Bone loss in the distal femur is a common problem in knee revision surgeries. The problem is exacerbated in the context of an active infection. In extreme cases this bone loss could compromise the feasibility of a two-stage exchange protocol using dynamic spacers due to the inherent instability of this type of spacers. Use of a hip prefabricated spacer in a “reverse” way forming a ball-and-socket joint is a therapeutic option in cases of massive bone defect and infection.
Material and methodsA retrospective review was performed of our institutional database to identify all cases of massive distal femoral defect in which this technique was used from January 2010 to December 2013. A record was made of the epidemiological data, characteristics of the infection (clinical and microbiological), and adverse event during the spacer stage. The main end-point was the infection eradication rate (minimum: 18 months of follow-up). The complications associated with the technique were also assessed. Finally, each patient completed a visual analogue pain scale, and a satisfaction questionnaire (SAPS).
ResultsThis technique was successfully used in six cases so far, controlling the infection in all cases. Mean femoral defect was 117cm (range: 32–191cm). Mean time with spacer was 7.6 months, with no major complications. All but one patient reached second stage reconstruction with a mega-prosthesis, and mean time since second stage was 34.7 months. All patients stated high levels of satisfaction with the technique employed, as well as and low pain scores (mean visual analogue pain scale: 1; range: 0–4).
ConclusionA reproducible and safe technique is described. Patients report a high level of satisfaction with the procedure, and there were no cases of recurrence of the infection after a minimum follow-up of 18 months.
Los defectos óseos del fémur distal son un problema común en la cirugía de revisión de rodilla. El problema se agrava en el contexto de una infección activa. En casos extremos, esta pérdida de hueso compromete la viabilidad de un protocolo de recambio en dos tiempos usando espaciadores dinámicos, debido a la inestabilidad inherente de estos espaciadores. El uso de un espaciador prefabricado de cadera usado de manera «inversa» con una articulación tipo ball-and-socket es una opción terapéutica en casos de defecto óseo masivo e infección.
Material y métodosSe realiza una revisión retrospectiva de nuestra base de datos institucional desde enero del 2010 hasta diciembre del 2013 para localizar todos los casos de defecto femoral distal masivo en un contexto séptico en el que esta técnica ha sido utilizada. Se recogen datos epidemiológicos, características de la infección (clínicas y microbiológicas) y eventos entre los tiempos quirúrgicos. Evaluamos como objetivo principal la tasa de erradicación de la infección tras al menos 18 meses de evolución del 2.° tiempo. También evaluamos las complicaciones relacionadas con la técnica. Finalmente cada paciente completó una escala analógica visual de dolor, y un cuestionario de satisfacción (SAPS).
ResultadosFinalmente seis pacientes cumplieron los criterios de inclusión. La mediana del defecto femoral fue de 117cm (rango: 32-191cm). El tiempo medio con espaciador fue de 7,6 meses. Entre las complicaciones relacionadas con la técnica solo tuvimos un caso de luxación del espaciador. A todos los pacientes, excepto uno, se le realizó el segundo tiempo, reconstruyendo la articulación con una megaprótesis cementada, con un seguimiento medio tras el segundo tiempo de 34,7 meses. Al final del seguimiento se controló la infección en todos los casos. Todos los pacientes manifestaron altos grados de satisfacción con la técnica empleada y un correcto control del dolor, con una media de la escala analógica visual de 1 (rango 0-4).
ConclusiónPresentamos una técnica reproducible, segura y con una baja tasa de complicaciones. Los pacientes refieren una gran satisfacción con el procedimiento y no tuvimos casos de recidiva de la infección tras un seguimiento mínimo de 18 meses.
Total knee replacement has been one of the most successful operations in the recent history of orthopaedic surgery,1 improving quality of life for millions of people. One of the most feared complications with this procedure is peri-prosthetic infection, which has published rates of chronic infection that vary from 1% to 15%.1–4 Due to different reasons the need for knee and hip replacement implants is expected to grow exponentially; this, together with the existence of an increasingly long-lived population, will mean that the needs for septic as well as aseptic revision surgery will increase enormously.5 At each revision the surgeon faces the problem of an increasingly small bone reserve and a possible increase in infection rates. The coexistence of both problems increases the complexity of the revision.
Bone loss is a common problem in the context of peri-prosthetic knee infection.6 This loss of bone tissue is caused by a combination of infection per se (osteolysis due to frustrated phagocytosis) and previous surgery. In cases of multiple attempts at replacement bone loss may become dramatic.7,8 In such extreme situations functional arthrodesis9,10 or amputation above the knee may be the sole valid options.11
The current standard treatment for chronic peri-prosthetic infection of the hip or knee, and which achieves high rates of infection control,1,12 is two-stage replacement of the prosthesis. The temporary use of an antibiotic-impregnated spacer is an important step in this protocol. In the case of what are known as articulated or dynamic spacers,13,14 the existence of a massive bone defect will subject the said spacers to a complex biomechanical situation caused by massive bone loss; such spacers will hardly be able to adapt to the lack of residual bone.13
This work has the aim of retrospectively analysing our results with a technique developed in a specialised unit for septic pathology and reconstruction of the legs. This technique allows us to use dynamic spacers in case of massive lack of distal femur in a septic context. As well as allowing us to control the infection, we hypothesise that this technique will permit the patient to benefit from the dynamic properties of an articulated spacer.8
Material and methodsStudy designA retrospective revision was undertaken using the prospective database of the unit, including all of the septic cases with massive femoral defect (January 2010 to December 2013) in which this technique was used. Our centre is a university hospital with more than 1000 beds, containing a national reference unit for multi-skeletal septic pathology. No approval was sought from the CEIC, as this study was carried out as a part of the routine working of our unit; the patients were studied retrospectively and treated according to the treatment standards in our centre. All of the patients signed their informed consent for the procedure. Epidemiological data were recorded, together with patient comorbidities and clinical and microbiological infection characteristics. The femoral bone defect was evaluated and adverse events during surgery were recorded together with the characteristics of revision surgery. The main aim was to evaluate the rate of infection eradication following at least 18 months of evolution after the revision surgery. Complications in connection with the surgical technique studied were also evaluated.
DefinitionsThis series only includes cases of chronic peri-prosthetic infection or chronic osteomyelitis of the distal femur within a time limit of 4 weeks from implantation. This aims to differentiate between chronic and acute cases according to Tsukayama's classification.2
The final diagnosis of infection is established when the patient fulfils at least one of the following infection criteria, as proposed by the Infectious Diseases Society of America15 (IDSA) published in 2013: (1) the existence of a fistula that communicates with the joint; (2) intraarticular pus without any other explanation; (3) ≥ 2 positive cultures with the same microorganism; (4) a histology that is compatible with acute inflammation (Feldman's criterion).16
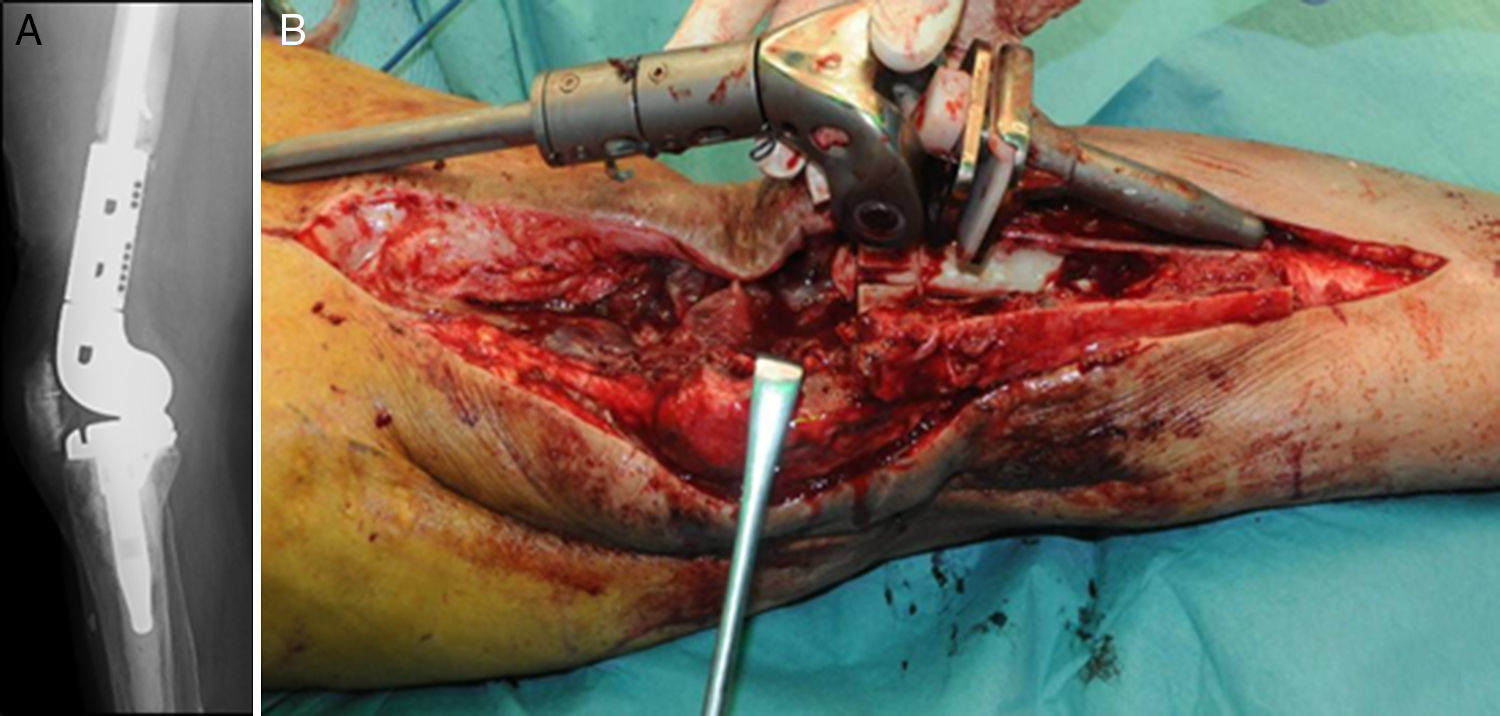
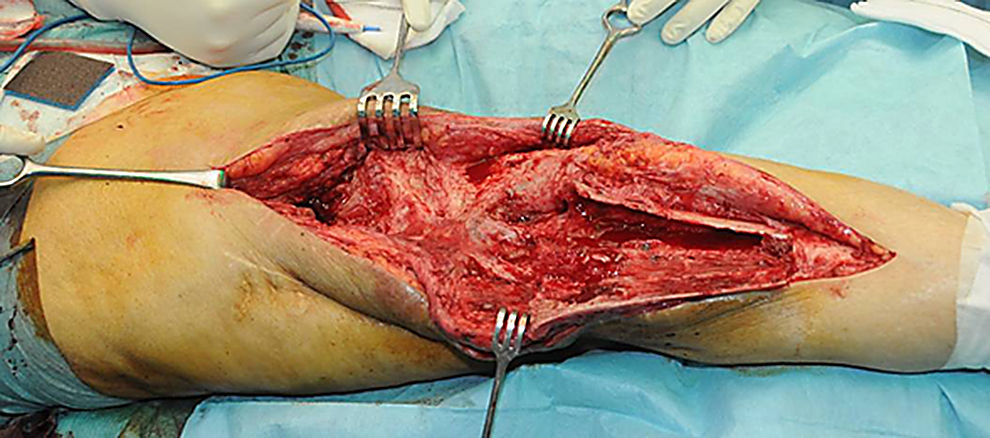
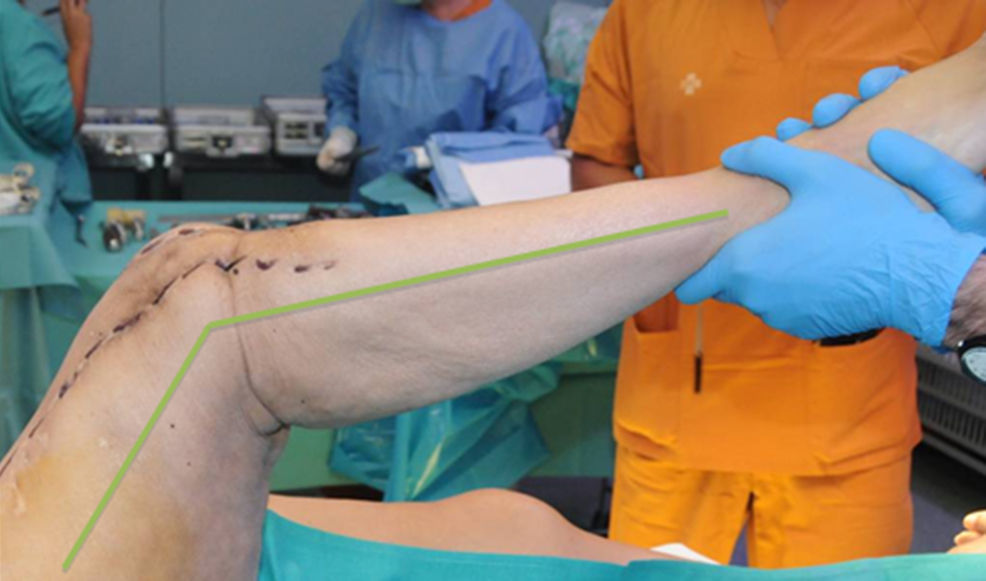
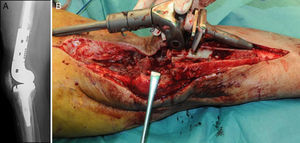
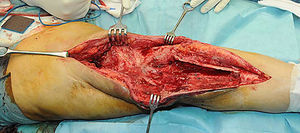
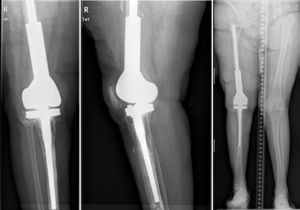
Description of the surgical technique studiedInfected components are removed in a standard way (Fig. 1A and B). Exhaustive debridement is then performed, including the endomedullar channel in the case of prosthesis with a rod. Before administering intraoperative antibiotics at least 6 samples are taken for microbiological culture. Low pressure irrigation of the whole exposed surgical field then takes place. The final bone defect is evaluated after debridement (Fig. 2). In cases where we find extensive loss of distal femur, we use a prefabricated hip spacer (Vancogenx®, Tecres, Somatocammpagna, Italy) to construct a ball-and-socket type joint. The hip spacer is industrially produced in a factory and looks similar to a hip hemiarthroplasty made of acrylic cement impregnated with gentamicin and vancomicin. The internal part of the spacer consists of a stainless-steel rod that creates mechanical stability. These spacers are available in 6 versions, with 3 sizes of head (46, 54 and 60mm) and with a long rod (275–290mm) or a short one (153–168mm). The spacers are impregnated with a 1:1 antibiotic concentration, so that depending on the size of the head and the length of the rod, they will contain from 1.1 to 3.2g of both antibiotics (Table 1). The size of prefabricated spacer selected depends on the residual defect. The spacer with a small head was used in the majority of cases (46mm). The hip spacer is used “reversed”, with the head against the surface of the remaining tibial plateau. The spacer head becomes the “ball” component of this ball-and-socket joint. Antibiotic-impregnated cement is used to construct the recipient on the surface of the proximal tibia. This mixture is prepared without a vacuum13 using high doses of two thermostable synergic antibiotics, selected according to the antibiotic sensitivity profile of the microorganism.14 In the majority of cases 4g vancomicin powder and 4g tobramycin powder are added to each 40g bag of bone cement. When there is infection with a prosthesis that has a rod the tibial endomedullar channel is filled with a bar of cement containing antibiotic prepared during surgery; this will add to the stability of the spacer and make it possible to fill the dead space of the tibial medullar channel.
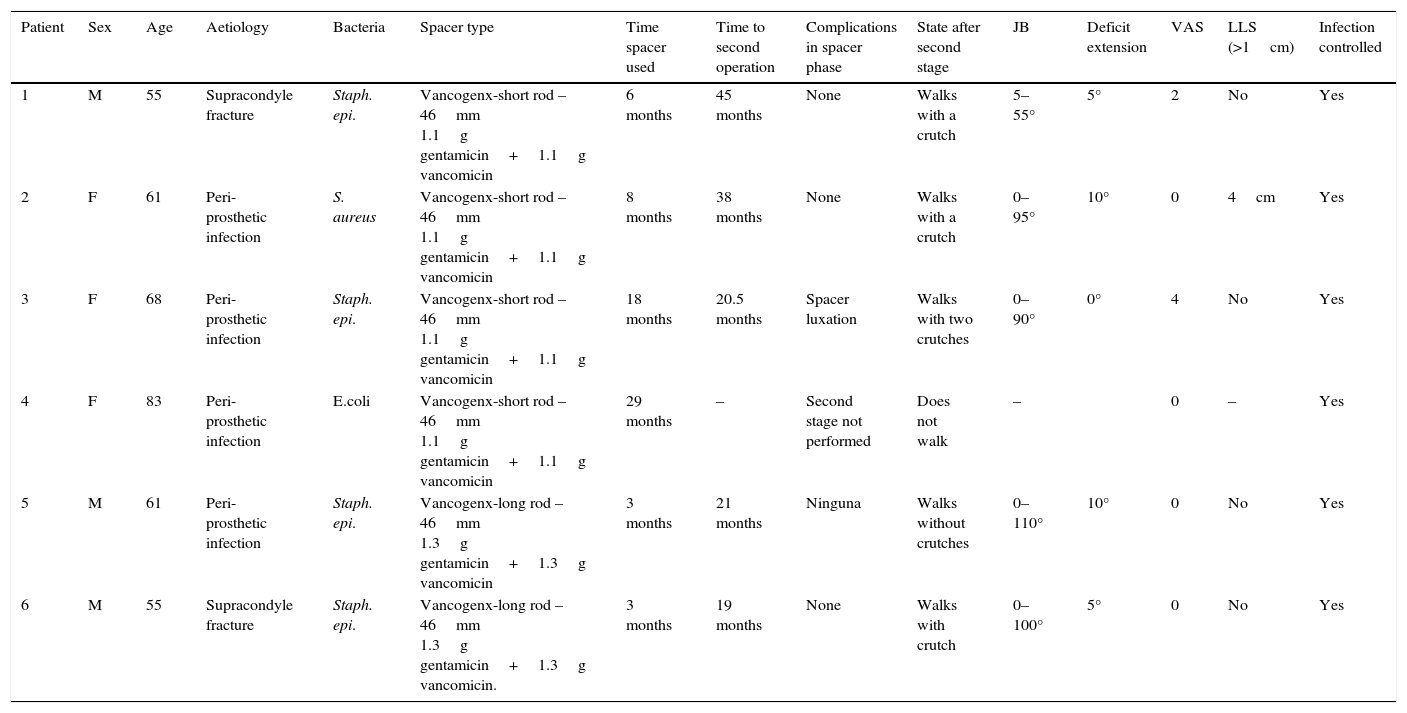
The clinical and surgical characteristics of the patients studied.
| Patient | Sex | Age | Aetiology | Bacteria | Spacer type | Time spacer used | Time to second operation | Complications in spacer phase | State after second stage | JB | Deficit extension | VAS | LLS (>1cm) | Infection controlled |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 55 | Supracondyle fracture | Staph. epi. | Vancogenx-short rod – 46mm 1.1g gentamicin+1.1g vancomicin | 6 months | 45 months | None | Walks with a crutch | 5–55° | 5° | 2 | No | Yes |
| 2 | F | 61 | Peri-prosthetic infection | S. aureus | Vancogenx-short rod – 46mm 1.1g gentamicin+1.1g vancomicin | 8 months | 38 months | None | Walks with a crutch | 0–95° | 10° | 0 | 4cm | Yes |
| 3 | F | 68 | Peri-prosthetic infection | Staph. epi. | Vancogenx-short rod – 46mm 1.1g gentamicin+1.1g vancomicin | 18 months | 20.5 months | Spacer luxation | Walks with two crutches | 0–90° | 0° | 4 | No | Yes |
| 4 | F | 83 | Peri-prosthetic infection | E.coli | Vancogenx-short rod – 46mm 1.1g gentamicin+1.1g vancomicin | 29 months | – | Second stage not performed | Does not walk | – | 0 | – | Yes | |
| 5 | M | 61 | Peri-prosthetic infection | Staph. epi. | Vancogenx-long rod – 46mm 1.3g gentamicin+1.3g vancomicin | 3 months | 21 months | Ninguna | Walks without crutches | 0–110° | 10° | 0 | No | Yes |
| 6 | M | 55 | Supracondyle fracture | Staph. epi. | Vancogenx-long rod – 46mm 1.3g gentamicin+1.3g vancomicin. | 3 months | 19 months | None | Walks with crutch | 0–100° | 5° | 0 | No | Yes |
E. coli: Escherichia coli; F: female; JB: joint balance; LLS: lower limb shortening; M: male; S. aureus: Staphylococcus aureus; Staph. epi.: Staphylococcus epidermidis; VAS: visual analogue scale.
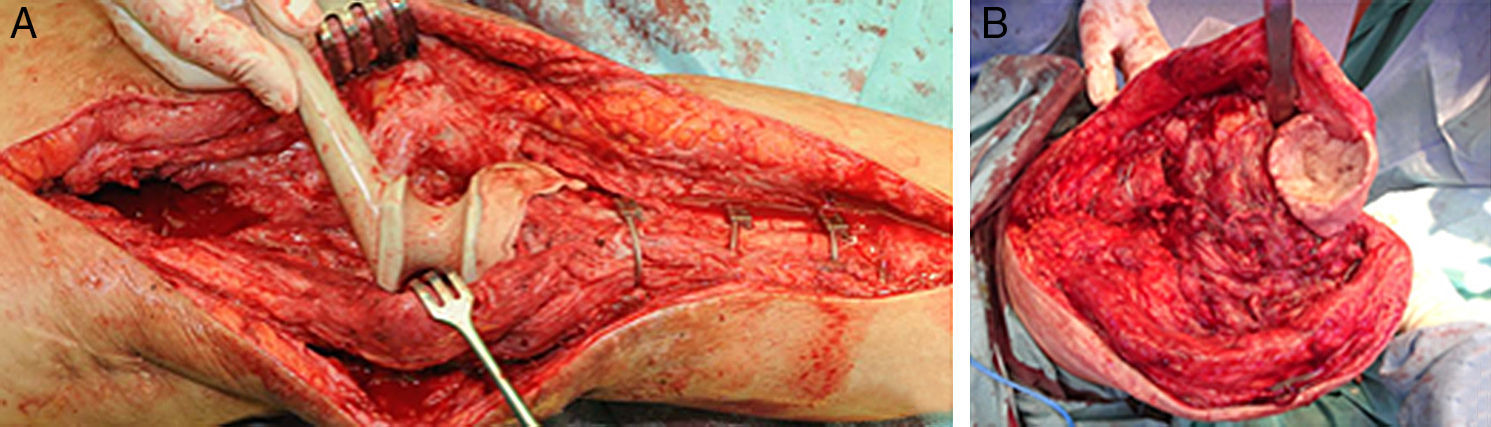
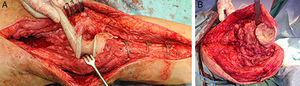
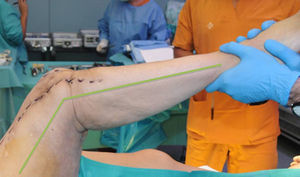
While the antibiotic-impregnated cement is in mouldable phase it is placed around and inside the metaphyseal tibial defect and joined to the tibial rod, if one is present. This forms a cement recipient that can be articulated with the head of the spacer. The spacer head is used as a mould to ensure a matching joint (Figs. 3A and B). The hip spacer rod is inserted into the femoral diaphysis and its most distal part is cemented to facilitate the removal of the spacer in a second operation. The hip spacer may be inserted as far as is necessary (Fig. 4) to achieve the desired tension in the soft tissues and prevent shortening of the limb. The most prominent part of the spacer may be cut as necessary. Stability and amplitude of movement are evaluated after the cement has polymerised (Fig. 5).
A splint is worn after the operation until the wound has healed correctly. A post-surgical articulated knee is then fitted for varus-valgus control. The knee is allowed to flex depending on the tolerance of the patient up to a maximum of 90°. Although the patient is unable to place any weight on the spacer while it is in place, he is able to walk with crutches or a frame and undergo rehabilitation. Patients are discharged to home with outpatient check-ups. According to our protocol,14 the second operation takes places after at least 8 weeks of targeted antibiotic treatment under the supervision of our infectious disease specialists, and when PCR and VSG levels have normalised for a period of at least 2 weeks without antibiotics.
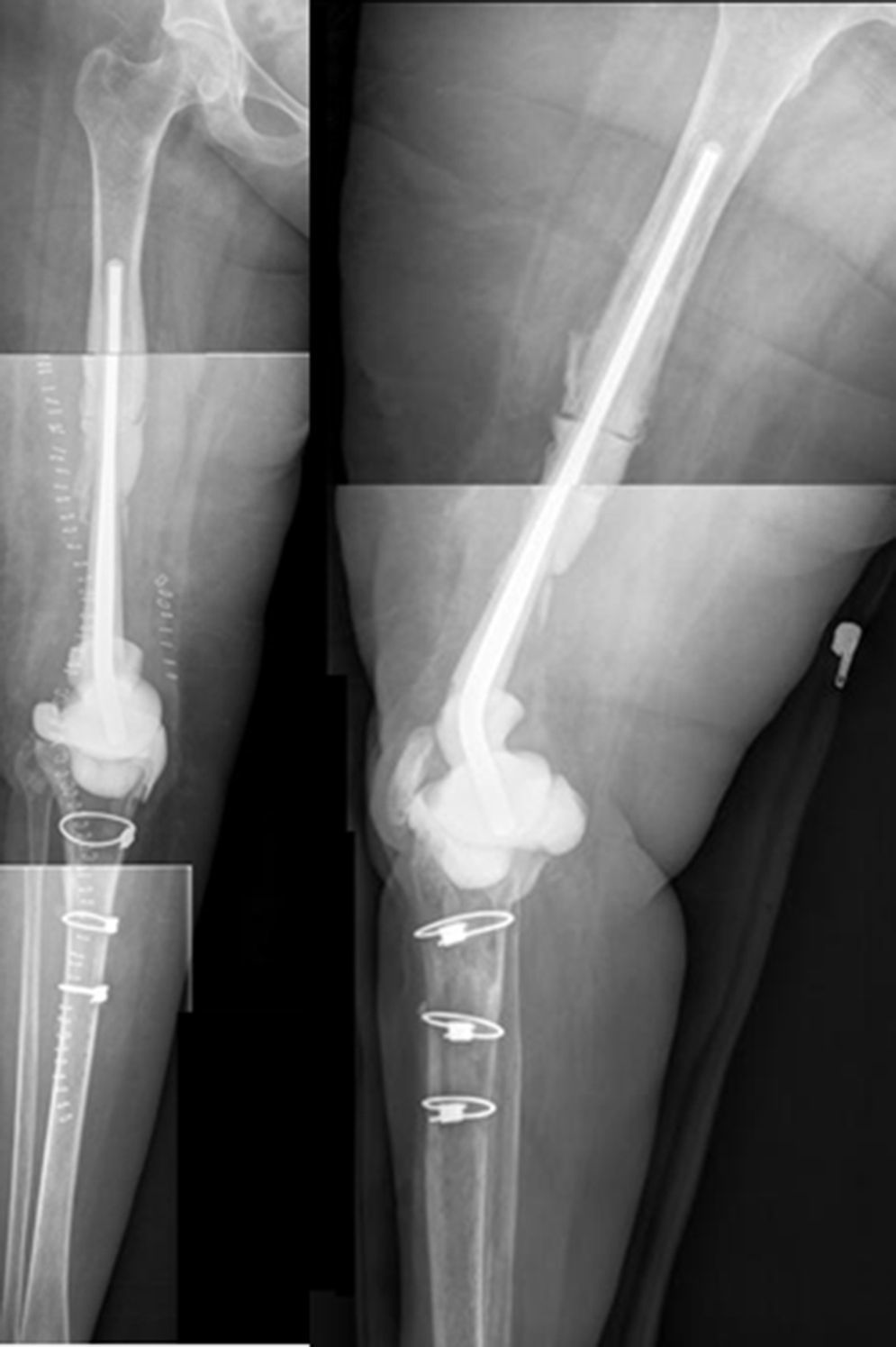
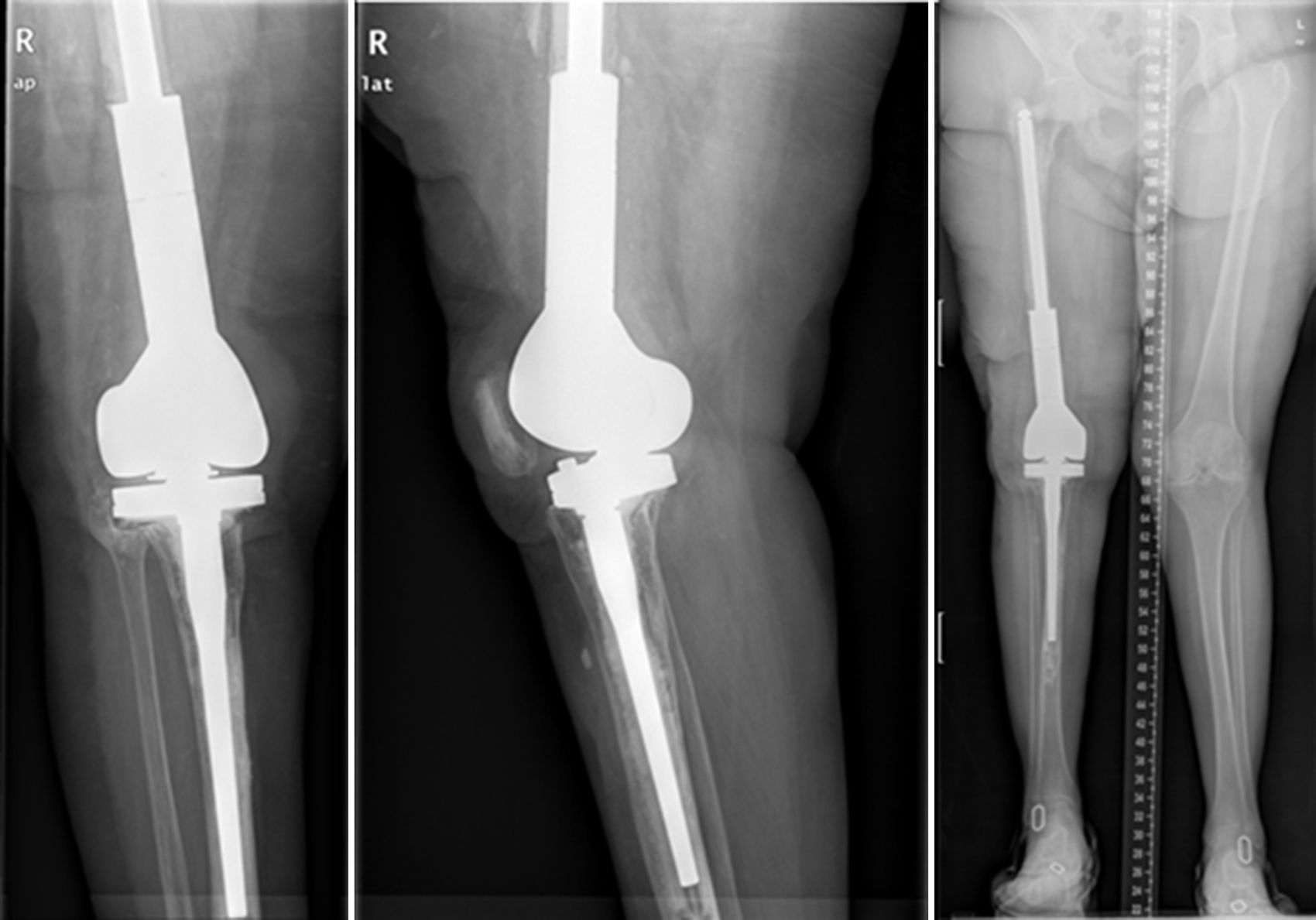
The second operation (Fig. 6) follows the standard protocol of the unit. Prior to the surgical incision we administer an antibiotic prophylaxis targeted according to the previous pathogen. The spacer components are removed and a new and exhaustive debridement is performed. After the removal of the implants at least 6 samples are taken from significant deep tissues for microbiological culture. Removed spacers are not cleaned sonically. The type of reconstruction is selected according to the defect in the remaining bone, the state of the soft tissues and the type of patient. Different techniques may be used to perform this reconstruction in the second operation. The majority of them are based on the use of modular mega-prostheses to resolve major bone defects. No silver-coated implants were used in this series, although they may be an option when patients of this type have been operated several times and for whom relapse would be dramatic. The patient continues with treatment targeted at the previous pathogen until the provisional result of the cultures is received; if they are negative the treatment is suspended after 5–7 days, unless there is any infection by anaerobic organisms.
Follow-upThe minimum time requisite for patients to be included in this study was 18 months after the second operation. This study centres on the eradication of the infection. Patients had to fulfil the following internationally accepted criteria for their treatment to be termed a success17: (1) eradication of the infection, characterised by a wound that had healed correctly without any fistula or drainage, without recurrence of the infection by the same pathogen; (2) no necessity for any new surgery after the second operation, due to infection, and (3) no death associated with the peri-prosthetic infection.
Those patients who lacked the minimum follow-up time required or who could not be suitably followed-up were excluded from our analysis. Finally, in the last follow-up check each patient filled out an analogue visual pain scale18 and a self-administered satisfaction questionnaire19 (SAPS).
Statistical methodologyData were processed anonymously and confidentially. A database created using Microsoft® Excel® 2008 for Mac (version 12.3.6) was used, and this program was also used for statistical analysis. Given the sample size, only retrospective descriptive analysis was performed of the patients and series variables.
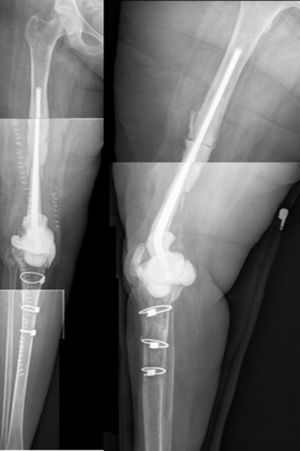
ResultsWe used this technique to treat 7 patients from January 2010 until December 2013. 6 patients finally fulfilled the selection criteria for inclusion in this study. Our series includes 3 men and 3 women with an average age at the time of surgery of 66.8 years old (range: 55–83 years old). In four cases the technique was used to resolve massive distal femoral defects following chronic peri-prosthetic infection of the knee, and in two cases it was used to treat septic pseudoarthrosis after supracondylar fracture of the femur (Table 1). The mean femoral bone defect was 117cm (range: 32–191cm). In all of the patients a pathogenic microorganism was isolated in the intraoperative samples from the first operation, and Staphylococcus epidermidis was the pathogen isolated the most frequently (66.6%). All of the patients except one were subjected to the second operation, due to the worsening of their underlying congestive heart failure. The average time spent with the spacer was 7.66 months, and during this period we only had one complication in connection with the technique: luxation of the spacer in an obese patients, which reduced closed. The correct evolution of the skin was monitoring during the follow-up, and there were no problems with coverage. After ceasing the antibiotic treatment the infection was considered to be controlled in all of the patients and their second operation was planned. During the second operation a completely matching joint surface was observed, without any signs of instability, wear or breakage. No technical difficulties arose in extracting the spacer and cement, or when implanting the revision knee prosthesis. After a new debridement and sample taking, in the second operation a cemented modular tumoral prosthesis was implanted (Mega-C®, Link, Hamburg, Germany). All of the intraoperative cultures in this second operation were negative. The mean follow-up time after the second operation was 34.7 months (range: 19–45 months). Passive joint balance is above 0–90° in all of the patients, except in one patient who suffered adherence of the extensor apparatus after the second surgical operation (patient 1). Three patients presented a lower active extensor deficit (<15°). All of the patients except for patient number 4 are walking without pain, with or without a frame. Only one patient (patient 2) presented a clinically relevant discrepancy between the lengths of their limbs, and this was corrected by a raised insole in their shoe. The average VAS score is 1 (range: 0–4). To measure satisfaction we used an analogue of the patient satisfaction scale,19 using only the first three elements (without evaluating the fourth area of assessment which refers to sports or recreational activity, as we considered this to be illogical in patients of this type). 83% (5/6) were highly satisfied with the results of surgery, and only one (patient number 4) expressed moderate satisfaction. 83% (5/6) were highly satisfied with pain control, while only 17% (1/6) were partially satisfied. When they were asked about their capacity to perform everyday tasks, 4/6 were highly satisfied, 1/6 was partially satisfied and 1/6 was dissatisfied following the procedure. However, when they were asked whether they would repeat the surgery, they all responded affirmatively.
Two patients were subjected to surgery after the second operation, although neither case was connected with infection; the reason in one case was luxation of the patella (patient 5), while in the other it was due to severe adherences of the extensor apparatus following the second operation (patient 1). Both have progressed favourably 7 and 8 months after their last check-up.
It is worthwhile to explain the history of patient number 4, the only one who was not subjected to the second operation. She is an 83 year-old woman who suffered left transfemoral amputation due to a bilateral peri-prosthetic infection in another hospital. She was sent to our hospital in an attempt to save her remaining leg. The decision was taken to perform a two-stage replacement using this technique. Nevertheless, while waiting for the second operation she suffered an acute deterioration in her congestive heart failure. She is now free of pain and has no signs of infection, and she uses a wheelchair due to her severe dyspnoea. She has conserved her joint balance at above 0–90°. Together with her and her family it was decided not to perform the second operation because of the high surgical risk involved and the fact that she would not walk again due to her dyspnoea and the contralateral amputation. She is highly satisfied with the surgery and the control of her pain, in spite of her restrictions.
DiscussionThe aim of this study was to investigate the initial results of a new technique for the treatment of massive bone defects in the distal femur within a septic context. We implemented the technique studied in an attempt to make us of the advantages of a prefabricated dynamic spacer. Following the initial analysis of the results, we are able to state that this technique is reproducible, safe and that it gives acceptable results in these cases, which are so complex. The patients are highly satisfied after the procedure, and there were no cases of infection relapse after a follow-up of at least 18 months, with a very low rate of complications associated with the technique.
In the 21st century, due to the growing number of knee replacements and the increasing life expectancy of patients, the incidence of knee revision surgery due to septic as well as aseptic loosening is expected to increase markedly.20,21 Peri-prosthetic infection is known to be the first cause of knee prosthesis revision surgery,22 and the risk of infection is also known to increase with each revision.20 Additionally, with each revision the surgeon faces an increasingly large bone defect.8,23 This is why in “terminal” cases of peri-prosthetic infection the bone defect may be catastrophic. The factors that contribute to the loss of bone stock are the type of implant, peri-prosthetic osteolysis, loosening of the component, the existence of infection or iatrogenic causes during prosthesis extraction surgery.23,24 Treatment of the loss of bone reserve includes the use of bone cement, modular metal spacers, impacted grafts, structural allografts and, more recently, trabecular metal supplements, depending on location, the size of the defect and the surgeon's preferences.6 The majority of these options are contraindicated in septic revision surgery, so that the question of simultaneous treatment of an infection and a bone defect is a long way from being resolved.25 Nowadays no ideal treatment has been established for these cases.
Two-stage replacement is the most widely accepted protocol for the eradication of chromic prosthetic infection.12,23 Other options in the case of a massive defect could be functional arthrodesis or false arthrodesis,9 while the final option may be amputation.8 Spacers containing antibiotics are a fundamental part of the two-stage replacement protocol. The spacer has the purpose of supplying a suitable and prolonged concentration of antibiotics,26 preventing retractions and maintaining the tension in soft tissues and the extensor apparatus; it permits a certain degree of functioning in the case of dynamic spacers27 and above all aids the second surgical operation by reducing peri-operative fibrosis.28 They also seem to give better functional results during the time spent waiting for the definitive reconstruction.29 However, the use of dynamic or static spacers does not seem to influence the final result, except for a possible increase in the final balance of the joint,27,28 although this last point has not been totally confirmed. Other possible advantages of dynamic spacers include their facilitating mobility and transfers, contributing to a better quality of life while awaiting a second surgical operation. Different types of dynamic spacers have been used, one of which is the type known as “industrially prefabricated spacers”. These spacers are impregnated with a certain type and amount of specific antibiotic. The said spacers have been widely used in Europe, and they have recently started to be distributed in the United States (InterSpace®, Inc. Exactech, Gainesville, Florida). Nevertheless, it must be remembered that their use is restricted by their high cost and lack of modularity in the case of massive bone defects. This is why we have witnessed an increase in the use of spacers made during the operation to resolve this problem.8,26,30 These “personal techniques” are not free of restrictions, such as their mechanical safety, their antibiotic emitting properties, loss of operating time and their economic cost. On the other hand, one of the recognised benefits of industrially manufactured spacers is precisely their proven mechanical reliability and safe pharmacological profile (i.e., they supply a known local liberation of antibiotic) and, very importantly, using them may save valuable operating time during the procedure. This is why the use of prefabricated articulated spacers is the technique of choice in our unit.13,14
The spacer we describe is based on the ball-and-socket principle described and validated by MacAvoy et al.30 This uses a bulb syringe as the mould for the femoral compartment and a 55mm impregnated bipolar head to form the tibial recipient at the other end. This technique has the potential risk of breakage of the femoral component or loosening, given that it is incompatible with a long stem.
Some of the drawbacks of the described technique may be its multidirectional mobility and instability of the artificial joint in a mechanically incompetent knee. Anticipating this complication, we restricted the movement of the joint to controlled flexion-extension, by using an articulated kneepad. In our initial series we only had one case of luxation, in a patient with morbid obesity (where the use of a stabilising kneepad is compromised).
Others possible subjects for discussion are the construction of a cement – cement coupling in which friction occurs, and the effects of wear particles. It has even been suggested that these particles due to wear may be a possible cause of the persistence of the infection.31 It must be said that this joint surface is used by other spacer models without any special problem, and that moreover it is used for a short period of time, without being subjected to a full weight load, thereby limiting wear.
This technique has been shown to be safe, with few complications. We had no case of implant breakage or loosening of the system. The patients were able to walk partially, with or without support, and all of them except one (due to her state of health) arrived at the second stage of surgery. The infection markers normalised in all of our patients, and none of them suffered cutaneous complications, confirming the resolution of the infection which is the core aim of any septic reconstructive surgery. Their physical and mental functioning was highly positive after the procedure. They were all highly satisfied with surgery, probably due to control of the infection after a long battle, and they would all repeat the procedure.
We recognise the limitations of this study. On the one hand it is a retrospective study, with all of the limitations inherent to this design, above all not being able to access all of the potentially useful data. On the other hand, although this is a small sample of only 6 cases, we are aware that we are presenting extreme cases here, ones that rarely occur in everyday clinical practice. Another limitation may be the definition of peri-prosthetic infection that was selected; there is currently controversy within the scientific community regarding the ideal definition of prosthetic infection; as we use an internationally accepted (IDSA) definition we consider that this will not be a relevant limitation for the study.
ConclusionIn the light of the above description, we consider this technique to be a useful tool that may be a valid resource in the case of a peri-prosthetic knee infection associated with a bone defect in the distal femur, or in cases of massive femoral defect in a septic context.
Level of evidenceLevel of evidence IV.
Ethical disclosuresProtection of people and animalsThe authors declare that for this research no experiments took place in human beings or animals.
Confidentiality of dataThe authors declare that they followed the protocols of their centre of work on the publication of patient data.
Right to privacy and informed consentThe authors obtained the informed consent of the patients and/or subjects referred to in this paper. This document is held by the corresponding author.
Conflict of interestsThe authors have no conflict of interests to declare.
This work would not have been possible without the aid of Dr. Dolors Rodríguez-Pardo and Dr. Carles Pigrau of the infectious diseases unit of our hospital. We would also like to thank Dr. Juan Carlos Juárez of the pharmacy and Dr. Mayli Lung of the microbiology department.
Please cite this article as: Flores X, Vicente M, Haddad S, Amat C, Carrera L, Corona PS. Espaciador de cadera «invertido» para defectos masivos de fémur distal en infecciones periprotésicas de rodilla. Rev Esp Cir Ortop Traumatol. 2016;60:346–354.