The in vitro creation of hyaline joint cartilage is a challenge since, to date, the ex vivo synthesis of a structured tissue with the same biomechanical and histological properties of the jint cartilage has not been achieved. To simulate the physiological conditions we have designed an in vitro culture system that reproduces joint movement.
Material and methodWe have developed a cell culture bioreactor that prints a mechanical stimulus on an elastin matrix, in which mesenchymal stem cells (MSC) are embedded. The first phase of study corresponds to the development of a bioreactor for hyaline cartilage culture and the verification of cell viability in the elastin matrix in the absence of stimulus. The second phase of the study includes the MSC culture under mechanical stimulus and the analysis of the resulting tissue.
ResultsAfter culture under mechanical stimulation we did not obtain hyaline tissue due to lack of cellularity and matrix destructuring.
ConclusionThe stimulus pattern used has not been effective in generating hyaline cartilage, so other combinations should be explored in future research.
La creación in vitro de cartílago hialino articular supone un reto ya que, a día de hoy no se ha conseguido la síntesis ex vivo de un tejido estructurado con las mismas propiedades biomecánicas e histológicas del cartílago articular. Para simular las condiciones fisiológicas hemos diseñado un sistema de cultivo in vitro que reproduce el movimiento articular.
Material y métodoHemos desarrollado un biorreactor de cultivo celular que imprime un estímulo mecánico sobre una matriz de elastina, en la que están embebidas células troncales mesenquimales (MSC). La primera fase de estudio corresponde al desarrollo de un biorreactor para cultivo de cartílago hialino y la comprobación de la viabilidad celular en la matriz de elastina en ausencia de estímulo. La segunda fase del estudio engloba el cultivo de MSC bajo estímulo mecánico y el análisis del tejido resultante.
ResultadosTras el cultivo bajo estímulo mecánico no obtuvimos tejido hialino por falta de celularidad y desestructuración de la matriz.
ConclusiónEl patrón de estímulo utilizado no ha resultado efectivo para la generación de cartílago hialino, por lo que se deberán explorar otras combinaciones en futuras investigaciones.
Hyaline articular cartilage has a complex tissue structure that gives it its biomechanical properties. There have been many attempts at in vitro culture to obtain this tissue, but none has yet succeeded in reproducing the tissue architecture resulting in its qualities of high resistance to compression, sliding and lubrication forces.
Chondrocytes, cells whose origin is in mesenchymal stem cells (MSC), are distributed in a matrix of collagen II and proteoglycans that they produce themselves following the pattern described by Benninghoff.1
The interest in in vitro culture of this tissue lies in the increasing prevalence of chondral lesions, both due to population ageing and the development of osteoarthritis, as well as the increase in cartilage injury-prone sports activities. Having healthy tissue available to replace in patients would be the beginning of tissue therapy and bioengineering in the treatment of cartilage disease.
As many previous studies have already shown, mechanical stimulation encourages the production of extracellular matrix (ECM) of hyaline cartilage.2,3 It also produces an increase in the proportion of proteoglycans and collagen II. The reorganisation of these fibres into bundles has not been achieved.
There are previous studies in which mechanical stimulus has been applied (direct compression, shear, hydrostatic pressure, surface fluid flow), for which bioreactors of different types have been designed.4 Most bioreactors are monoaxial and only a few combine pressure and shear.5–7
We start from the hypothesis that cyclical mechanical stimulus helps cellular differentiation towards chondrocyte and the reorganisation of the extracellular matrix. Using 3D printing technology, we have designed a bioreactor that allows direct compression and shear stimulation to be performed independently.
For cell nutrition there are many culture media available, from which we chose Dulbecco's Modified Eagle Medium (DMEM), on which studies have already been conducted under pressure of mesenchymal cells and chondrocyte cultures,5,7,8 and NH-Chondrodiff® for differentiation of mesenchymal cells to chondrocytes, a medium that has also been tested in other studies for this purpose.9
The mesenchymal cells or chondrocytes should be included in a matrix that serves as support. The ideal matrix should have sufficient resistance, enable survival (biocompatible) and cell expansion, and be degradable so that it can be replaced in the long term by the product of our cells.10 Tissue engineering has studied multiple biomaterials - natural, polymers and ceramics - for cell culture. The natural biomaterials include collagen matrices, hyaluronic acid or decellularized extracellular matrix. Ceramic biomaterials, such as hydroxyapatite or calcium phosphate, as osteoinductive materials. Finally, the polymers, such as polyglycolic acid (PGA), polylactic acid (PLA), very hydrophobic, or hydrogel polymers, with high capacity for hydration and less involvement of the ECM.11 For our study we looked for a hydrogel capable of withstanding the forces to which it would be subjected and which would have already demonstrated cell viability for cultures, choosing an elastin-like recombinamer.
Material and methodsCulture systemThe cell culture system used consists of bioreactor, culture matrix and cell elements.
Development of the bioreactorFor our experiment we created a bioreactor that simulates as much as possible the natural environment of the chondrocyte. To that end, we designed a bioreactor that meets the necessary requirements to maintain the viability of cells in culture and also comprises the mechanical elements that apply compression and shear stimuli while maintaining sterility and incubation conditions. The bioreactor was designed using Tinkercad® software. Afterwards, the parts were printed in 3D. We used polyethylene glycol terephthalate (PETG) filament. This material was chosen because it is not toxic to the cells and is compatible with the ambient conditions of incubation (temperature 37C, 5% CO2, humidity 80%). The bioreactor consists of a mechanism with moving parts, by means of which a piston comes into contact with the cell culture surface and is capable of performing axial compression and rotation (shear) movements. This mechanism takes place by three servos and one processor (HiTec HS-65MG®, Arduino UNO). The duration and magnitude of these stimuli is controlled using a computer programme created by the authors. This programme allows us to adjust the code to modify the time intervals between stimuli, as well as the magnitude of compression and degrees of shear (Fig. 1).
To ensure that the magnitude of the compression stimulus was similar in all cultures, a calibration process was carried out by measuring the compression forces in a well created for this purpose with a resistive sensor (FRS).
The pressure to which to subject our culture was chosen based on previous experiments in which the start of collagen II matrix production has been demonstrated and cellular production of glycosaminoglycans and proteoglycans typical of articular cartilage when the MSCs were subjected to a wide range of pressures (.5−10 MPa) 8 and more frequently to 5Mpa.12–14 This, translated into units of mass-force, would be equivalent to stimuli between approximately 50 and 1100 g force/cm.2 We chose a maximum pressure figure that would be in the range (300 g/cm2) that the servo motor is capable of printing.
We based the shear frequency on most publications in which the stimulus is performed at 1 Hz, since it is the most frequently used in previous publications.15
Prior to its use, the entire assembly (bioreactor, culture wells and electronic components) was sterilized by ethylene oxide and stored in sterile sealed packages.
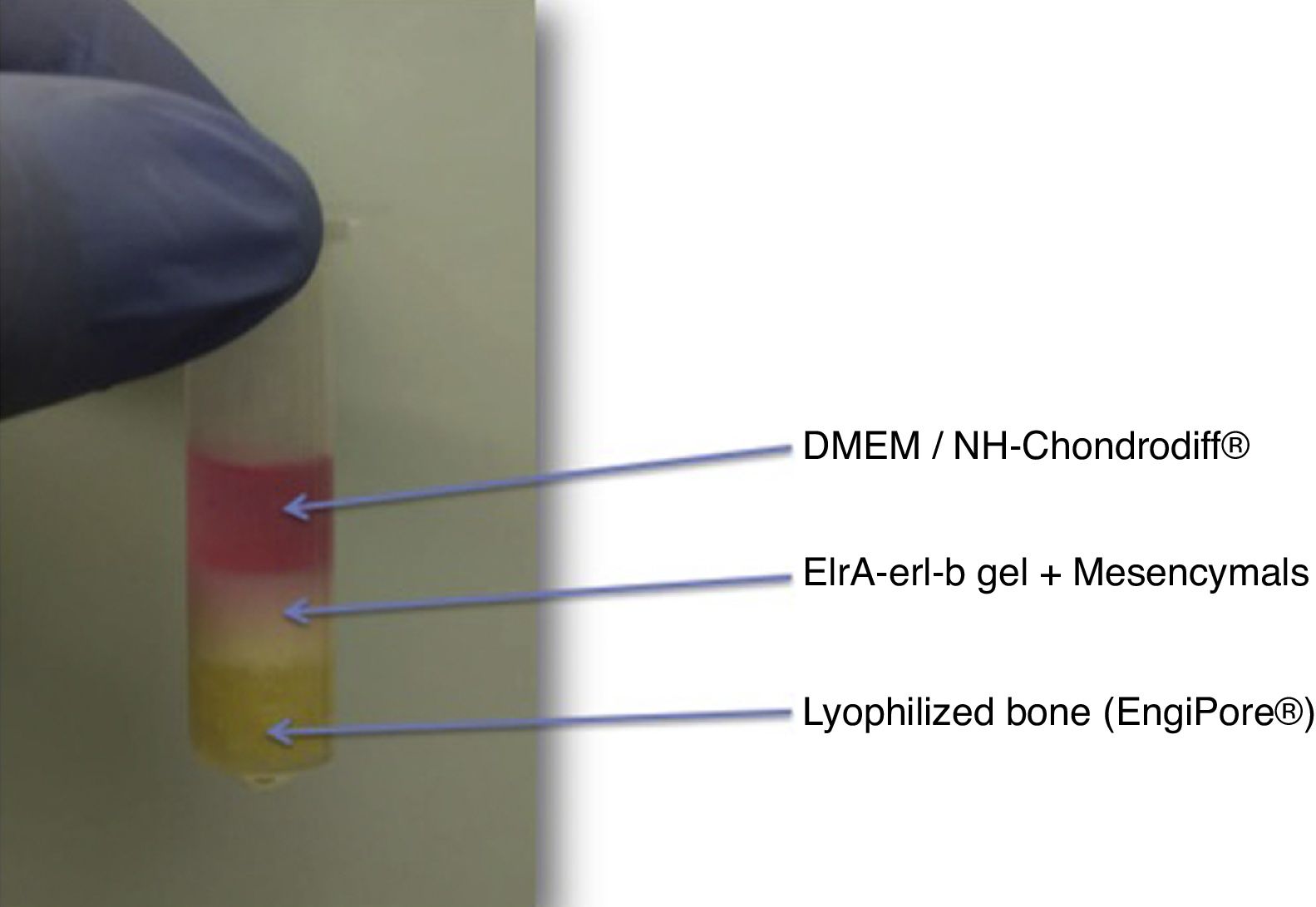
Culture matrixThis is the support medium in which we will grow the cells. This construct consists of three components: a base of demineralized bone matrix putty, a hydrogel in which the MSCs are embedded, and the culture medium that it diffuses to provide nutrition to the cells (Fig. 2).
In the initial phases, the design consisted of a hydroxyapatite base (hydroxyapatite discs obtained by cutting a cylinder of Engipore® hydroxyapatite). In a first test excessive porosity was observed that resulted in de-structuring of the matrix, and therefore this element was replaced by demineralized bone matrix of human bone in putty DBM putty SteriFuse®), which is less porous and prevented migration of the culture gel. The rest of the cultures were made using this method.
ELRc-ELRa (Bioforge®) gel was the matrix chosen to plant the MSCs. This is an elastin-like recombinamer gel (ELR) that at low temperatures is in a liquid state and solidifies from the transition temperature (4 °C), going on to a hydrophobic state, providing the gel with the biomechanical properties that will tolerate the stress of the bioreactor. The ELRc-ELRa (Bioforge®) is prepared in eppendorf at low temperature (introduced in dry ice). While in liquid state we can plant the MSC. Once solidified, at room temperature, the cells are distributed by the elastin-like recombinamer gel.
As culture media we use Dulbecco's Modified Eagle Medium (DMEM) as standard and NH-Chondrodiff® as chondral differentiation media.
Obtaining mesenchymal stem cellsThe MSC samples were obtained, after informed consent and approval by the Ethics Committee, by means of bone marrow aspiration from the anterior iliac crest from healthy patients. The technique and extraction protocols were the same as those described in our previous studies.9,16
The MSCs will be classified as per the criteria described by the International Society for Cellular Therapy.17
Control culturesA control culture was then performed in a standard bioreactor. The aim of this control was to check the behaviour and viability of the MSCs in the ELR gel and the presence of the hydroxyapatite disk.
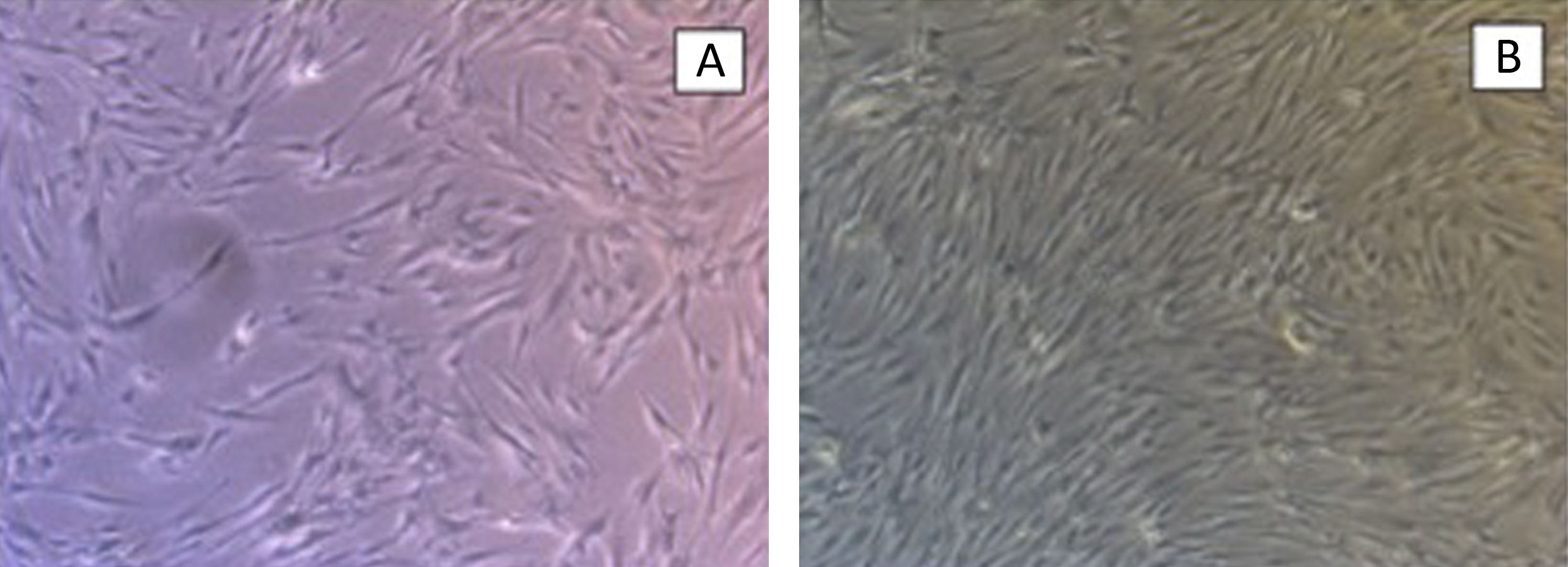
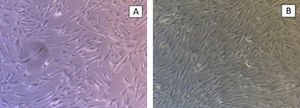
The hydroxyapatite base, the elastin recombinamer gel with the MSCs embedded in it and DMEM are placed in the culture well. The culture is maintained for 21 days changing the medium 3 times a week. The culture is removed to perform histological sections and haematoxylin-eosin stain to check cell viability (Fig. 3).
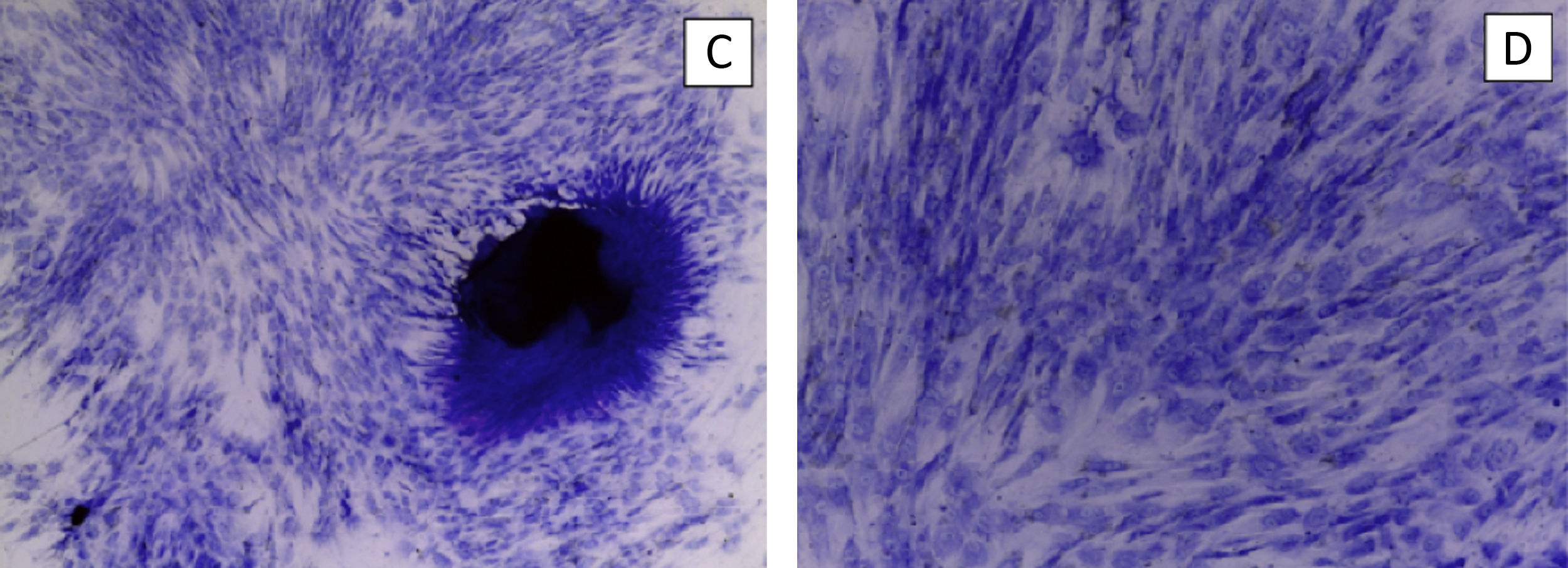
We perform the same operation with NH-Chondrodiff® culture medium, also observing cell survival and MSC to chondrocyte differentiation (Fig. 4).
Cultures under mechanical stimulationTwo culture lines were made: A) MSC in ELR gel with DMEM medium, subjected to compression and shear cycles, and B) MSC in ELR gel with NH-Chondrodiff® medium, subjected to compression and shear cycles. Only the research corresponding to line A will be presented in this paper.
After expansion of mesenchymal cells and seeding them in the ELR gel at a concentration of 80,000 cells/400 μl, we leave the culture in the well with DMEM for 7 days to allow cell stability in the new culture medium. From day 8 the culture well is assembled in the mechanical stimulation bioreactor and we start the pressure and shear cycles.
The mechanical compression stimulus was programmed to apply a pressure of 300 mg/cm2, and the shear stimulus for a rotation of 180°in maximum compression position for 3 cycles, for a total of 30 s. This process is repeated every 10 min, for 3 weeks, pausing the process only to change the culture medium.
On day 30, we remove the bioreactor and disassemble the samples from the well, putting them into paraffin, to perform histological cuts and haematoxylin-eosin stain.
Throughout the entire time of culture, the DMEM medium is changed every 2–3 days and the bioreactor and cells are handled under a laminar flow hood. The bioreactor works in the incubator at 37 °C.
ResultsThe control groups were cultured for 21 days, observing adequate proliferation and cell survival of MSC and chondrocytes, without mechanical stimulus.
Then, a total of 5 cultures was made, all corresponding to the MSC group in ELR gel with DMEM medium subjected to compression and shear cycles (group A).
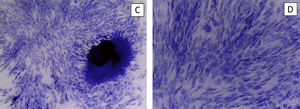
The first culture was that corresponding to the initial design with hydroxyapatite disc. After the first week we observed that the hydroxyapatite had broken down into granules and the gel was interdigitated between them. We could not perform histological cuts, and therefore we used the sample to analyse it with scanning electron microscope. We did not observe cells on the surface of the hydroxyapatite and the gel was de-structured.
In view of these results, as previously mentioned, the design of the study was changed, and the hydroxyapatite disc was replaced by a freeze-dried human bone putty (DBM putty SteriFuse®).
The following three cultures were fully completed. In the samples obtained at the end of the process we observed a minimal cellular presence in the gel and breakage of the hydroxyapatite-gel ELR interface. In one of the samples the cells were distributed as cellular aggregates. It was not possible to perform cell differentiation checks.
The last sample was contaminated with fungi in the second week of culture and was discarded.
In all the samples the ELR matrix from the few days after the beginning of the mechanical stimulus appeared thin and weak and did not exceed a thickness of 1 mm.
After the five experiments of line A we stopped the study, due to the problems detected.
DiscussionOur hypothesis is based on observing foetal mobility during pregnancy. General foetal movements begin at 9 weeks’ gestation. These movements allow the delineation of the joint zones and the development of the synovial joints from mesenchymal cell nuclei. Thus, intrauterine pressure, added to the shear mobility and the pressure exerted by the foetal musculature on the area where the future joint will be housed, will allow the development of the joints and, consequently, of the joint cartilage.18–20
There are already numerous publications in which mechanical stimulation of chondral cell cultures is carried out to obtain articular hyaline cartilage.
The mechanical stimuli found will be direct pressure, shear, hydrostatic pressure and fluid flow on the surface.3,21–23
Many of the models present unidirectional stimulus designs. Others use hydrostatic pressure without directly compressing the cells. And those in which the mechanism is combined do not differentiate the shear component from that of pressure.7,24,25 In some of the models in which pressure and shear are combined, one of the surfaces is curved, so that the pressures are not homogeneous throughout the culture surface.5,6
Tissue with a histological structure that presents the order of fibres in vertical bundles at depth and parallel at the surface is not obtained in any of them, most cultivated at 21 days.
Our bioreactor allows independent shear and pressure movement, simulating a slight hydrostatic pressure until the vertical stimulus column descends. When it contacts the culture, the stimulus starts by direct pressure.
Regarding the chosen cell source, MSCs are mainly present in the bone marrow and have a great plasticity to differentiate them from adipose tissue, cartilage and bone.
We decided to use this cell source since these are the cells that we find in the embryo when it begins to move.26 In addition, they are easy to expand and conserve and have already been used under mechanical stimuli, demonstrating the capacity to produce extracellular matrix of cartilaginous tissue.2,3
In choosing the matrix, we looked for one that was resistant to mechanical stimuli, solid at 37 °C and that would allow cell survival and proliferation, as well as the diffusion of nutrients. Nanotechnology has developed recombinamer polymer matrices capable of generating elastin meshes to which degradation sequences can be added. These matrices, resistant to a greater or lesser extent to compression and shearing stimuli, liquid at temperatures below 4 °C and solid at 37 °C, met our needs.27 Their capacity for self-degradation is also interesting, since, once the chondrocytes begin to synthesize matrix, this should start to provide the structure with consistency and tolerance capacity to physical stimulus. Cell survival in them was adequate in the experiments without pressure or shear, but we found a thinning of the matrix a few days after the beginning of the mechanical stimulus. We also observed low cellularity in the histological sections.
With regard to the resistance of the matrix, to date there are no studies to establish the maximum load that it tolerates, but having a transparent well would have allowed us to see its collapse and relate it to an excess of axial compression.
To investigate the low cellularity of the histological sections of the cultures made under the described pressure and shear conditions, we looked for the places where MSCs could be. On the one hand, we subjected the PETG well to washing and trypsinization. By trypsinizing it (a process by which the MSCs detach from the PETG) and we saw many MSCs under an optical microscope. We realized that the cells had not died or disappeared, they had adhered to the PETG, a plastic for which MSCs are naturally very greedy. We believe that the cells migrated through the pores of the gel, helped by the compression exerted on them. When they reached the walls of the well, they adhered to them.
The rest of the cells that we did not find on trypsinization, we believe could have been lost in DMEM medium changes or by the well overflowing.
The first objective of hydroxyapatite is to imitate the environment of the chondroblast that in the deepest layers is close to the bone tissue, and, as a challenge, to achieve a transition layer from bone to cartilage giving the project a translational nature, since it would be possible to implant an osteochondral graft generated in vitro.
In our case we had difficulty in the histological section of large particles of hydroxyapatite which we believe may be the cause of cell migration to the mineral phase prior to the stabilisation of the chondral culture, which would explain the low cellularity in the histological sections. In the next culture protocol, we will eliminate this factor until the matrix and cell stabilisation are controlled.
Other authors have started to give solutions to the creation of an interface with the use of nanoparticles or with chemical concentration gradients more favourable for chondral or bone differentiation.28–30
We believe that the fact that the matrix de-structured could be due to an excess of pressure on the culture. We will have to readjust the weight exerted until the matrix starts to develop mechanical properties with the activation of the MSCs and its own production of extracellular matrix.
With regard to the shear periods, the pause periods between which the movement cycles should occur are not defined. If we look again at the foetal saccadic movement, the pauses between movement cycles range from 13 to 45 min depending on the time of gestation. If these movements are those that stimulate joint formation, and thus hyaline cartilage, we should adapt these pause periods in the pressure and shear movement cycles.18,19
ConclusionsMechanical stimulus promotes the formation of hyaline cartilage extracellular matrix products, as has already been demonstrated in previous experiments, but to date the structural organisation of collagen in Benninghoff arches has not been achieved. The effects of compression and shear remain unknown.
Our experiment failed in that low cellularity and matrix de-structuring occurred due to an error in the bioreactor design, low gel consistency and the difficulty of processing samples in the presence of hydroxyapatite.
For the next phase, we have already made changes in the bioreactor to correct the shortcomings of the one described in this study. The wells are taller to prevent leakage of culture medium and are made of polystyrene instead of PETG, which is transparent and generates less cell adhesion.
The gel is going to be replaced by another of greater strength and less porosity (we are studying the available gels that are best suited to our needs) and we are going to remove the hydroxyapatite or freeze-dried bone component.
We hope that a new study with the introduction of these changes will bring satisfactory results.
FundingThe work was financially possible thanks to the SECOT Research Grant. Likewise, the company BioForge (University of Valladolid) provided their elastin-like recombinamer matrices for the construction of the culture matrices.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Sánchez-Pérez C, Fernández-Santos ME, Chana-Rodríguez F, Vaquero-Martín J, Crego-Vita D, Carbó Laso E, et al. Cultivo condral in vitro bajo estímulos de compresión y cizallamiento. De las células troncales mesenquimales al cartílago hialino. Rev Esp Cir Ortop Traumatol. 2020;64:380–387.