
| Organic psychosis | 0.0% | 1.7% | 0.0% | 0.7% |
| Depression syndrome | 18.3% | 18.3% | 28.6% | 19.9% |
| Malignant neoplasms | 11.7% | 3.3% | 4.8% | 7.1% |
| Varicose veins in lower limbs | 16.7% | 13.3% | 28.6% | 17.0% |
| Osteoporosis | 16.7% | 10.0% | 14.3% | 13.5% |
| Mean Charlson index | 1.5 (0.9) | 1.4 (0.9) | 1.7 (0.9)a | 1.4 (0.9) |
| Ranges | ||||
| 0 | 13.3% | 25.0% | 9.5% | 17.7% |
| 1 | 40.0% | 36.7% | 33.3% | 37.6% |
| 2 | 30.0% | 33.3% | 38.1% | 32.6% |
| ≥3 | 16.7% | 5.0% | 19.0% | 12.1% |
COPD: chronic obstructive pulmonary disease.
Values expressed as percentage or mean (SD: standard deviation).
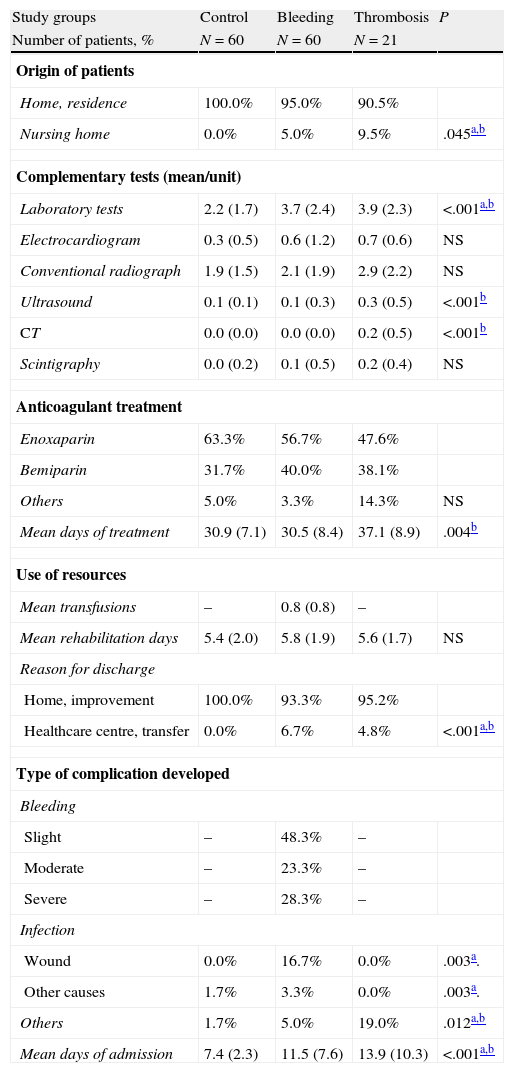
Table 3 shows the general variables, resources and complications occurring during hospitalisation for each study group. Out of all patients studied, 3.5% came from a nursing home (thrombosis: 9.5%) and 58.2% were given anticoagulant therapy with enoxaparin. In the group of patients who suffered bleeding, 55.0% underwent a blood transfusion (26.7% underwent 1 transfusion, whereas 28.3%undewent 2 or more transfusions) and 20.0% presented an intrahospital infection. Other complications developed were: allergic/adverse reaction to drugs (3), severe pain (3) and cardiac arrhythmia (2). The mean length of stay in the bleeding and thrombosis groups was higher than in the control group (11.5±3.5 and 13.9±3.8 vs 7.4±2.3 days, respectively; P<.001). However, there were no marked differences between patients undergoing TKA or THA. As an example, the mean hospital stay was 10.5±3.1 vs 9.1±3.6 days, respectively.
General variables, use of resources and complications occurring during hospitalisation.
| Study groups | Control | Bleeding | Thrombosis | P |
| Number of patients, % | N=60 | N=60 | N=21 | |
| Origin of patients | ||||
| Home, residence | 100.0% | 95.0% | 90.5% | |
| Nursing home | 0.0% | 5.0% | 9.5% | .045a,b |
| Complementary tests (mean/unit) | ||||
| Laboratory tests | 2.2 (1.7) | 3.7 (2.4) | 3.9 (2.3) | <.001a,b |
| Electrocardiogram | 0.3 (0.5) | 0.6 (1.2) | 0.7 (0.6) | NS |
| Conventional radiograph | 1.9 (1.5) | 2.1 (1.9) | 2.9 (2.2) | NS |
| Ultrasound | 0.1 (0.1) | 0.1 (0.3) | 0.3 (0.5) | <.001b |
| CT | 0.0 (0.0) | 0.0 (0.0) | 0.2 (0.5) | <.001b |
| Scintigraphy | 0.0 (0.2) | 0.1 (0.5) | 0.2 (0.4) | NS |
| Anticoagulant treatment | ||||
| Enoxaparin | 63.3% | 56.7% | 47.6% | |
| Bemiparin | 31.7% | 40.0% | 38.1% | |
| Others | 5.0% | 3.3% | 14.3% | NS |
| Mean days of treatment | 30.9 (7.1) | 30.5 (8.4) | 37.1 (8.9) | .004b |
| Use of resources | ||||
| Mean transfusions | – | 0.8 (0.8) | – | |
| Meanrehabilitation days | 5.4 (2.0) | 5.8 (1.9) | 5.6 (1.7) | NS |
| Reason for discharge | ||||
| Home, improvement | 100.0% | 93.3% | 95.2% | |
| Healthcare centre, transfer | 0.0% | 6.7% | 4.8% | <.001a,b |
| Type of complication developed | ||||
| Bleeding | ||||
| Slight | – | 48.3% | – | |
| Moderate | – | 23.3% | – | |
| Severe | – | 28.3% | – | |
| Infection | ||||
| Wound | 0.0% | 16.7% | 0.0% | .003a. |
| Other causes | 1.7% | 3.3% | 0.0% | .003a. |
| Others | 1.7% | 5.0% | 19.0% | .012a,b |
| Mean days of admission | 7.4 (2.3) | 11.5 (7.6) | 13.9 (10.3) | <.001a,b |
CT: computerised tomography; NS: not significant; P: statistical significance.
Values expressed as percentage or mean (SD: standard deviation).
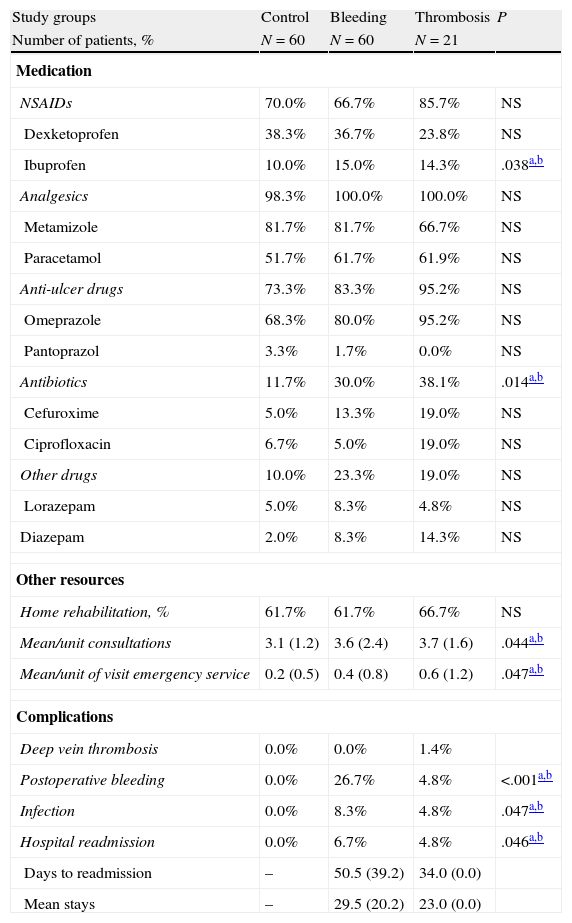
Table 4 details the medication administered, the use of resources and the complications developed during the outpatient follow-up period (3 months post-hospitalisation). Compared with the control group, the bleeding and thrombosis groups required an increased use of medication (30.0% and 38.1% vs 11.7%; P=.014), outpatient visits (3.6% and 3.7% vs 3.1%; P=.044) and visits to the emergency department (0.4% and 0.6% vs 0.2%; P=.047), respectively. In the bleeding group, 26.7% of patients developed haematomas/bleeding of the surgical wound and 4 were readmitted. In the thrombosis group, 2 patients suffered a new DVT and 1 was readmitted. Surgical wound infections or other infections (urinary: 2) were treated as outpatients (total: 5.1%). There were no marked differences between patients undergoing TKA or THA.
Medication administered, use of resources and complications occurring during outpatient follow-up (3 months post-hospitalisation).
| Study groups | Control | Bleeding | Thrombosis | P |
| Number of patients, % | N=60 | N=60 | N=21 | |
| Medication | ||||
| NSAIDs | 70.0% | 66.7% | 85.7% | NS |
| Dexketoprofen | 38.3% | 36.7% | 23.8% | NS |
| Ibuprofen | 10.0% | 15.0% | 14.3% | .038a,b |
| Analgesics | 98.3% | 100.0% | 100.0% | NS |
| Metamizole | 81.7% | 81.7% | 66.7% | NS |
| Paracetamol | 51.7% | 61.7% | 61.9% | NS |
| Anti-ulcer drugs | 73.3% | 83.3% | 95.2% | NS |
| Omeprazole | 68.3% | 80.0% | 95.2% | NS |
| Pantoprazol | 3.3% | 1.7% | 0.0% | NS |
| Antibiotics | 11.7% | 30.0% | 38.1% | .014a,b |
| Cefuroxime | 5.0% | 13.3% | 19.0% | NS |
| Ciprofloxacin | 6.7% | 5.0% | 19.0% | NS |
| Other drugs | 10.0% | 23.3% | 19.0% | NS |
| Lorazepam | 5.0% | 8.3% | 4.8% | NS |
| Diazepam | 2.0% | 8.3% | 14.3% | NS |
| Other resources | ||||
| Home rehabilitation, % | 61.7% | 61.7% | 66.7% | NS |
| Mean/unit consultations | 3.1 (1.2) | 3.6 (2.4) | 3.7 (1.6) | .044a,b |
| Mean/unit of visit emergency service | 0.2 (0.5) | 0.4 (0.8) | 0.6 (1.2) | .047a,b |
| Complications | ||||
| Deep vein thrombosis | 0.0% | 0.0% | 1.4% | |
| Postoperative bleeding | 0.0% | 26.7% | 4.8% | <.001a,b |
| Infection | 0.0% | 8.3% | 4.8% | .047a,b |
| Hospital readmission | 0.0% | 6.7% | 4.8% | .046a,b |
| Days to readmission | – | 50.5 (39.2) | 34.0 (0.0) | |
| Mean stays | – | 29.5 (20.2) | 23.0 (0.0) | |
NS: not significant; NSAID: Non steroidal anti-inflammatory drugs; P: statistical significance.
Values expressed as percentage or mean (SD: standard deviation).
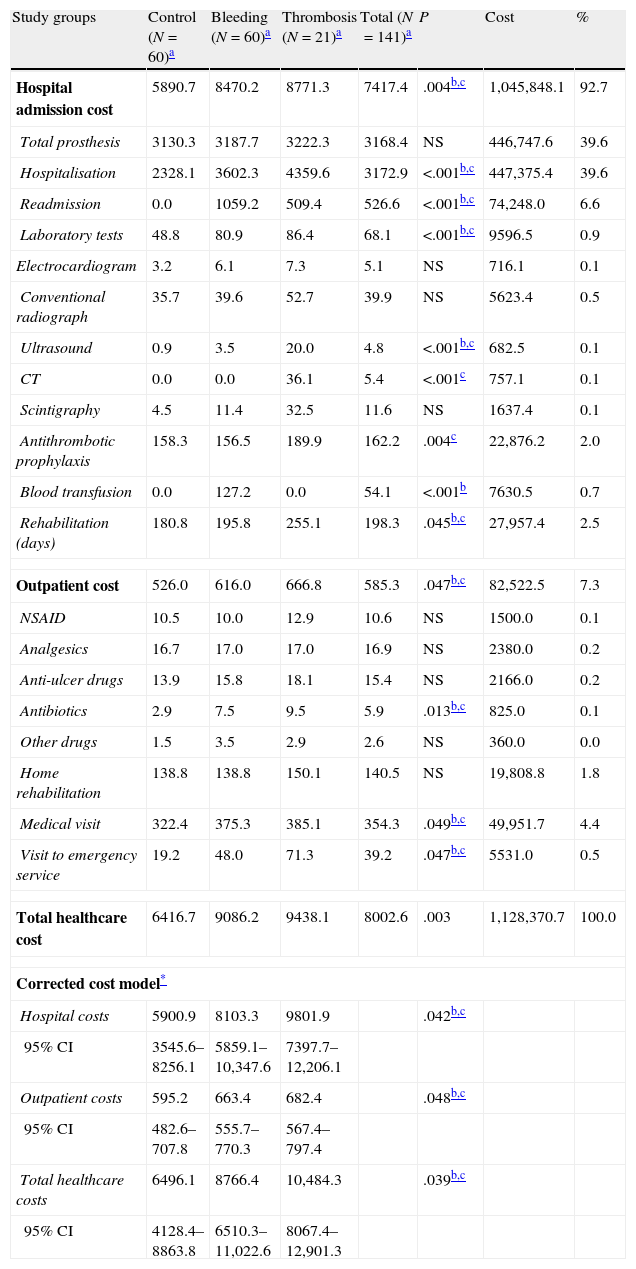
The gross and corrected costs model associated with primary total arthroplasty by study groups is shown in Table 5. The total cost of healthcare for patients amounted to € 1,128,371. The mean cost per patient was € 8003±4875 (outpatient: € 585±267; hospital: € 7417±4808). The most significant costs generated in specialised care corresponded to the days of hospitalisation and placement of prostheses, with both representing 39.6% of the total cost. In the multivariate model adjusted for age, gender and comorbidity, the mean costs were higher in the group with thrombosis (total: € 10,484; hospital € 9802; outpatients: € 682), followed by the bleeding group (total: € 8766; hospital: € 8103; outpatients: € 663), compared with the control group (total: € 6496; hospital: € 5901; outpatients: € 595), with results being statistically significant in all cases.
Cost model associated to primary total arthroplasty by study groups.
| Study groups | Control (N=60)a | Bleeding (N=60)a | Thrombosis (N=21)a | Total (N=141)a | P | Cost | % |
| Hospital admission cost | 5890.7 | 8470.2 | 8771.3 | 7417.4 | .004b,c | 1,045,848.1 | 92.7 |
| Total prosthesis | 3130.3 | 3187.7 | 3222.3 | 3168.4 | NS | 446,747.6 | 39.6 |
| Hospitalisation | 2328.1 | 3602.3 | 4359.6 | 3172.9 | <.001b,c | 447,375.4 | 39.6 |
| Readmission | 0.0 | 1059.2 | 509.4 | 526.6 | <.001b,c | 74,248.0 | 6.6 |
| Laboratory tests | 48.8 | 80.9 | 86.4 | 68.1 | <.001b,c | 9596.5 | 0.9 |
| Electrocardiogram | 3.2 | 6.1 | 7.3 | 5.1 | NS | 716.1 | 0.1 |
| Conventional radiograph | 35.7 | 39.6 | 52.7 | 39.9 | NS | 5623.4 | 0.5 |
| Ultrasound | 0.9 | 3.5 | 20.0 | 4.8 | <.001b,c | 682.5 | 0.1 |
| CT | 0.0 | 0.0 | 36.1 | 5.4 | <.001c | 757.1 | 0.1 |
| Scintigraphy | 4.5 | 11.4 | 32.5 | 11.6 | NS | 1637.4 | 0.1 |
| Antithrombotic prophylaxis | 158.3 | 156.5 | 189.9 | 162.2 | .004c | 22,876.2 | 2.0 |
| Blood transfusion | 0.0 | 127.2 | 0.0 | 54.1 | <.001b | 7630.5 | 0.7 |
| Rehabilitation (days) | 180.8 | 195.8 | 255.1 | 198.3 | .045b,c | 27,957.4 | 2.5 |
| Outpatient cost | 526.0 | 616.0 | 666.8 | 585.3 | .047b,c | 82,522.5 | 7.3 |
| NSAID | 10.5 | 10.0 | 12.9 | 10.6 | NS | 1500.0 | 0.1 |
| Analgesics | 16.7 | 17.0 | 17.0 | 16.9 | NS | 2380.0 | 0.2 |
| Anti-ulcer drugs | 13.9 | 15.8 | 18.1 | 15.4 | NS | 2166.0 | 0.2 |
| Antibiotics | 2.9 | 7.5 | 9.5 | 5.9 | .013b,c | 825.0 | 0.1 |
| Other drugs | 1.5 | 3.5 | 2.9 | 2.6 | NS | 360.0 | 0.0 |
| Home rehabilitation | 138.8 | 138.8 | 150.1 | 140.5 | NS | 19,808.8 | 1.8 |
| Medical visit | 322.4 | 375.3 | 385.1 | 354.3 | .049b,c | 49,951.7 | 4.4 |
| Visit to emergency service | 19.2 | 48.0 | 71.3 | 39.2 | .047b,c | 5531.0 | 0.5 |
| Total healthcare cost | 6416.7 | 9086.2 | 9438.1 | 8002.6 | .003 | 1,128,370.7 | 100.0 |
| Corrected cost model* | |||||||
| Hospital costs | 5900.9 | 8103.3 | 9801.9 | .042b,c | |||
| 95% CI | 3545.6–8256.1 | 5859.1–10,347.6 | 7397.7–12,206.1 | ||||
| Outpatient costs | 595.2 | 663.4 | 682.4 | .048b,c | |||
| 95% CI | 482.6–707.8 | 555.7–770.3 | 567.4–797.4 | ||||
| Total healthcare costs | 6496.1 | 8766.4 | 10,484.3 | .039b,c | |||
| 95% CI | 4128.4–8863.8 | 6510.3–11,022.6 | 8067.4–12,901.3 | ||||
CI: confidence interval; CT: computed tomography; NS: not significant; NSAID: non steroidal anti-inflammatory drugs; P: statistical significance.
Values expressed as mean (euros).
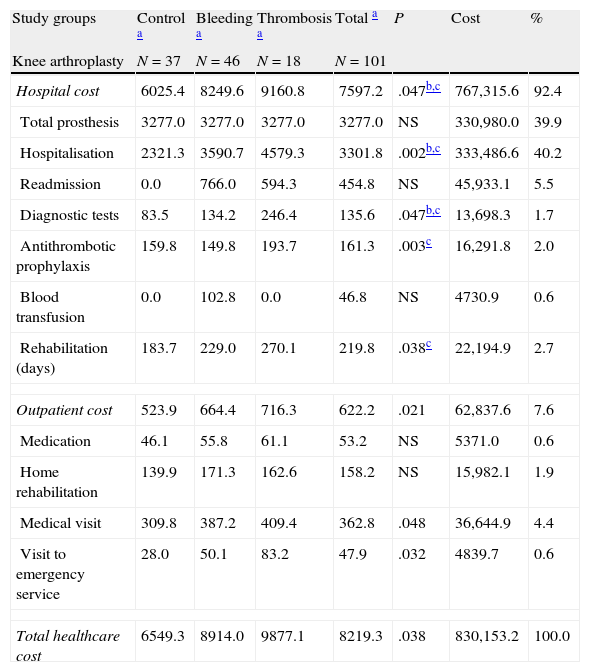
Table 6 details the model of gross and corrected costs associated with TKA and THA. The distribution of costs by study groups and variables does not show marked differences. In the ANCOVA model in patients undergoing TKA, total costs were higher in the group with thrombosis (€ 10732) and bleeding (€ 8411) compared with the control group (€ 6571), P<.05. In patients with THA, the total cost of the control group was € 6401 whereas that of the bleeding group was € 8615. These results were similar to those of the TKA group.
Gross and corrected costs model associated to primary total knee and hip arthroplasty by study groups.
| Study groups | Control a | Bleeding a | Thrombosis a | Total a | P | Cost | % |
| Knee arthroplasty | N=37 | N=46 | N=18 | N=101 | |||
| Hospital cost | 6025.4 | 8249.6 | 9160.8 | 7597.2 | .047b,c | 767,315.6 | 92.4 |
| Total prosthesis | 3277.0 | 3277.0 | 3277.0 | 3277.0 | NS | 330,980.0 | 39.9 |
| Hospitalisation | 2321.3 | 3590.7 | 4579.3 | 3301.8 | .002b,c | 333,486.6 | 40.2 |
| Readmission | 0.0 | 766.0 | 594.3 | 454.8 | NS | 45,933.1 | 5.5 |
| Diagnostic tests | 83.5 | 134.2 | 246.4 | 135.6 | .047b,c | 13,698.3 | 1.7 |
| Antithrombotic prophylaxis | 159.8 | 149.8 | 193.7 | 161.3 | .003c | 16,291.8 | 2.0 |
| Blood transfusion | 0.0 | 102.8 | 0.0 | 46.8 | NS | 4730.9 | 0.6 |
| Rehabilitation (days) | 183.7 | 229.0 | 270.1 | 219.8 | .038c | 22,194.9 | 2.7 |
| Outpatient cost | 523.9 | 664.4 | 716.3 | 622.2 | .021 | 62,837.6 | 7.6 |
| Medication | 46.1 | 55.8 | 61.1 | 53.2 | NS | 5371.0 | 0.6 |
| Home rehabilitation | 139.9 | 171.3 | 162.6 | 158.2 | NS | 15,982.1 | 1.9 |
| Medical visit | 309.8 | 387.2 | 409.4 | 362.8 | .048 | 36,644.9 | 4.4 |
| Visit to emergency service | 28.0 | 50.1 | 83.2 | 47.9 | .032 | 4839.7 | 0.6 |
| Total healthcare cost | 6549.3 | 8914.0 | 9877.1 | 8219.3 | .038 | 830,153.2 | 100.0 |
| Hip arthroplasty | N=23 | N=14 | N=3 | N=40 | P | Cost | % |
| Hospital cost | 5673.9 | 9195.0 | 6434.0 | 6963.3 | .023b | 278,532.6 | 93.4 |
| Total prosthesis | 2894.2 | 2894.2 | 2894.2 | 2894.2 | NS | 115,767.6 | 38.8 |
| Hospitalisation | 2339.1 | 3640.5 | 3041.2 | 2847.2 | .011b,c | 113,888.8 | 38.2 |
| Readmission | 0.0 | 2022.5 | 0.0 | 707.9 | NS | 28,314.9 | 9.5 |
| Diagnostic tests | 108.7 | 165.5 | 166.3 | 132.9 | NS | 5314.8 | 1.8 |
| Antithrombotic prophylaxis | 155.8 | 178.5 | 167.3 | 164.6 | NS | 6584.3 | 2.2 |
| Blood transfusion | 0.0 | 207.1 | 0.0 | 72.5 | NS | 2899.6 | 1.0 |
| Rehabilitation (days) | 176.2 | 86.8 | 165.1 | 144.1 | NS | 5762.6 | 1.9 |
| Outpatient cost | 529.5 | 456.9 | 369.9 | 492.1 | NS | 19,684.9 | 6.6 |
| Medication | 44.8 | 47.3 | 56.0 | 46.5 | NS | 1860.0 | 0.6 |
| Home rehabilitation | 137.0 | 32.2 | 75.0 | 95.7 | NS | 3826.7 | 1.3 |
| Medical visit | 342.7 | 336.3 | 238.8 | 332.7 | NS | 13,306.8 | 4.5 |
| Visit to emergency service | 5.0 | 41.2 | 0.0 | 17.3 | NS | 691.4 | 0.2 |
| Total healthcare cost | 6203.4 | 9652.0 | 6803.9 | 7455.4 | .037b | 298,217.4 | 100.0 |
| Corrected cost modeld | |||||||
| Total healthcare cost (knee) | 6571.1 | 8410.6 | 10,731.8 | .047b,c | |||
| 95% CI | 3198.6–9943.6 | 5680.4–11,140.7 | 7820.2–13,643.5 | ||||
| Total healthcare cost (hip) | 6401.2 | 8614.7 | 7533.4 | NS | |||
| 95% CI | 4721.3–8080.9 | 4386.4–12,843.1 | 4520.4–10,546.2 | ||||
CI: confidence interval; NS: not significant; P: statistical significance.
Values expressed as mean (euros).
In the linear regression model, the total healthcare cost was associated with the number of hospital stays (β=0.651), readmission (β=0.536), Charlson index (β=0.185), presence of thrombosis (β=0.173) and nosocomial infection (β=0.157); P<.001. All group results were comparable in the hospitals studied (Hospital Municipal de Badalona, Hospital Universitario Puerta de Hierro and Hospital de Alzira). As an example, the mean ages were 69.7±10.2, 68.2±10.7 and 69.1±10.4 years, P=.653; the percentages of females were 68.2%, 67.9% and 71.2%, P=.821; and the total gross costs were € 8287±4954, € 7805±4879 and € 8123±4997, P=.333, respectively. The differences were not significantly significant.
DiscussionThe study shows the high use of healthcare resources and the mean cost associated with the diagnosis and treatment of DVT and bleeding in patients undergoing THA and/or TKA compared with a control group during 3 months follow-up in everyday clinical practice within a Spanish healthcare environment (hospital and outpatients). It should be noted that, without adequate standardisation of the methodology in terms of patient characteristics and the number and measurement of the variables studied, the results should be interpreted with caution and we should be weary of their external validity.
Possible study limitations may be due to a bias in patient classification, the method of cost calculation (attributable to the information system employed by hospitals) and the follow-up period. Group comparability may be affected, since we were forced to extend the selection period of the thrombosis group by 4 years (deliberate selection), in order to obtain the necessary number of cases according to the inclusion/exclusion criteria of the study. However, the impact of this factor may be minimal for the purpose of the study. Another possible limitation is the inclusion of indirect costs, including the cost for patients of attending rehabilitation every day, days of absence from work, etc., or failing to take into account the type of surgical technique (traditional, minimally invasive) and prosthesis used. However, due to the considerable diversity of existing types of prostheses, it is difficult to compare results between them. We must also consider that the follow-up period was short and, therefore, some of the possible complications may have not been recorded, although this would affect all groups equally. Therefore, the results show the inherent limitations of retrospective studies, such as possible underestimation of some variables or possible variability among professionals. In addition, the small sample size may lead to poor comparability between groups. Another aspect to consider is that the observed results have no data other than symptomatic thrombosis, so the actual thrombosis may have not been assessed correctly. It is noteworthy that prevention methods of VTE do not only include heparin or oral anti-thrombotic agents. Among other factors, physical methods, rehabilitation processes (postoperative mobilisation time) and the type of anaesthesia and/or pain control were not considered among the analysed data, so this aspect should be construed as another limitation of the study. A further limitation to consider is that we were unable to differentiate whether bleeding patients showed a higher rate of infections or if bleeding was a sign of acute infection, although in our opinion the first circumstance was probably true. However, despite these limitations, our study may be applicable in economic assessment models and for general knowledge of the disease costs, since it incorporates a substantial base of knowledge which was previously unexplored.
Recent studies have shown that the introduction of organised practice guidelines obtained through consensus among the various clinical services involved in the diagnosis and treatment of these patients, leads to a decrease of mean length of stay, intrahospital complications and healthcare costs, thus reducing process variability. These guidelines contemplate the prioritisation of arthroplasty according to explicit criteria, implementation of guaranteed service times or the incorporation of strategies to improve the indication for intervention.25–27 The overall results of our study show a certain degree of homogeneity among the variables studied, so the differences between the 3 participating hospitals were acceptable within the context of clinical practice and reduction of uncertainty.1,13
The overall study results show that, out of all patients undergoing THA or TKA, 8.8% suffered some type of bleeding (55.0% underwent blood transfusion and 0.9% suffered DVT). Of the patients studied, 5.1% presented some kind of nosocomial infection. It seems clear that clinical complications cause greater consumption of hospital resources.1,28
Intraoperative bleeding is one of the leading complications of arthroplasty.1,16,17 The literature contains reports of some factors related to the duration of surgery, type of procedure, intraoperative optimisation of intravascular volume, age, platelet count or previous illness of patients, in about 5–8% of arthroplasties. In our study, we found an incidence of bleeding and transfusion similar to those described by Cuenca29 or Swain30 and slightly higher than that described by Singh.31 Nevertheless, these results are difficult to compare because they are from total primary arthroplasties and there may also be bias due to selection and misclassification of patients, since the proposed objective was the identification of costs in the 3 study groups analysed. In this respect, the relationship between bleeding and nosocomial infections seems clear as a cause of increased health costs.
Thromboembolic disease has been described as a complication linked to an increased risk of perioperative mortality after arthroplasty, so prophylactic measures have come to occupy a central role in the treatment of surgical patients, with LMWH being the most prominent.14–17,22 Our results are difficult to compare due to the lack of similar studies. However, in our study we found a low incidence, given the need to extend the patient inclusion period. The available evidence shows rates below 2% in patients treated with LMWH.32 The case series of Rodriguez-Alonso33 reported DVT rates of 1.7% with 0.9% of PE. These results are similar to those reported by other authors.34 The association with nosocomial infections, causing an increased use of both hospital and outpatient resources, is also noteworthy.35 It seems clear that, despite administering treatment, it remains a common complication.36 Depending on the results and in spite of the prophylactic efforts made to eradicate DVT, it appears that there is still some room for improvement.
Our results showed that infection was the main complication and was associated with the group of patients with bleeding and thrombosis. In addition, we noted a greater consumption of antibiotics, which could also pose an additional risk due to development of bacterial resistance. In the literature, the incidence among primary THA cases varies between 0.4 and 5%, in accordance with the study.37–39 It should be mentioned that surgical procedures have evolved constantly and, at present, minimally invasive techniques have been developed in which the surgical incision is greatly reduced. It is possible that using a smaller incision reduces blood loss, surgical time and surgical trauma, favours rapid rehabilitation and decreases hospital stay and likelihood of infection.40 However, our study failed to quantify this impact.
The mean length of stay in the thrombosis, bleeding and control groups was 13.9, 11.5 and 7.4 days, respectively, while costs were € 10,484, € 8766 and € 6496, respectively, with € 8003 being the mean overall cost. The cost was mainly associated to the number of hospital stays (including readmissions) caused by intrahospital complications, especially nosocomial infection. These results seem easy to interpret, although difficult to compare, given the absence of studies with a similar methodology. In our country, Martí-Valls et al.41 described a process cost of € 5051, related to the length of stay and cost of the prosthesis in a series of 332 cases of total hip replacement. In a series of 80 cases of THA and TKA (40 for each joint), Navarro-Espigares and Hernandez-Torres42 reported a cost of € 7891 and € 6866, respectively, and showed that functional status and perceived health improved after the intervention. Riu et al.43 obtained a total process cost of € 7592 in a series describing the healthcare impact of a knee arthroplasty functional unit. In general terms, these results can be interpreted as being similar to those found in our study, although in our case there were differences between the study groups analysed. These differences were due to the likelihood of developing some type of complication during the follow-up period. The use of resources for arthroplasty has become more efficient in recent years, partly due to clinical practice guidelines. Internationally, the mean cost of the process ranges between € 5500 and € 12,500. The variations found are mainly due to the variables included, the method of cost calculation, the type of surgical procedure employed and the healthcare and organisational polices.44–48 We should note that in our study we used mean/unit costs, so the assessment was independent of the time of analysis. This means that the final variation in cost was entirely due to changes in the volume of resources, without incorporating changes related to the price variation of production factors resulting from organisational changes or management of human resources. Regarding hospital readmissions, most of the reviewed studies reported around 3–5%.7 These results are consistent with our study, so they should not be considered as a variable which maximises healthcare costs.
Future investigations will require cost/effectiveness,49 delayed diagnosis and surgical indication studies, in addition to replicating the study in other healthcare organisations. Another aspect to consider would be the effect of risk factors on poor surgical outcomes and intrahospital complications (outpatient follow-up had little impact), resulting in a reduction of hospital stay and costs. We should note that the wide range of prostheses available, the limited information available about their long-term results and impact of review surgery on the health of patients and healthcare costs has led some countries to develop arthroplasty registers. Given the number of alternatives in existence, it would be advisable to create a consensus guide for decision-making aimed at selecting the most appropriate type of prosthesis according to the previous health status and diagnosis of patients. In short, establishing organised consensus action programs, developed by multidisciplinary teams which are familiar with this illness, would lead to a reduction in variability50 and, therefore, in the cost of treatment.
In conclusion, primary total hip and knee arthroplasties are surgical procedures which generate significant use of hospital healthcare resources. These costs are higher for patients who develop thrombosis and bleeding, respectively. The costs are mainly associated with extended length of stay and nosocomial infections.
Level of evidenceLevel of evidence I.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation did not require experiments on humans or animals.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that this work does not reflect any patient data.
Conflict of interestsThis study was jointly funded by Pfizer S.L.U. and Bristol-Myers Squibb Inc. Marina de Salas is an employee of Pfizer S.L.U.; Irene Lizano was an employee of Pfizer S.L.U. at the time of preparation of the manuscript; Lourdes Betegón is an employee of Bristol-Myers Squibb Inc. The remaining authors have no conflicts of interest to declare.
The authors wish to thank all the professionals at the 3 participating hospitals.
Please cite this article as: Sicras-Mainar A, et al. Utilización de recursos sanitarios y costes asociados al diagnóstico y tratamiento de cada episodio de trombosis venosa profunda y sangrado en pacientes intervenidos de cirugía ortopédica de cadera o rodilla. Rev Esp Cir Ortop Traumatol. 2012;56:341–53