Following medical device alerts published in different countries of problems with metal-on-metal total hip replacements, the Spanish Agency of Medicines and Medical Devices (AEMPS) in collaboration with the Spanish Hip Society Surgery designed a national survey to gather information on the use and behaviour of these hip implants.
MethodsThe survey consisted of a questionnaire sent by e-mail to 283 clinical centre recipients of metal-on-metal hips to be filled in by surgeons with expertise in the field.
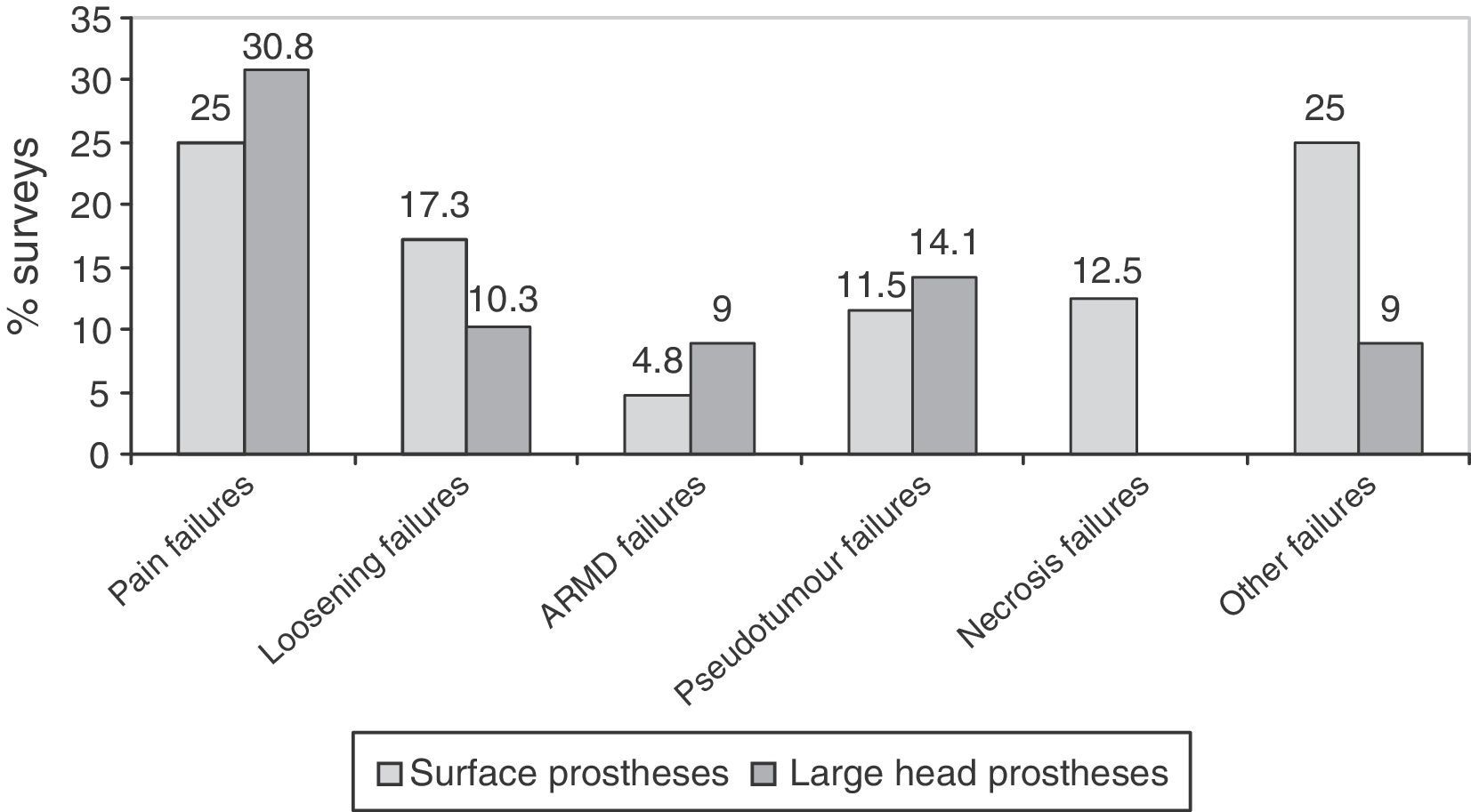
ResultsA total of 257 questionnaires were completed. The response rate of the clinical centres was 36.7%. A total of 97.7% of the responses reported that clinical and radiological follow-ups are carried out, and 79.6% undertook metal ion analyses (chromium and cobalt). A large majority (83.6%) of the responders who had used surface implants, and 70% of those with large-head implants reported peri-operative complications. The most common complication was pain (25% with surface implants and 30.8% with large-head implants). Currently 80.8% of those responding were considering abandoning implanting of these hip replacements.
ConclusionsDespite the many limitations to this study, the survey has allowed us to obtain in a quick first view of the implant scenario of Metal on Metal hip implants in Spain, and to determine the type of patient implanted, the time of implantation, and the experience/expertise of the surgeons, and the type of follow-up carried out.
La publicación de alertas en distintos países sobre posibles complicaciones del par metal-metal en las prótesis totales de cadera, indujo a la Agencia Española de Medicamentos y Productos Sanitarios en colaboración con la Sociedad Española de Cirugía de Cadera a diseñar una encuesta de ámbito nacional, con el objetivo de obtener información sobre el uso y comportamiento de estos implantes.
Material y métodoLa encuesta utilizó como instrumento un cuestionario que fue enviado por correo electrónico a 283 centros receptores de prótesis de cadera metal-metal para que fuese cumplimentado por los cirujanos con experiencia en este tipo de implantes.
ResultadosEl número total de encuestas cumplimentadas fue 257. La tasa global de respuesta de los centros fue del 36,7%. El 97,7% de las encuestas refirieron realizar seguimientos clínicos y radiográficos y el 79,6% analíticos. El 83,6% de las encuestas que habían implantado prótesis de superficie y el 70% de las que habían implantado prótesis con cabeza grande refirieron complicaciones perioperatorias. El fallo más frecuente referido fue el dolor tanto en prótesis de superficie como en prótesis de cabeza grande. Actualmente el 80,8% de las encuestas han referido haber abandonado este tipo de implantes.
ConclusionesA pesar de las numerosas limitaciones del estudio, la encuesta ha permitido obtener de forma rápida un primer contacto con la realidad de la implantación de prótesis de cadera metal-metal en España, y conocer así el perfil de pacientes intervenidos, el periodo de implantación, la experiencia de los cirujanos y el tipo de seguimiento realizado.
The theoretical advantages of hip prostheses with a metal-on-metal friction coupling and their widespread adoption in clinical practice1–6 have subsequently been questioned due to reports of local adverse effects,7–9 as well as an increase in the levels of metallic ions in blood and urine with possible systemic reactions.10–13 The potential hazards of these implants became better known after health alerts were published in various countries14–16 and at a national level,17 even causing significant public concern in some cases.18
The publication of these alerts about possible complications related to metal-on-metal couplings in total hip prostheses (THP) and their possible repercussion on the population, led the Spanish Drug and Healthcare Products Agency (Agencia Española de Medicamentos y Productos Sanitarios, AEMPS) to evaluate the use and behaviour of these implants in our country. Due to the variability in the results obtained by research studies, which hindered an analysis of both effectiveness and safety of metal-on-metal couplings in THP, as well as an absence of available information regarding the monitoring of implanted patients caused by the lack of a National Registry of Arthroplasties, AEMPS collaborated with the Spanish Hip Surgery Society (Sociedad Española de Cirugía de Cadera, SECCA) in the design of a survey on the use and performance of hip prostheses with metal-on-metal couplings in Spain.
The survey on the use and performance of hip prostheses with metal-on-metal couplings was conducted at a national level to obtain information about the behaviour of metal-on-metal THP in our country. The specific objectives were to describe the characteristics of the intervened population and of the prostheses used, to know the experience in implantation, analyse some of the complications derived from their use and to know the type of monitoring conducted on implanted patients.
This work presents the main results obtained in the survey on the use and performance of metal-on-metal hip prostheses in Spain with the limitations inherent to this type of research procedure.
Material and methodsDesignThe survey was designed by AEMPS in collaboration with SECCA between the months of September and October 2013.
The target population of the survey were surgeons with experience in implantation of THP with metal-on-metal couplings who worked at public and private centres throughout the national territory.
The sample was calculated using the Registry of Healthcare Products of AEMPS, listing distributors of metal-on-metal coupling THP in Spain. Based on this registry, AEMPS identified 283 centres receiving hip prostheses with metal-on-metal couplings in Spain. All 283 centres were included in the study.
The questionnaire produced is structured into 15 questions, mainly with closed answers. The questions were defined to gather the following information:
- 1.
Profile of patients. Age, gender and hip pathology of implanted patients.
- 2.
Period of implantation. Year when metal-on-metal hip prostheses started to be implanted and year when this type of implants were abandoned.
- 3.
Type of metal-on-metal hip prostheses. Surface prostheses, small and large head prostheses. The total prosthesis with a 28 or 32mm head was defined as a small head prosthesis and the total prosthesis with a head larger than or equal to 36mm19 was defined as a large head prosthesis.
- 4.
Experience in implantation. The experience in implantation with metal-on-metal hip prostheses was measured through the volume of implantation, defined as the total number of implants carried out throughout the professional career of the surgeons. The experience was grouped into 3 categories, low implantation or scarce experience in implantation when the total number of implants carried out was less than 10, medium implantation when the total number of implants carried out was between 10 and 50, and high implantation or extensive experience in implantation, when the total number carried out was greater than 50.
- 5.
Complications. The complications were broken down into perioperative complications, number of replacements carried out, and causes of failures. Failures observed were grouped into 6 categories: pain, loosening, Adverse Reactions to Metal Debris (ARMD), pseudotumours, necrosis of the femoral head and other failures.
- 6.
Type of monitoring. The type of monitoring was broken down into clinical, radiological, analytical, and determination of chromium (Cr) and cobalt (Co) ions in blood and urine. The figures considered as normal reference values for Cr and Co concentration were ≤5μg/L for Cr and ≤2μg/L for Co.20
AEMPS sent the questionnaire by e-mail together with a cover letter explaining the study to the contact points of the Monitoring System for Healthcare Products of each Spanish political region, which were in charge of distributing it to the heads of monitoring at the 283 centres receiving THP with metal-on-metal couplings. These, in turn, forwarded it to the surgeons of each centre.
The field work was conducted between November 2013 and January 2014. Once completed by the surgeons, the questionnaires were returned to AEMPS by e-mail or fax.
Data collection and analysisThe data obtained through the questionnaire were stored in an MS Excel spreadsheet, and then captured and logged for subsequent analysis using the statistics software package SPSS for Windows version 19 (SPSS Inc., Chicago, IL, USA).
Qualitative variables were presented as distribution of absolute frequencies and percentages. The quantitative variable “time of implantation” was not adjusted to a normal distribution, and was expressed as median and interquartile range, minimum and maximum. The comparison of “experience in implantation” (surface prostheses) and “type of centre” (public or private), was done using the chi-squared test, or Fisher exact test when the expected frequencies under 5 were more than 20%. The level of statistically significant difference was set at values of P lower than 5% (P<.05).
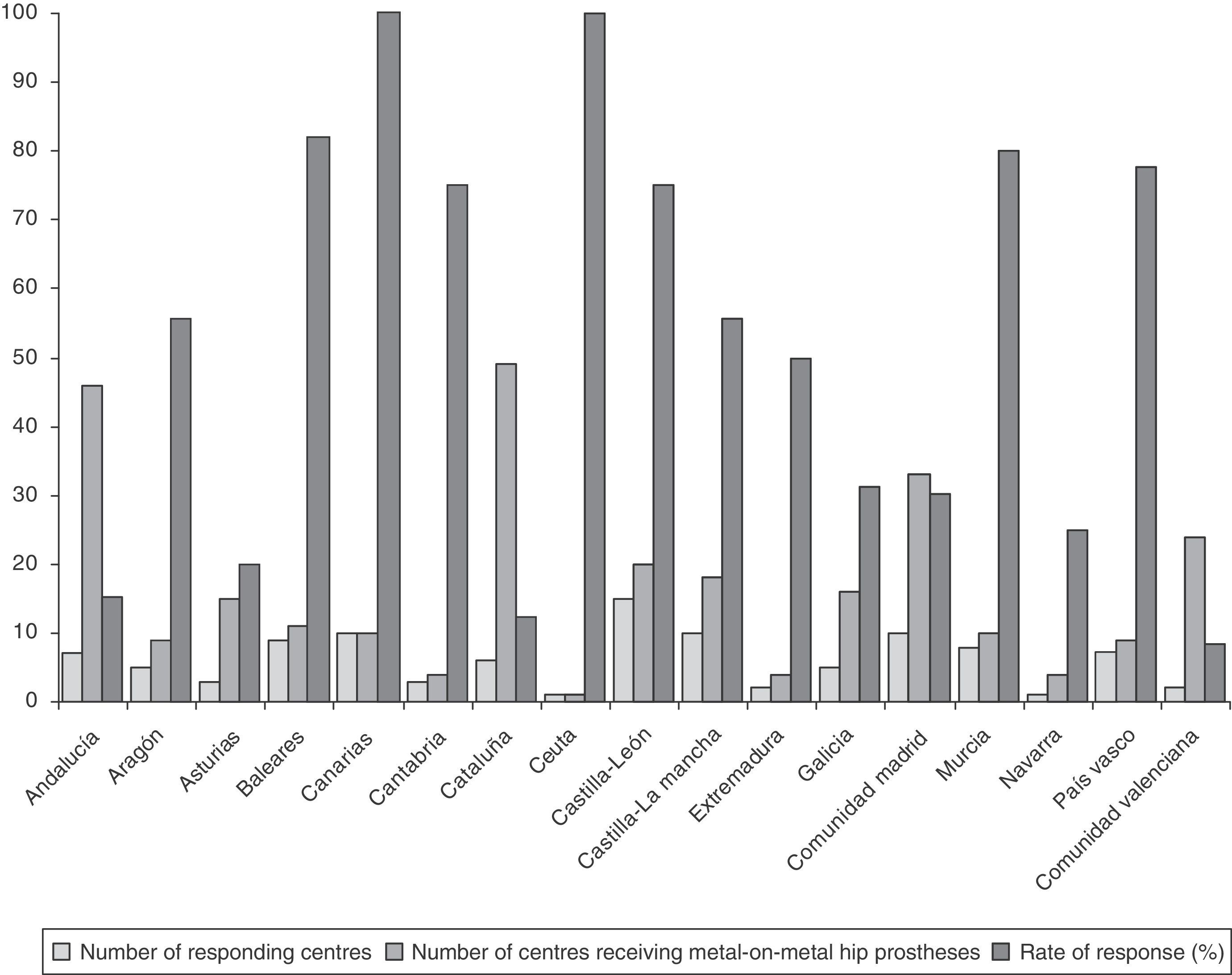
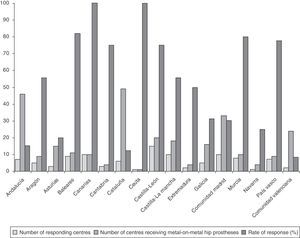
ResultsRate of response to the survey by healthcare centresOut of the 283 centres receiving THP with metal-on-metal couplings, 104 centres replied to the survey, with the overall rate of response by centres being 36.7%. The rate of response of the centres broken down by regions is reflected in Fig. 1.
Rates of response of 100% were obtained in the regions of the Canary Islands and Ceuta (Fig. 1).
Rates of response between 75% and 82% were obtained in the regions of the Balearic Islands, Murcia, Basque Country, Castilla y León (C y L) and Cantabria (Fig. 1).
The lowest rates of response were obtained in the regions with the highest number of centres receiving THP with metal-on-metal couplings, that is, Andalusia (15.2%), Catalonia (12.2%) and Valencia (8.3%) (Fig. 1).
Experience with metal-on-metal total hip prosthesesA total of 257 completed surveys were collected from 104 centres receiving THP with metal-on-metal couplings. Out of these 257 completed surveys, 50.6% (130) of the surveys reported having implanted metal-on-metal total hip prostheses, whilst 49.4% (127) reported never having implanted this kind of prosthesis.
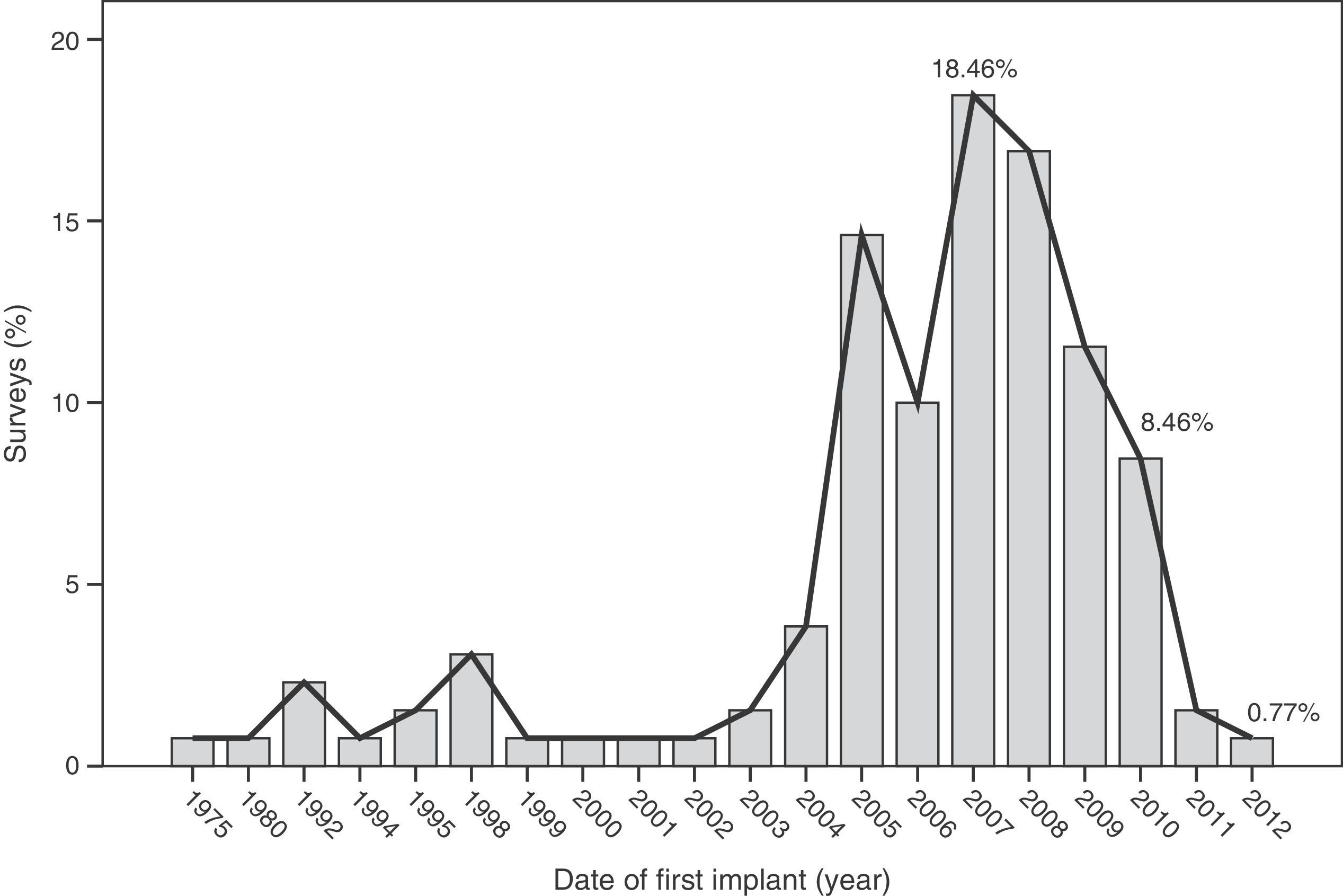
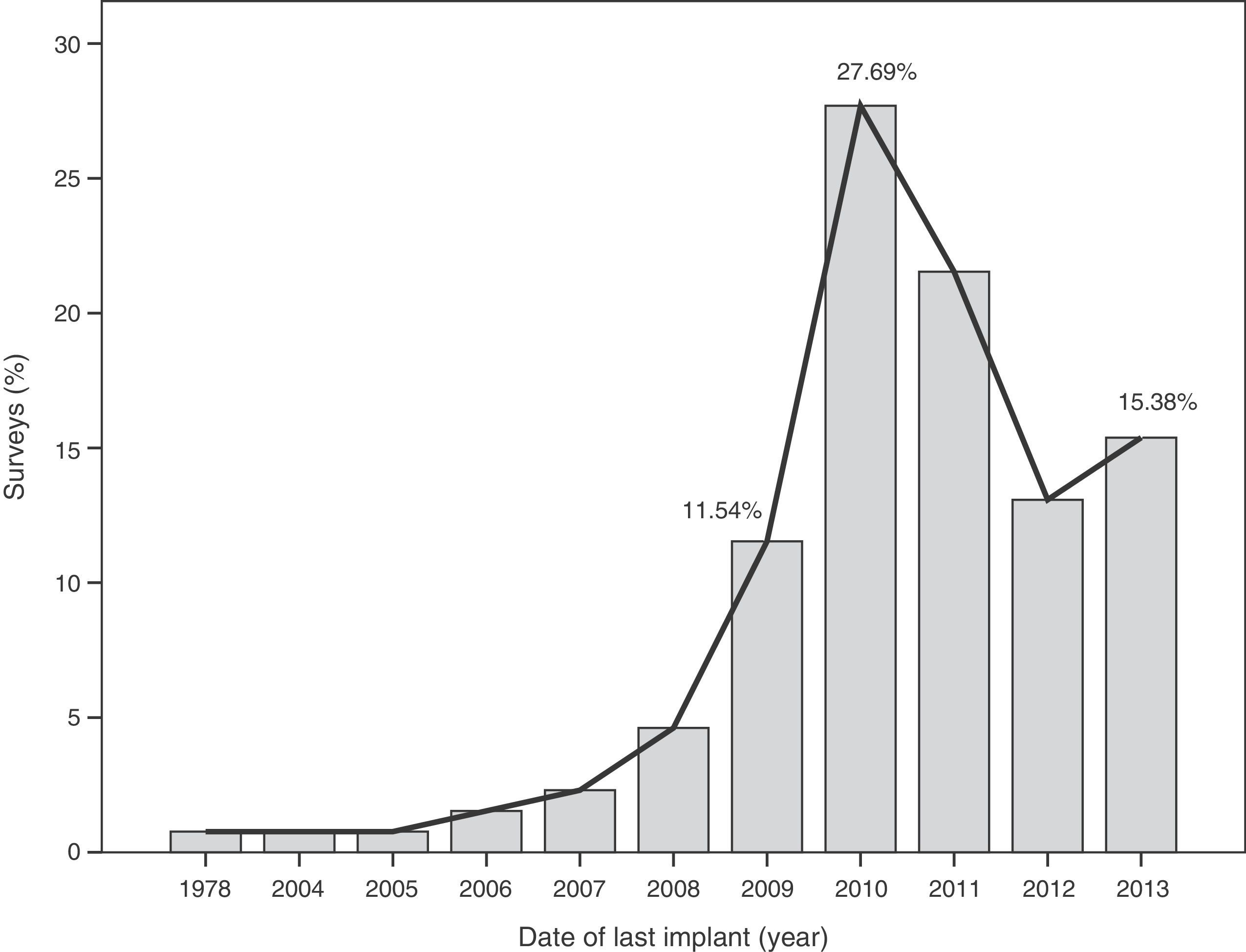
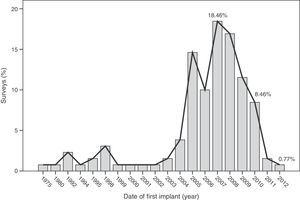
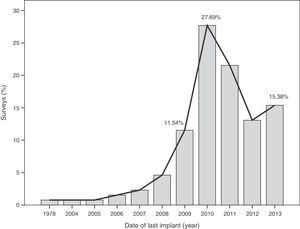
The median time of implantation of metal-on-metal hip prostheses was 3 years (interquartile range=2–6 years), with a minimum implantation of 1 year and a maximum implantation of 28 years. The start of implantation time of these prostheses increased from 2004 and decreased after 2010 (Fig. 2), the year when 27.7% of respondents reported abandoning the implantation of this type of prostheses (Fig. 3).
Regarding the volume of implantation, 49% (50) of the surveys indicated having implanted less than 10 surface prostheses, 44% (46) of the surveys between 10 and 50 surface prostheses, and only 6.9% (7) of the surveys indicated having implanted more than 50 surface prostheses. Regarding the volume of implantation of prostheses with a large head, 67.9% (53) of the surveys indicated having implanted less than 10 prostheses, 24.4% (19) of the surveys between 10 and 50, and only 7.7% (6) of the surveys indicated having implanted more than 50 large head prostheses.
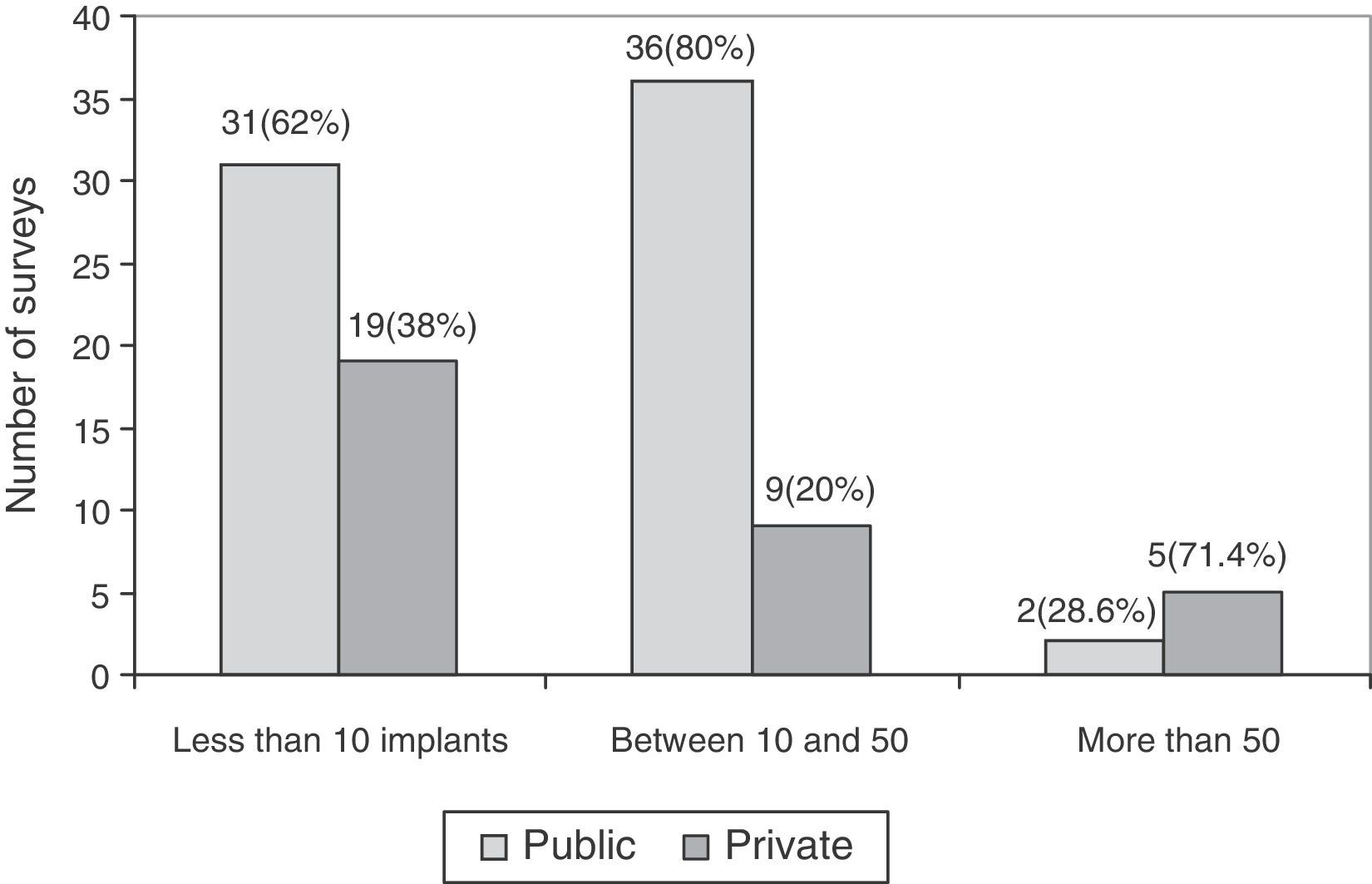
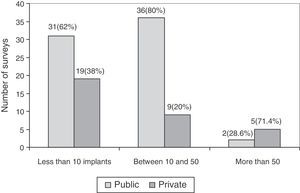
The association between experience in implantation of surface prostheses and the type of centre, public or private, was also studied. The experience in implantation of surface prostheses was dependent on the type of centre (P<.05), so that when the volume of implantation was high (more than 50 implants), the majority of surgeons belonged to private centres (Fig. 4).
Profile of intervened patientsThe main indication for implantation of a metal-on-metal hip prosthesis reflected in the surveys was hip osteoarthritis.
Regarding the indication for metal-on-metal hip prosthesis based on the patient characteristics, 68.5% (87) of the surveys reported indicating them exclusively in males, whilst 22% (28) reported indicating them in both males and females.
In total, 67.6% (98) of the surveys considered the age group under 55 years as the most suitable for implantation of metal-on-metal hip prostheses, 29% (42) indicated this type of implant in the age group between 56 and 65 years, and only 3.4% (5) indicated it in those aged over 65 years.
Type of prostheses usedOut of the 130 surveys with experience in implantation of metal-on-metal hip prostheses, 80% (104) reported having used surface prostheses, 59.2% (77) reported having used total hip prostheses with a large head, and only 24.6% (32) reported having used total hip prostheses with heads of 28 or 32mm. The majority of respondents had used more than one type of metal-on-metal hip prosthesis.
ComplicationsOut of the 104 surveys referring implants of surface prostheses, 11.5% (12) of the surveys reported not having observed any perioperative complication, 82.7% (86) reported less than 10, and only 0.9% (1) reported more than 10 complications. Out of the 77 surveys referring implantation of total prostheses with large heads, 18% (14) reported not having observed any complication, 67.5% (52) reported less than 10 complications, and 2.5% (2) reported more than 10 complications.
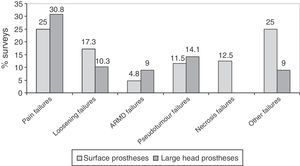
The most frequent failures reported in surveys were pain, followed by loosening, in surface implants with large heads (Fig. 5). In prostheses with small heads, the most frequent failure was loosening, in 35.2% (12) of the surveys, followed by failure due to pain, in 25.7% (9). Surveys with experience in prostheses with small heads did not observe ARMD reactions and only 1 survey reported a pseudotumour.
The majority of surveys reported having replaced less than 10 implants, with very similar percentages between the different types of prostheses: 76.3% (74) of the surveys for surface prostheses, 70.8% (46) for large head prostheses and 73.1% (19) for small head prostheses. The number of replacements registered between 10 and 50 was low, 2.1% (2) of the surveys reported this interval of replacements for surface prostheses, 1.5% (1) of the surveys for large head prostheses, and no replacement in this interval for small prostheses. The surveys reported not having used more than 50 replacements of metal-on-metal prostheses.
Monitoring of patients with metal-on-metal hip prosthesesA total of 97.7% (126) of the surveys conducted clinical and radiological monitoring of patients with a THP with metal-on-metal coupling, whilst 75.3% (98) of the surveys carried out analytical monitoring to determine the levels of Cr and Co ions.
Out of the 98 surveys that carried out metal ion monitoring, 79.6% (78) of the surveys observed an increase in the levels above 5μg/L for Cr and/or 2μg/L for Co in less than 10 patients, 6.1% (6) between 10 and 50 patients, 1% (1) in more than 50 patients, and 13.3% (13) reported not having detected these levels in any patient.
In total, 86.2% (112) of the surveys reported having carried out monitoring of patients for 2 years, whilst 77.7% (101) of the surveys reported having done so for 5 years.
Current implantationOut of the 130 surveys reporting experience in implantation of metal-on-metal hip prostheses, as of January 2014, 80.8% (105) of the surveys had abandoned this type of prosthesis and only 19.2% (25) continued implanting this type of prosthesis.
Out of the 105 surveys that had abandoned implantation of metal-on-metal prostheses, 100 surveys had detailed the causes of abandonment, whilst 5 surveys had not specified a motive. The causes for abandonment were failures and complications in 56%, economic reasons and/or removal of the catalogue within the centre in 26%, due to literature references and/or publication of healthcare alerts in 21%, and due the complexity of the surgical technique in 7%.
DiscussionThe use of surface prostheses is an attractive treatment due to its reduced femoral bone resection, particularly in young and active patients with primary osteoarthritis, which led to its use becoming widespread, especially in some European countries,5,6 in the last decade. The survey on the use and performance of metal-on-metal hip prostheses in Spain also reflects that the patient profile in which implantation of a THP with metal-on-metal coupling was most commonly indicated were males aged under 55 years.
The survey has provided information on the period of implantation of metal-on-metal prostheses in Spain, such as an increase in its use since 2004 and subsequent decrease after 2010, the year in which the highest number of surveyed surgeons abandoned these implants.
This decrease in implantation after 2010 and highest rate of abandonment on that same year may have been caused by the removal from the worldwide market of the Durom (Zimmer®) and ASR (Depuy Johnson&Johnson®) surface prostheses, as well as the XL (Depuy Johnson&Johnson®) large diameter heads of the ASR model following the publication of various studies13,22 and healthcare alerts in different countries,15,17,21 indicating a high level of failures.
The main drawback of the THP with metal-on-metal coupling is a consequence of wear. By articulating metal against metal, the passage of time leads to a release of metal particles into the joint space and circulatory system, which, when elevated, can give rise to local adverse reactions, pseudotumours7,9 and inflammatory phenomena around the prosthesis, causing an increase in the rate of revision surgery.23 The survey highlighted failures caused by pain, loosening and pseudotumour, as well as failures due to femoral head necrosis in surface prostheses. In addition, the majority of respondents, between 76.3% and 70.8%, had replaced less than 10 metal-on-metal prostheses in a median implantation period of 3 years. This short period of implantation could be one of the causes of the high number of complications, since the majority were centres with surgeons in a learning curve for this type of implants. These figures should be considered with caution, since, given the limitations of the survey, it was not possible to distinguish surgeons who performed 1 revision from those who performed 10.
There have also been reports of possible systemic adverse reactions among patients with metal-on-metal prostheses due to a deposit of metal particles in different organs.24–26 The hematopoietic and urogenital systems may be affected in the medium term, 10–20 years, and solid organs may be affected in the long term, 20–40 years.
Due to the possibility of these adverse effects, various national and international scientific societies, through multidisciplinary expert panels, have established consensus action criteria, and elaborated recommendations for the monitoring of patients implanted with metal-on-metal prostheses. At a national level, in 2011 SECCA published a guide for the management of patients with metal-on-metal prostheses.27 At a European level, in 2013 the European Federation of National Associations of Orthopaedics and Traumatology (EFORT) published its own recommendations.28 In general, there is a consensus regarding the need to determine the levels of Cr/Co ions in the blood and/or urine of intervened patients, as well as the need to conduct complementary imaging tests in the monitoring of these patients. The survey reflected that surgeons followed the recommendations of scientific societies; nearly 100% carried out clinical and radiological monitoring, and a high percentage (75.3%) carried out monitoring of Cr and/or Co ions in implanted patients.
Regarding the experience in implantation of THP with metal-on-metal coupling, the survey reflected that the majority of respondents did not have considerable experience in such implantations; only 7 surveys for surface prostheses and 6 surveys for large head prostheses reported having implanted more than 50 throughout their professional careers. It is worth noting the higher frequency of implantations of metal-on-metal prostheses at private centres compared to public ones. One of the causes could be the increased administrative control by the National Healthcare Service of the use of different models, compared to private centres.
Limitations of the studyOne of the difficulties faced by the study of surveys on the use and performance of metal-on-metal hip prostheses was knowing the real number of centres where metal-on-metal hip prostheses had been implanted. At present, there is no national database that registers which centres implant hip prostheses. In the absence of a registry of centres implanting metal-on-metal hip prostheses, AEMPS had to estimate the sample size indirectly, through the list of centres receiving metal-on-metal hip prostheses, by means of information available to AEMPS through distributor companies for these products in Spain. In Spain, Royal Decree 1591/200929 establishes that healthcare products, like hip prosthesis implants must be accompanied by an implantation card. This implantation card must contain information on the product, the patient and the centre where the implantation took place, and should be completed by the healthcare centre, which should keep a copy in the medical history of the patient, provide a second copy to the patient and a third copy to the manufacturer or distributor of the product. However, in everyday practice these cards often do not reach the manufacturer, who then cannot know whether a specific prosthesis was finally implanted. In the survey, it is striking that 49.4% of respondents belonging to centres receiving metal-on-metal hip prostheses reported not having implanted this type of prosthesis; however, the fact that a centre receives a product does not necessarily mean that it finally implants it. The lack of a National Registry of Arthroplasties motivated this survey, with its inherent limitations, and leads us to insist in the need to implement this type of registries, which already exist in Catalonia, and which have proven their usefulness.30,31
Among the limitations of the study, it is worth highlighting that it is only a survey describing the experience of surgeons, so it does not reflect specific data regarding the number and type of implanted prostheses.
The survey on the use and performance of metal-on-metal hip prostheses in Spain also presented the usual limitations expected from a method using surveys as a research technique, and using as measurement instrument a questionnaire which was not administered in person: the rate of response obtained was low (36.7%) and, consequently, it may be that the results are only representative of the studied population and cannot be extrapolated, thus affecting the external validity of the study. In addition, there may have been a memory bias, as the data obtained did not come from documented sources, but instead depended on the accuracy of the responses.
Another limitation is that, in some cases, the survey was completed by the centre rather than at an individual level, thus encompassing the activity carried out by the entire centre, whereas in other cases it was completed by each individual surgeon working in the centre. Therefore, the result in terms of the number of surveys with experience in THP with metal-on-metal coupling may have been underestimated.
ConclusionsThe survey has allowed us to obtain a rapid first contact with the reality of implantation of hip prostheses with metal-on-metal friction coupling in our country. It has provided data on the period of implantation of metal-on-metal prostheses in Spain, on the profile of intervened patients and the type of monitoring conducted, describing the most commonly used type of prosthesis and the experience in implantation.
The analysis of these findings allows us to conclude that, in spite of the limitations entailed by extracting conclusions from a survey, there was a clear increase in the use of metal-on-metal hip prostheses in Spain starting from 2004, followed by a decrease after 2010. The majority of surgeons carried out periodic monitoring of their patients. Complications were not infrequently observed, particularly pain (surface and large head prostheses) and loosening (small head prostheses).
At present, most respondents report having abandoned these implants due to different causes, with the most common being failures and/or complications.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation adhered to the ethical guidelines of the Committee on Responsible Human Experimentation, as well as the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data.
Right to privacy and informed consentThe authors declare having obtained written informed consent from patients and/or subjects referred to in the work. This document is held by the corresponding author.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors wish to thank Dr. Elena González-Burgos for her technical support during the development of this work.
Please cite this article as: Calcerrada N, Fernández-Vega A, Valls-León C, Garcia-Cimbrelo E. Encuesta sobre el uso y comportamiento de las prótesis de cadera metal-metal en España. Rev Esp Cir Ortop Traumatol. 2015;60:20–28.