To demonstrate whether there is a difference in the time that total knee arthroplasty (TKA) takes according to the instrumentation system used.
Material and methodsRetrospective analysis of the duration of 243 interventions (skin-to-skin time and ischaemia time) performed by the same surgeon. 72 cases operated with conventional instruments (CI), 68 operated with computer assisted surgery (CAS) and 103 with personalized instrumentation system (PSI).
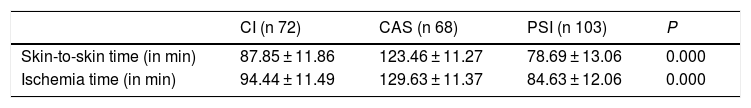
ResultsCI skin-to-skin 87.85 min (SD 11.86). CI ischaemia 94.44 min (SD 11.49). CAS skin-to-skin 123.46 min (SD 11.27). CAS ischaemia 129.63 min (SD 11.37). PSI skin-to-skin 78.69 min (SD 13.06). PSI ischaemia 84.63 (DE 12.06). There is a significant difference between PSI and the other instrumentation systems (p 0.000).
ConclusionsIn our study, the time taken for TKA was significantly lower when we used customized cutting blocks rather than other systems.
Demostrar si existe diferencia en el tiempo que se prolonga la cirugía para implantar una prótesis de rodilla (PTR) atendiendo al sistema de instrumentación empleado.
Material y métodoAnálisis retrospectivo de la duración de 243 intervenciones (tiempo piel-piel y tiempo de isquemia) realizadas por el mismo cirujano. 72 casos intervenidos con instrumental convencional (IC), 68 asistidos por navegador (CAS, de computer assisted surgery) y 103 con bloques de corte personalizados (PSI, de personalized instrumentation system).
ResultadosIC piel-piel 87,85 min (DE 11,86). IC isquemia 94,44 min (DE 11,49). CAS piel-piel 123,46 min (DE 11,27). CAS isquemia 129,63 min (DE 11,37). PSI piel-piel 78,69 min (DE 13,06). PSI isquemia 84,63 (DE 12,06). Existe una diferencia significativa favorable a PSI respecto a los otros sistemas de instrumentación (p 0,000).
ConclusionesEn nuestro estudio, el consumo de tiempo para la implantación de una PTR ha sido significativamente inferior cuando hemos empleado bloques de corte personalizados, que cuando hemos empleado otros sistemas.
There are different types of instrumentation systems for implanting a knee prosthesis, a total knee replacement (TKR). The conventional systems, the most widely used, are based on intramedullary mechanical guide devices at the femoral level and intra- or extramedullary devices at the tibial level, over which cutting blocks are anchored for the relevant osteotomies.1 Computer assisted surgery (CAS) works with anatomical references that a computer system analyses to guide the cutting block placement.2–4 Another alternative is designing the surgery over virtual 3-dimensional (3D) models obtained from a computed tomography or magnetic resonance study (that is, a preoperative guide) and the use of personalised cutting blocks or placers as instruments (patient-specific instruments (PSI).5,6 Each of these technologies involve a sequence of different surgical steps and actions, so an intervention can last more or less time. Knowing the length of interventions can help to optimise the performance of each surgical session.7,8 There are various studies9,10 that suggest that replacing conventional instruments with patient-specific instruments reduces the time that the intervention lasts and consequently has a positive effect on the cost-efficiency and cost-effectiveness analyses. The objective of this study was to demonstrate if there was a significant difference in the time knee prosthetic surgery lasts depending on the instrumentation system used.
Materials and methodWe carried out a retrospective analysis of the information registered in the database of the web form specifically designed for TKR surgery. The durations of 243 interventions performed by the same senior surgeon (XXX) between 2008 and 2012 were analysed. The study included cases affected by bi- or tricompartmental knee osteoarthritis, with no prior interventions on the affected knee, in which the same prosthetic model was implanted. Patients not operated by XXX, patients that received posterior prosthesis implants or those stabilised medially, cases without patellar replacement, cases with cementing in two stages, osteotomy modifications, use of stems and/or supplements, patients with one-stage bilateral interventions and osteotomies with anterior tibial tuberosity for the approach (that is, the cases in which non-standard actions during the intervention might cause a variation in the intervention time) were all excluded from the study. The first 15 cases undergoing an intervention with each system were also excluded.
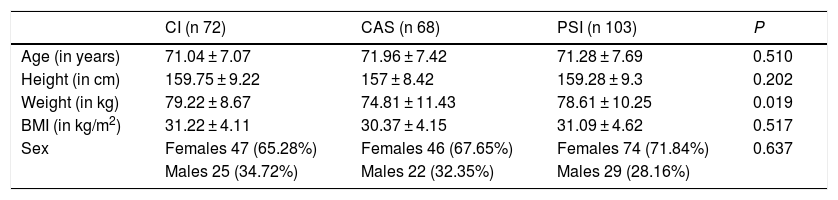
To implant the prosthesis, conventional mechanical instruments (CI) were used in 72 cases (29.63%), computer assisted surgery (CAS) was used in 68 cases (27.98%) and patient-specific instruments (PSI) were used in 103 cases (42.39%). The surgeries with CI were performed simultaneously with those carried out with CAS and PSI. The ones performed with PSI and CAS were consecutive, given that either 1 system or the other was used. The cases were not selected on the basis of radiographic, clinical or anthropometric characteristics. The demographic and anthropometric characteristics of the series are presented in Table 1. There were no statistically significant differences with respect to the variables of age, sex distribution, height and body mass index among the 3 groups. There was a difference in weight (P = 0.019).
Anthropometric and demographic characteristics of the series.
| CI (n 72) | CAS (n 68) | PSI (n 103) | P | |
|---|---|---|---|---|
| Age (in years) | 71.04 ± 7.07 | 71.96 ± 7.42 | 71.28 ± 7.69 | 0.510 |
| Height (in cm) | 159.75 ± 9.22 | 157 ± 8.42 | 159.28 ± 9.3 | 0.202 |
| Weight (in kg) | 79.22 ± 8.67 | 74.81 ± 11.43 | 78.61 ± 10.25 | 0.019 |
| BMI (in kg/m2) | 31.22 ± 4.11 | 30.37 ± 4.15 | 31.09 ± 4.62 | 0.517 |
| Sex | Females 47 (65.28%) | Females 46 (67.65%) | Females 74 (71.84%) | 0.637 |
| Males 25 (34.72%) | Males 22 (32.35%) | Males 29 (28.16%) |
Values expressed in mean and standard deviation or number and percentage.
BMI: body mass index; CAS: computer assisted surgery; CI: conventional instruments; PSI: patient-specific instruments.
Level of significance P < 0.05.
Independently of the instrumentation system used, the prosthesis implanted was always the cemented Global Medacta Knee (GMK®, Medacta International SA, Castel San Pietro, Switzerland), with an ultra-congruent insert. The kneecap was implanted in all cases. The postoperative alignment objective in all cases, regardless of the instruments used, was to obtain a mechanical femorotibial angle (mFTa) of 180°.
Ischemia was performed using a sterile exsanguinations tourniquet (S-MART® or HemaClear®, OHK Medical Devices, Haifa, Israel). This was applied just before the incision and removed after bandaging the limb.
In the cases undergoing an intervention using conventional mechanical instruments, intramedullary femoral and tibial alignment and the ligament balancing system were used to establish femoral external rotation. Computer assisted surgery was performed with the iMNS system — Medacta Navigation System (Medacta International S.A., Castel San Pietro, Switzerland). Cutting blocks were used following intervention planning on virtual 3D models obtained after computed tomography study, with the MyKnee® system (Medacta International S.A., Castel San Pietro, Switzerland). Using computer-aided design (CAD) programs, the patient-specific instruments were designed and then produced using selective laser sintering of a precision polyamide powder.
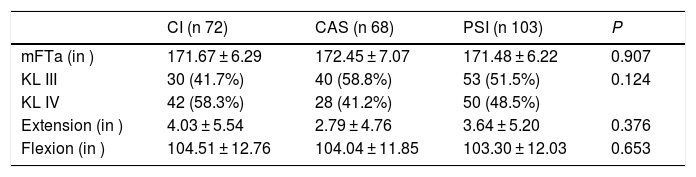
VariablesSkin-to-skin time (time from the beginning of the approach until the surgical wound is closed) and ischemia time (sterile ischemia just before the incision and removal after limb bandaging) were determined. The time the intervention lasted was obtained by exploitation of the database of the web form specifically designed for the capture of activity related to TKR surgery. This information was crossed with that registered in the web form for operating theatre management, used by the nursing staff. In addition, we analysed limb deformity by means of the preoperative standing mFTa angle determined via telemetry, the degree of osteoarthritis according to the Kellgren and Lawrence classification11,12 and the preoperative joint movement (existence of the inability to extend the knee and maximum active flexion movement), as can be seen in Table 2. There were no statistically significant differences between the groups. We determined the postoperative coronal plane alignment in the 3 groups. Because the variables to analyse became anonymous upon their downloading from the databases, it was not necessary to obtain express informed consent from the patients for the publication of these data.
Radiographic characteristics and joint movement.
| CI (n 72) | CAS (n 68) | PSI (n 103) | P | |
|---|---|---|---|---|
| mFTa (in ) | 171.67 ± 6.29 | 172.45 ± 7.07 | 171.48 ± 6.22 | 0.907 |
| KL III | 30 (41.7%) | 40 (58.8%) | 53 (51.5%) | 0.124 |
| KL IV | 42 (58.3%) | 28 (41.2%) | 50 (48.5%) | |
| Extension (in ) | 4.03 ± 5.54 | 2.79 ± 4.76 | 3.64 ± 5.20 | 0.376 |
| Flexion (in ) | 104.51 ± 12.76 | 104.04 ± 11.85 | 103.30 ± 12.03 | 0.653 |
Values expressed in mean and standard deviation or number and percentage.
CAS: computer assisted surgery; CI: conventional instruments; KL III & KL IV: the degree of osteoarthritis according to the Kellgren and Lawrence classification11,12; mFTa: mechanical femorotibial angle; PSI: patient-specific instruments.
Level of significance P < 0.05.
The predictive analytical software Minitab® Statistical Software v.18 and IBM SPSS v.25 for Windows were used for the analytical treatment. The absence of normal distribution of values, as demonstrated by the Kolmogorov–Smirnov test, made it necessary to use the Kruskal–Wallis test for more than 2 independent samples, the Pearson chi-square test for qualitative variables and the Pearson correlation coefficient. Intervals of confidence were set to 95% and the value of significance to P = 0.05.
ResultsWe obtained a statistically significant difference (P = 0.000) in favour of the cutting blocks compared with the other 2 instrumentation systems, as is shown in Table 3, with a 9-minute reduction with respect to CI and a 44-minute reduction with respect to CAS. There was also a statistically-significant difference in favour of the cases in which CI were used, as opposed to using computer assisted surgery, of 35 min (P = 0.000). In no case of the patients operated using PSI was it necessary to interrupt the technique and change to CI. We established a weak positive correlation between mFTa and the time the intervention lasted. The greater the valgus of the limb, the greater the skin-to-skin time (P = 0.049) and the greater the ischemia time (P = 0.038). There was no correlation between preoperative joint movement and operative time. The percentage of cases with postoperative alignment (mFTa) in the range of 180° ± 3° was as follows: 73.4% with CI, 90.2% with CAS and 88.6% with PSI (with a mean standard deviation outside of the range of 1.98° ± 1.73° for the PSI cases).
Results.
| CI (n 72) | CAS (n 68) | PSI (n 103) | P | |
|---|---|---|---|---|
| Skin-to-skin time (in min) | 87.85 ± 11.86 | 123.46 ± 11.27 | 78.69 ± 13.06 | 0.000 |
| Ischemia time (in min) | 94.44 ± 11.49 | 129.63 ± 11.37 | 84.63 ± 12.06 | 0.000 |
Values expressed in mean and standard deviation or number and percentage.
CAS: computer assisted surgery; CI: conventional instruments; PSI: patient-specific instruments.
Level of significance P < 0.05.
The most relevant observation in our study is the statistically significant reduction in operative time required for TKR implantation when patient-specific instruments (PSI) are used instead of conventional mechanical instruments or direct computer assisted surgery. Our records are comparable with those published by other authors.13–19
It has been estimated that using PSI to implant a TKR reduces the surgical procedure by up to 21 steps, with the consequent reduction in time.20 In a systematic review and metaanalysis performed by Gong et al.,21 9 studies that published operative time were evaluated.10,22–29 According to their result, surgery with PSI significantly reduced operative time an average of 7 min, compared with the use of CI (confidence interval [CI] of 95% from −10.95 to −3.75 with P < 0.0001 and ratio of total variation attributable to study heterogeneity [I2] of 78%). The currently-available literature not analysed in the study by Gong et al.21 is not consistent about the reduction of operative time with the use of patient-specific instruments. Several studies13–16,24,30 indicate that there is a significant reduction in time when the intervention is carried out using PSI. Other authors17,18 show a certain decrease in the length of surgery, without a statistically-significant difference, between the cases operated using CI and those using PSI. To the contrary, Hamilton et al.31 have published a prospective randomized study in which the surgeries are filmed for later detailed analysis of 15 sequential steps in prosthesis implantation. In their study, implanting the prosthesis by means of PSI takes a few minutes more when conventional instruments are used (61.47 ± 5.48 min against 57.27 ± 4.58 min with P = 0.006). Interpreting the results, Hamilton postulates that they stem from having experience with more than 1500 interventions using conventional instruments and with only 20 cases using guide blocks before the study. In a series of 86 cases, Chinnappa et al.19 establish a significant reduction in operative time after the learning curve, with 85 ± 11.1 min in the first 30 cases compared with 78 ± 8.7 min in the next 56 (P = 0.001). Steimle et al.32 also publish a non-significant greater time consumption when patient-personalised instruments are used (102.2 ± 13.4 min compared with 99 ± 21.3 min with conventional instruments and P = 0.721). Focusing on the economic aspect, Watters et al.33 publish a comparison of costs and efficiencies between CI, PSI and CAS, from the supplier’s perspective. These authors state that the cost per case is higher with PSI than with conventional instruments, but less than with CAS. This is in spite of estimating, with respect to the conventional technique, a 13-min reduction of operative time when patient-specific instrumentation is used and a 39-min increase when CAS is used. In contrast, Tibesku et al.9 apply activity-based costing and determine that prosthetic knee surgery using patient-specific instruments is economically effective, as long as the time savings (they estimate 10 min in the surgery and 20 min in the operating theatre preparation) is used effectively to perform additional procedures.
In our study, we have observed a mean decrease of 9 min when comparing PSI with CI, and of 44 min when comparing PSI with CAS. Computer assisted surgery involves the capture of various anatomical references for computer processing and offers real-time information, allowing corrections in the osteotomies, with an obvious time consumption. It is true that 9 min, in spite of the statistical significance, might appear irrelevant. However, if we consider one of the objectives of the surgery, such as obtaining a postoperative mFTa of 180° ± 3°, we can state that we achieve the objective (73.4% using CI, 90.2% using CAS and 88.6% using PSI) with shorter operative time. Our coronal alignment with PSI is comparable to that of other authors, using the same system (Koch et al.,34 87.6%, and Anderl et al.,35 90.4%, or Helmy et al.,36 81.4%).
There are a few limitations to our study. The main ones are the retrospective nature of the study and the lack of randomization in the cases undergoing an intervention with each instrumentation. All the surgeries have been performed at a single institution by the same surgeon. It is true that this factor avoids bias in differences in surgical technique among different surgeons, but it is no less true that it makes it impossible for the conclusions to be easily generalised. The study has been carried out in a hospital that currently handles a population of approximately 54,500 inhabitants. The surgeon has performed an annual mean of 49 ± 10 primary knee replacement surgeries in the last 10 years. We cannot extrapolate the result to institutions in which the surgeons perform a very different number of annual surgeries (institutions with a very limited or a very high volume of primary knee implants). In addition, only one type of patient-specific instrumentation has been used (the MyKnee® cutting block system from Medacta International SA, Castel San Pietro, Switzerland), so the results should not be extrapolated to the different devices of other manufacturers.
We can conclude that, in the conditions in which our study has been carried out, the length of time for implanting a TKR has been less when we have used PSI than when we have used other instrumentation systems. Quantitatively and with respect to the CI system, the time consumption does not seem to be an advantage of patient-specific instruments. Further studies will be necessary to be able to correlate the reduction in operative time with advantageous operating theatre utilisation and with the positive effect on cost-effectiveness and cost-efficiency.
Level of evidenceLevel of evidence III.
Conflict of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: León-Muñoz VJ, Lisón-Almagro AJ, López-López M. Influencia de la instrumentación sobre el tiempo quirúrgico para implantar una prótesis total de rodilla. Rev Esp Cir Ortop Traumatol. 2019;63:321–326.