The increasing antimicrobial resistance is promoting the addition of antibiotics with high antistaphylococcal activity to polymethylmethacrylate (PMMA), for use in cement spacers in periprosthetic joint infection. Linezolid and levofloxacin have already been used in in vitro studies, however, rifampicin has been shown to have a deleterious effect on the mechanical properties of PMMA, because it inhibits PMMA polymerisation. The objective of our study was to isolate the rifampicin during the polymerisation process using microencapsulation techniques, in order to obtain a PMMA suitable for manufacturing bone cement spacers.
Material and methodMicrocapsules of rifampicin were synthesised with alginate and PHBV, using Rifaldin®. The concentration levels of rifampicin were studied by UV–vis spectrophotometry. Compression, hardness and setting time tests were performed with CMW®1 cement samples alone, with non-encapsulated rifampicin and with alginate or PHBV microcapsules.
ResultsThe production yield, efficiency and microencapsulation yield were greater with alginate (p=0.0001). The cement with microcapsules demonstrated greater resistance to compression than the cement with rifampicin (91.26±5.13, 91.35±6.29 and 74.04±3.57MPa in alginate, PHBV and rifampicin, respectively) (p=0.0001). The setting time reduced, and the hardness curve of the cement with alginate microcapsules was similar to that of the control.
Discussion and conclusionsMicroencapsulation with alginate is an appropriate technique for introducing rifampicin into PMMA, preserving compression properties and setting time. This could allow intraoperative manufacturing of bone cement spacers that release rifampicin for the treatment of periprosthetic joint infection.
La creciente resistencia a antimicrobianos está impulsando la adición de antibióticos con elevada actividad antiestafilocócica al polimetilmetacrilato (PMMA), para su uso en los espaciadores de cemento en la infección periprotésica. El linezolid o el levofloxacino ya han sido utilizados en estudios in vitro; sin embargo, la rifampicina ha demostrado un efecto deletéreo sobre las propiedades mecánicas del PMMA, inhibiendo su polimerización. El objetivo de nuestro estudio fue aislar la rifampicina durante el proceso de polimerización mediante técnicas de microencapsulación, con el fin de obtener un PMMA apto para la fabricación de espaciadores articulares.
Material y métodoSe sintetizaron microcápsulas de rifampicina con alginato y PHBV, utilizando Rifaldin®. Se estudió la concentración de rifampicina mediante espectrofotometría UV-visible. Se realizaron ensayos de compresión, dureza y tiempo de fraguado con probetas de cemento CMW®1 solo, con rifampicina y microcápsulas de PHBV y alginato.
ResultadosEl rendimiento de producción, la eficiencia y el rendimiento de microencapsulación fueron mayores con alginato (p = 0,0001). El cemento con microcápsulas mostró mayor resistencia a la compresión que el cemento con rifampicina (91,26 ± 5,13, 91,35 ± 6,29 y 74,04 ± 3,57MPa en alginato, PHBV y rifampicina, respectivamente) (p = 0,0001). El tiempo de fraguado disminuyó, siendo la curva de dureza del cemento con microcápsulas de alginato similar a la de control.
Discusión y conclusionesLa microencapsulación con alginato es una técnica adecuada para introducir rifampicina en el PMMA preservando las propiedades de compresión y el tiempo de fraguado. Su obtención permitiría fabricar espaciadores que liberasen localmente rifampicina para el tratamiento de la infección periprotésica.
Periprosthetic joint infection is a serious complication, the prevalence of which has risen in the last few decades. It has been estimated that this will be the most common cause of prosthetic revision surgery in the next 2 or 3 decades.1,2 The appearance of multi-resistant germs and the medical complexity of patients have imposed modification of existing surgical protocols which are insufficient for optimum management of such a complex problem, in addition to investigating new forms of treatment.
The majority of microorganisms which cause periprosthetic infection are producers of biofilm. The bacteria inside the biofilm radically change their phenotype leading to bacterial growth with a metabolically low activity and enabling bacteria to survive during chronic infection.3 This means that in conditions of mature biofilm, the active in vitro doses of the antibiotic are 200–1000 times higher than the standard dosis,4 making it impossible to treat periprosthetic infections with antibiotics alone, administered systemically. Elevated concentrations of antibiotic on the infection site raises the probability of success of the treatment and this may be achieved through the use of antibiotic-impregnated cement spacers.
The two-stage exchange arthroplasty is considered to be the gold standard treatment for chronic periprosthetic infection. After the removal of the joint prosthesis and debridement of infected tissues, a bone cement spacer is temporally inserted with a high dose of antibiotics aimed at preserving the joint space and achieving high local levels of antibiotics. Amino glucosides and vancomycin are the most commonly used antibiotics added to the bone cement, but the appearance of multi-resistant strains of bacteria is threatening their efficacy in local treatment.5
The role of rifampicin against staphylococcal infections associated with implants has been demonstrated.6 Rifampicin, in combination with other antibiotics is considered to be the antibiotic of choice in the treatment of staphylococcal infections associated with joint replacements due to its bacterial activity against stationary phase bacteria, its intracellular activity and its capacity for diffusion in biofilm.7 Theoretically, its topical use on periprosthetic tissues would offer advantages but the addition of rifampicin to polymethylmethacrylate (PMMA) delays the polymerisation of the cement preventing its use for the manufacture of spacers. The proposed mechanism is that the rifampicin reacts with benzoyl peroxide (initiator) and/or with N,N-dimethyl-p-toluidine (activator), preventing the formation of benzoil radicals and from them forming covalent bonds in the methylmethacrylate.8
The aim of this experimental study was the isolation of rifampicin during the PMMA polymerisation process using microencapsulation techniques to preserve the mechanical properties and setting time of the bone cement.
Material and methodsMicrocapsule synthesis processMicrocapsules of rifampicin were synthesised with alginate and polyhydroxybutyrate (PHBV) using ionic jellification and evaporation of the solvent, respectively and using rifampicin, the trade name of which is Rifaldin® (Sanofi, Barcelona, Spain).
Ionic jellification is based on the ability of the alginate to reticulate and form hydrogels in the presence of counter ions (CaCl2 and chitosan). The alginate and rifampicin were added drop by drop, under constant agitation, to a solution with counter ions, forming complexes between the opposing load species and producing microcapsules which were collected by filtration.
The evaporation method of the solvent consisted in forming an emulsion whose internal phase was dissolved PHBV in an organic solvent with the rifampicin. After the organic phase this was dispersed into the watery solution which contained the surfactant. Once the emulsion had been formed, it was homogenised and the solvent evaporated to achieve the gradual precipitation of the PHBV with rifampicin.
The micro particles obtained were analysed with a scanning electron microscope. The morphology of the micro particles was spherical in the PHBV samples and irregular in the alginate particles. Eight samples of PHBV microcapsules and 4 of alginate were synthesised.
Traits of the microcapsulesTo determine the rifampicin content of the PHBV microcapsules we dissolved 5mg of each sample in 5ml of a solution of dichloromethane and methanol (10%/90%). In the case of alginate, we used a solution of 0.25M ethylene diamine tetra acetate since the alginate does not dissolve in dichloromethane and ethanol. To process the total dilution of the microcapsules, they were centrifuged at 4000rpm for 5min. The supernatant was analysed using visible ultraviolet spectrophotometry (Agilent Technologies, Santa Clara, CA, U.S.A.), with a previous construction of the calibrated curve of rifampicin at a wavelength of 334nm. Each sample was analysed in triplicate.
The microencapsulation process was characterised using 3 parameters: efficiency of rifampicin encapsulation or rifampicin content (% Rifampicin), performance of microcapsule production (ηMIC) and performance of rifampicin microencapsulation (ηRIF). The rifampicin content was defined as the quotient between the microensapculated rifampicin mass and the microcapsule mass obtained. The performance of microcapsule production was determined as the quotient between the microcapsule mass obtained and the mass of the quotient. The performance of the rifampicin microencapsulation was calculated as the quotient between the microencapsulated mass of rifampicin and the mass of rifampicin used.
Preparation of cement sample and mechanical trialsThe cement was manually prepared in accordance with international standards ASTM F451:99 (Standard Specification for Acrylic Bone Cement)9 and ISO 5833:2002 (Implants for surgery-Acrylic resin cements).10 The commercial cement CMW®1 (DePuy International Ltd., Blackpool, United Kingdom) was used. Samples with rifampicin (1.25% P/P) and microcapsules (5% P/P) were prepared following the recommendations of Frommelt to gain homogeneous distribution of the antibiotic. Control samples of cement without antibiotic were also prepared.
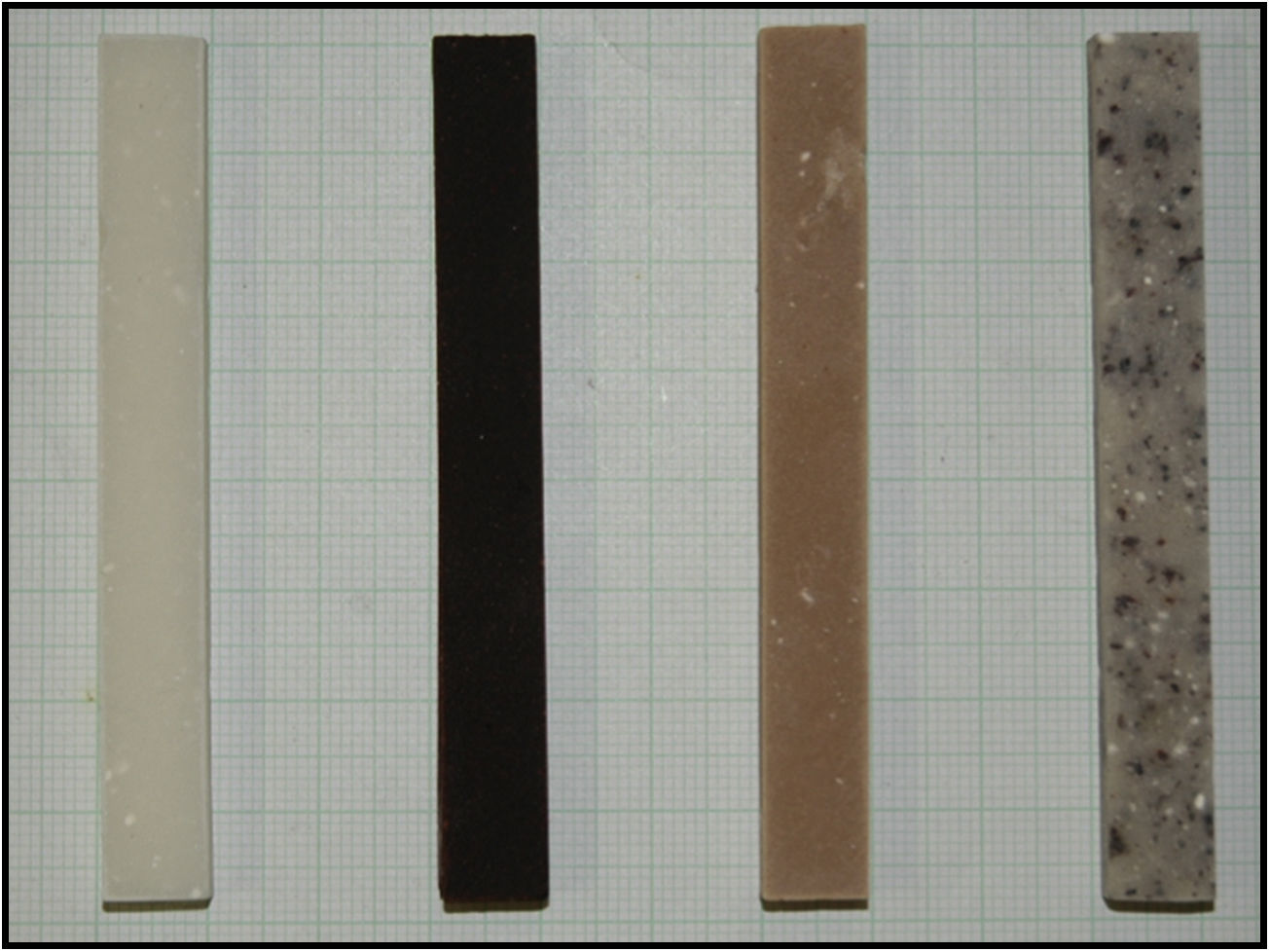
Cylindrical test tubes were made measuring 12.0±0.1mm in height and 6.0±0.1mm in diameter for compression tests (Fig. 1), in compliance with regulation ISO 5833:2002. We obtained between 4 and 6 test tubes for each sample. These were tested with the universal trial machine ELIB 20W® (Ibertest, Madrid, Spain) one week after their production, at a compression speed of 20mm/min and with a load cell of 20kN.
To study hardness, rectangular test tubes were made measuring 80.0±0.1mm in length, 10.0±0.1mm in width and 4.0±0.1mm in height (Fig. 2), according to the UNE-ISO 7619-1:2011 international standard (vulcanised rubber and thermoplastic. Determination of indentation toughness).11 The shore Bareiss® (Neurtek, Eibar, Spain) hardness tester was used to determine hardness at 15, 30, 45 and 60min, and at 2, 3, 4 and 24h, in 3 test tubes of each sample.
The data are presented as a mean±standard deviation. Statistical analysis was performed suing version 22.0 SPSS® (SPSS Inc., Chicago, IL, U.S.A.), using the ANOVA test for repeated means and the post hoc Bonferroni analysis. p<0.05 was defined as statistically significant.
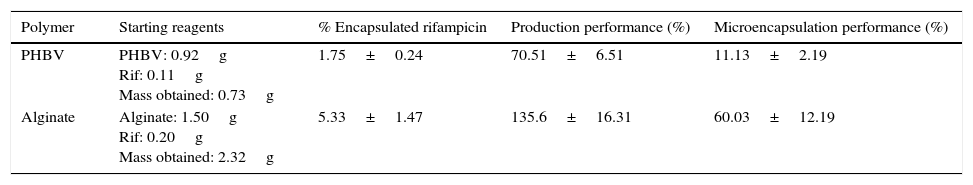
ResultsThe percentage of rifampicin, performance of microcapsule production and performance of rifampicin microencapsulation were higher with alginate than with PHBV, as is shown in Table 1, with differences being statistically significant (p=0.0001).
Mean values and standard deviation of the percentage of encapsulated rifampicin, production performance and microencapsulation of the 8 samples of PHBV microcapsules and the 4 alginate capsules.
| Polymer | Starting reagents | % Encapsulated rifampicin | Production performance (%) | Microencapsulation performance (%) |
|---|---|---|---|---|
| PHBV | PHBV: 0.92g Rif: 0.11g Mass obtained: 0.73g | 1.75±0.24 | 70.51±6.51 | 11.13±2.19 |
| Alginate | Alginate: 1.50g Rif: 0.20g Mass obtained: 2.32g | 5.33±1.47 | 135.6±16.31 | 60.03±12.19 |
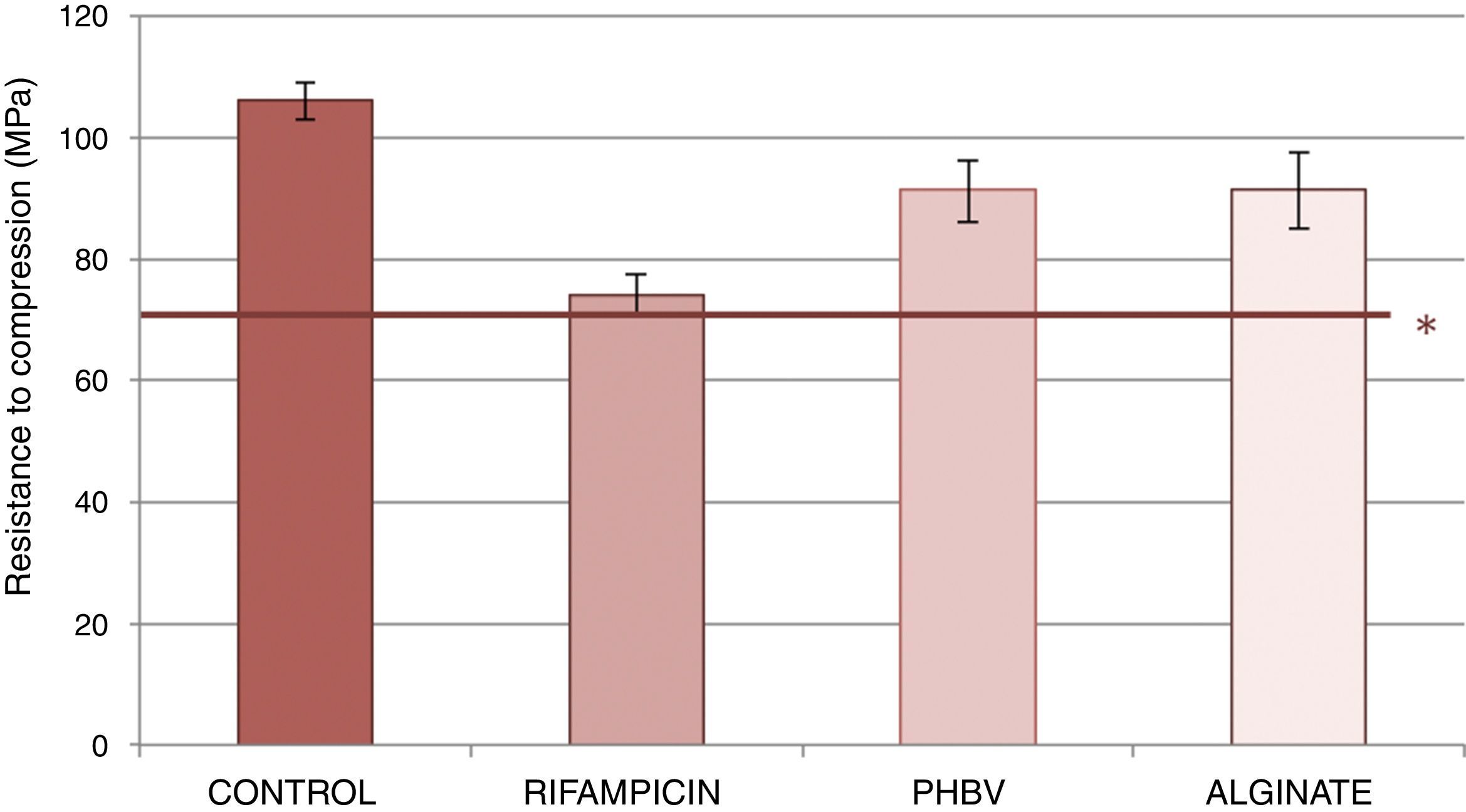
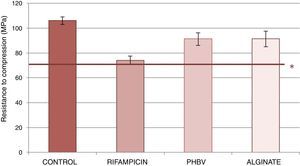
Fig. 3 shows the resistance to compression of the samples studied. The test tubes with of cement with PHBV and alginate microcapsules and alginate showed lower reduction in resistance to compression (reduction of 14% with respect to cement control) than the cement with non-encapsulated rifampicin (reduction of 30% compared with the cement). The data obtained from the trial are shown in Table 2. Significant statistical differences were detected in on comparing the mean values of resistance to compression between the rifampicin control cement, the alginate control, the PHBV-control, PHBV-rifampicin and alginate-rifampicin (p=0.0001 in all comparisons). No statistical significance was reached on comparing PHBV and alginate (p=1).
Mean values and standard deviation of the resistance to compression of the control cements, with non encapsulated rifampicin, with PHBV microcapsules and with alginate microcapsules.
| Resistance to compression (MPa) | Standard deviation | |
|---|---|---|
| Control | 106.2 | 2.97 |
| Rifampicin | 74.04 | 3.57 |
| PHBV | 91.26 | 5.13 |
| Alginate | 91.35 | 6.29 |
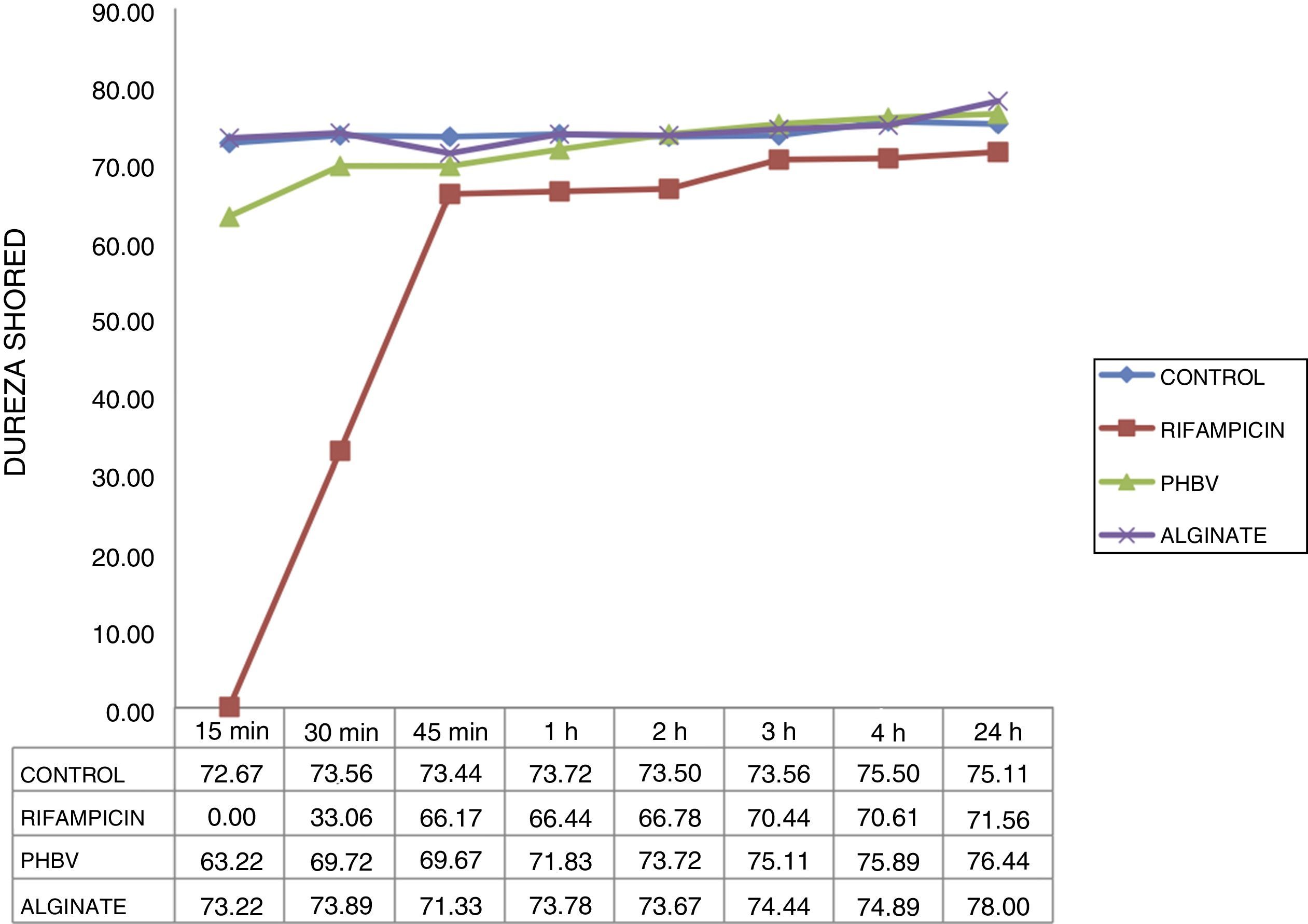
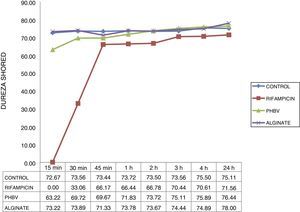
The hardness curve obtained with the cement with microcapsules of alginate was similar to that of the control cement at all the times studied (Fig. 4), reaching its maximum hardness after 15min (73 HD). Cement setting time with non-encapsulated rifampicin was greater than that of the cement with microencapsulated rifampicin and the control cement. The cement with non-encapsulated rifampicin could not be tested at 15min because it was still too liquid. It began to harden after 30min (33 HD) and did not achieve a similar hardness to the control cement until after 45min (67 HD).
DiscussionThere is a growing interest in adding antibiotics with elevated anti-staphylococcal activity to bone cement due to the concern in the increase in bacterial resistance to standard antibiotics. Linezolid and several quinolones such as levofloxacin have already been used in in vitro studies.12,13 However, the addition of rifampicin to PMMA prevents it complete polymerisation producing a sticky black material which is unsuitable for medical practice, despite having demonstrated to be a good at in vitro elution, and active against S. aureus.5,8,14
The first people to microencapsulate an antibiotic to add it to PMMA were Shi et al.,15 who described the manufacture of colistin microspheres with polylactic acid to control the release of the PMA antibiotic used in craniofacial bone defects. Since then there have been no references in the literature to antibiotic microencapsulation in PMMA.
Rifampicin had already been previously encapsulated for its use in the treatment of pulmonary tuberculosis. The polymers used were PHBV, alginate, colistin with polylactic acid and also liposomes and lipids.16–20 However, the use of microencapsulated rifampicin in bone cement aimed at the preservation of mechanical properties and setting characteristics has not been studied.
There is a need for between 5% and 10% P/P of antibiotic to obtain good levels of elution of bone cement antibiotic and thus achieve local therapeutic levels. However, the addition of elevated doses of antibiotics to the bone cement impairs its mechanical properties. He et al.21 studied the mechanical changes after adding 1g (2.4% P/P), 2g (4.8% P/P), 4g (9.1% P/P) and 6g (13% P/P) of gentamicin to the cement Palacos®, recommended to a maximum of 6.5% P/P of antibiotic for maintaining the properties of cement compression. Lautenschlager et al.22 estimated that the addition of 4.5g of gentamicin by 40g (10.1% P/P) would reduce the compression force to below 70MPa. Rifampicin, even in lower concentrations to those recommended for the manufacture of cement spacers seriously impairs the PMMA properties. In 1982, De Palma et al. were the first to refer to the inhibition of complete polymerisation of CMW® bone cement when rifampicin was added.23 Anguita-Alonso et al.5 and Gálvez-López et al.24 recently recognised the benefits of obtaining a cement which released rifampicin in the treatment of periprosthetic infection, but they experienced the same problem with regards to the setting delay. In our mechanical trials, compression resistance of the cement with 1.25% P/P of rifampicin (0.5g of rifampicin per 40g) was 74.04MPa, and that of the cement with 5% P/P of microcapsules (0.035 or 0.10g of rifampicin per 40g) was 91.26 and 91.35MPa in the PHBV microcapsules or alginate, respectively. There are no data in the references on resistance to PMMA compression with rifampicin.
Our study is the first one to offer a solution to the problem of the delayed setting time of PMMA on the addition of rifampicin. The polymerisation of the cement with non-encapsulated rifampicin took 45min, and exceeded the time which is normally available in the operating theatre for manufacture of cement spacers. However, microencapsulation with alginate gave us setting times which were equivalent to those of the control cement. Longer setting times, 122.5±31.1min, have been published after adding 1, 2 and 4g of rifampicin or a combination of 1g de rifampicin+1g of isoniazide, or 2g of rifampicin+2g of isoniazide to the CMW®314 cement. The results obtained indicate that microencapsulation could be an appropriate technique for isolating rifampicin during the PMMA polymerisation process, leading to its complete setting in a reasonable amount of time.
The main limitation of our study is that it was in vitro, which may not reflect in vivo conditions exactly. Further animal trials would be needed to become better acquainted with the effects of the cement with rifampicin in prosthetic infection. Another limitation is that all trials were conducted as monotherapy. It is well known that the use of rifampicin in monotherapy is not recommended due to the high risk of developing resistance. The synergy study of rifampicin with other antibiotics commonly used in bone cement, such as gentamicin or vancomicin, form part of our future research investigations. Neither was there any determination of the rifampicin release kinetics from the cement with microcapsules, since this did not form part of the objective of the initial stage of the study, which was to achieve the addition of rifampicin to PMMA.
The essential clinical application derived from this experimental study is the possibility of obtaining a bone cement which contains rifampicin, whilst preserving several valid mechanical properties for manufacturing cement spacers. The benefits of obtaining high local concentrations of rifampicin in the periprosthetic infection site are indubitable. The ionic jellification of alginate would be the microencapsulation technique of rifampicin with the most advantages with regards to setting time and PMMA compression properties.
The trials carried out in our study may be the starting point for the study of whether microencapsulation would offer advantages in the case of commonly used antibiotics in bone cement, enabling mechanical properties to be preserved despite an increase in concentration. Along these same lines, encapsulation could offer an advantage against other antibiotics whose addition to bone cement is disadvantageous, such as amphotericin,25 and in the spreading of tumour-inducing agents in bone metastases.26
To conclude, microencapsulation with alginate is the best technique for introducing rifampicin in the PMMA, since the properties of compression and setting time are preserved.
Level of evidenceLevel of evidence I.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments have been performed on humans or animals in this research.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis project was financed thanks to the Initial Research Project in Orthopaedic Surgery and Orthopaedics Grant, awarded by the Sociedad Española de Cirugía Ortopédica y Traumatología (SECOT), 2015. The title of the project was: “Experimental Study of rifampicin elution of bone cement (polymethylmethacrylate.” Main author: Esther Carbó Laso. Colaborators: Pablo Sanz Ruiz; Francisco Javier Vaquero Martín. Centre of work: Hospital Universitario Gregorio Marañón, Madrid.
Conflict of interestWe state there are no conflicts of interests.
We would like to thank Carmen de Pablos, student of the Mechanical Engineering Master's Degree of ICAI who collaborated in the undertaking of mechanical trials. Also to the Department of Microbiology of the Hospital General Universitario Gregorio Marañón, who supplied us with the means to conduct the microbiological trials.
Please cite this article as: Carbó-Laso E, Sanz-Ruiz P, del Real-Romero JC, Ballesteros-Iglesias Y, Paz-Jiménez E, Arán-Ais F, et al. Nuevo método de liberación de antibióticos del cemento óseo (polimetilmetacrilato): redefiniendo los límites. Rev Esp Cir Ortop Traumatol. 2018;62:86–92.