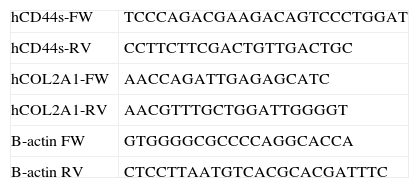To evaluate the in vitro effects of hyaluronic acid (HA) on adipose-derived stem cells (ASC) in order to consider the possibility of their combined use in the treatment of knee arthrosis.
Material and methodsThe ASC cells were grown both in the presence and absence of AH, and several studies were carried out: proliferation (WST8) and cell viability studies (Alamar Blue® and Trypan Blue), possible chondrogenic differentiation (collagen type 2 expression) by RT-PCR, AH receptor expression (CD44) by flow cytometry and RT-QPCR, and expression of inflammatory and anti-inflammatory factors (IL-6, TGFß, IL-10) by RT-QPCR.
ResultsThe number of ASC significantly increased after 7 days with HA (158±39%, p<0.05). Additionally, the cell viability of the ASC treated with HA after 1, 3, 5 and 7 days was similar to that of the control cells, being considered non-toxic. There were no changes observed in the expression of CD44 and chondrogenic differentiation. TGFß expression was not modified after AH treatment, but there was a 4-fold decrease in IL-6 expression and IL-10 expression increased up to 2-fold compared to control cells.
ConclusionsHyaluronic acid favours ASC proliferation without causing cellular toxicity, and inducing an anti-inflammatory profile in these cells. Hyaluronic acid appears to be a suitable vehicle for the intra-articular administration of mesenchymal stem cells.
Evaluar in vitro los efectos del ácido hialurónico (AH) en células madre mesenquimales derivadas de tejido adiposo (ASC) ante su posible uso combinado en el tratamiento de la artrosis de rodilla.
Material y métodoLas ASC fueron cultivadas en presencia o ausencia de AH, realizando estudios de proliferación (ensayo con WST8) y viabilidad celular (Alamar Blue® y Trypan Blue), posible diferenciación condrogénica de las células (expresión de colágeno tipo 2) por RT-PCR, así como el estudio de la expresión del receptor de AH (CD44) por citometría de flujo y RT-QPCR, y factores pro- y antiinflamatorios (IL-6, TGFß, IL-10) por RT-QPCR.
ResultadosEl número de ASC aumentó significativamente tras 7 días con AH (158±39%, p<0,05). Así mismo, la viabilidad de las ASC tratadas con AH a uno, 3, 5 y 7 días fue similar a la de las células control, considerándose que el tratamiento con AH no resultaba tóxico. No se observaron cambios en la expresión de CD44 tras el tratamiento con AH ni tampoco la inducción a la diferenciación condrogénica. La expresión de TGFß no se modificó con el tratamiento con AH; sin embargo, las ASC cultivadas con AH mostraron un incremento de 2 veces en la expresión de IL-10 y una reducción sobre el valour basal de 4 veces en la expresión de IL-6.
ConclusionesEl AH favorece la proliferación de las ASC en cultivo, no presentando toxicidad celular, e induciendo un perfil antiinflamatorio en estas células. Consideramos al AH un vehículo adecuado para la administración intraarticular de células madre mesenquimales.
Regenerative medicine is currently presented as one of the most promising alternatives in the treatment of osteoarticular disease. In particular, the use of cells combined with three-dimensional structures and bioactive molecules is showing promising results in tissular regeneration in chondral lesions. Of the different types of mesenchymal stem cells (MSC), those which derive from adipose tissue (ASC) have proved very useful in tissular regeneration due to their chondrogenic and osteogenic potential, which, along with their being relatively easy to obtain and isolate, makes them excellent candidates for use in regeneration studies in orthopaedic and traumatology surgery.1
One of the problems facing cellular therapy is determining the vehicle to bring the cells to the injured tissue. Hyaluronic acid (HA) is a basic element of the extracellular matrix and is found in most body tissues and fluids, such as hyaline cartilage and synovial fluid. This is a biodegradable polymer of high molecular weight which acts as scaffolding, and also has important biological functions such as regulating adhesion, mobility, cell differentiation and proliferation. These characteristics, along with its low immunogenicity, make it a very promising biomaterial for use in tissular engineering.2 HA gels, and substrates derived from it, have been tested for use as support structures for the culture of chondrocytes and the production of cartilage tissue.3 Recent animal experimentation studies have assessed the possibility of using pluripotential mesenchymal cells from bone marrow along with biphasic substrates of HA and tricalcium phosphate to repair osteochondral defects with encouraging results.4 Furthermore, recent studies highlight the importance of the interaction between HA and mesenchymal stem cells (MSC) in their therapeutic effect, and the production of HA by the MSC themselves as an essential part of their action mechanism.5 Finally, it is worth noting that HA has shown a better differentiating effect towards the chondrogenic line of MSC than other products used as scaffolding for their culture.6
Arthrosis is one of the most prevalent and widespread osteoarticular diseases and has a very negative impact, not only in terms of how it affects patients’ quality of life, but also because of its high economic burden on healthcare institutions.7 Arthrosis affects joint cartilage and through immunological and inflammatory reactions gradually produces a progressive lesion which eventually results in loss of joint function.8 Although various joints can be affected by this disease, the knee is one of the most commonly affected. In Spain, it is estimated that 10% of the population present symptoms associated with knee arthrosis.9
Taking all these aspects into consideration, the objective of this work was to evaluate in vitro the proliferation, viability and possible chondrogenic differentiation of ASC in the presence of HA, and to study HA receptor expression (CD44), the pro- and anti-inflammatory factors in these cells which might be altered in the presence of the polysaccharide, in order to assess the potential of HA as a vehicle for the intra-articular administration of ASC.
Material and methodThe ASC were obtained from Inbiobank (San Sebastian, Spain) and came from a young, healthy woman; they were routinely cultured in DMEM medium with 1g/l d-glucose (Gibco®-Life Technologies, Grand Island, NY, USA), and antibiotics (100UI/ml penicillin and 100μg/ml streptomycin) supplemented with 10% qualified foetal bovine serum (MSC Qualified Foetal Bovine Serum, FBS, Gibco®-Life Technologies), changing the medium every 3–4 days. The cells were kept in 75 or 150cm2 vials (Corning, Corning, NY, USA) and were passaged before reaching confluence by trypsinisation (TrypLE Express, Gibco®-Life Technologies). The ASC were characterised according to the criteria of the International Society for Cellular Therapy10,11: formation of fibroblastoid-type colonies, immunophenotype by flow cytometry (CD73+, CD90+, CD105+, CD11b−, CD45−, HLADR−), and osteoblastic and adipogenic differentiation (data not shown).
Diverse experiments were undertaken to evaluate the activity of HA (Adant®, 10mg/ml, Tedec-Meiji Farma, Alcalá de Henares, Spain) on cellular proliferation and viability, as well as its effect on the expression of the HA receptor, CD44, of type 2 collagen, and of pro- and anti-inflammatory cytokines on the ASC.
In the cellular proliferation study, the ASC were seeded on 96-well plates at a concentration of 2×103cells/well; after 24h, quiescence was induced by deprivation of serum (1%) and they were treated with different doses of HA (0.1, 0.3, and 1mg/ml) for 7 days, changing the medium after 4 days. When this time had passed, the number of cells was evaluated by means of a colorimetric test which assessed the formazan produced over 3h at 37°C from a tetrazolium salt (CCK-8, Sigma, Barcelona, Spain) measuring absorbance at 450nm in a microplate spectrophotometer (Epoch, Biotek Instruments, Winooski, VT, USA).
In the cellular viability study, the ASC were seeded on 48-well plates at a concentration of 2×103cells/well. The cells were treated at a concentration of 5mg/ml of HA in medium supplemented with serum for 1, 3, 5 and 7 days. After these times, the amount of viable cells was assessed by incubation with Alamar Blue® (AbD Serotec, Oxford, UK) for 2.5h at 37°C, measuring the fluorescence signal emitted (excitation wavelength: 530nm; emission wavelength: 590nm) on a multimodal spectrophotometer (Synergy 4 Hybrid, Biotek Instruments, Winooski, VT, USA). Once this test had been performed, the viable cells were counted with a Neubauer haemocytometer using trypsinisation and trypan blue stain.
AH receptor expression, CD44, in ASC in the presence and absence of HA was assessed by flow cytometry and reverse transcription polymerase chain reaction (RT-PCR). For flow cytometry, the cells were seeded in plates of a diameter of 100mm (6×103/cm2), and they were cultured for 7 days in medium with serum±1mg/ml HA. After this time, the cells were detached from the culture plate with TrypLE-Express to be subsequently resuspended in phosphate-buffered saline (PBS) at 106cells/100μl; and incubated at ambient temperature for 30min with 5μl anti-CD44-PE antibody (clone MEM-263, Novus Biologicals, Cambridge, UK). The fluorescence of the cells was quantified in a Navios flow cytometer (Beckman Coulter, Brea, CA, USA.). For RT-PCR or qPCR, after treatment of the cells ±1mg/ml HA for 7 days total RNA was extracted with TRIzol® reagent (Ambion-Life Technologies, Carlsbad, CA, USA), according to the manufacturer's instructions, after treatment with DNase (Turbo DNA free, Ambion-Life Technologies) to eliminate possible genome DNA contamination; complementary DNA was synthesised from 1μg total RNA by Multiscribe® reverse transcriptase reaction (Applied Biosystems-Life Technologies, Foster City, CA, USA) and oligo (dT)16.
A thermal cycler was used for standard PCR (Mastercycler, Eppendorf, Hamburg, Germany) with a reaction volume of 20μl, containing 1.5mM MgCl2, 0.5μM oligonucleotides (0.3μM for ß-Actin), and 1U Taq DNA Polymerase (Biotools, Madrid, Spain), amplifying the cDNA with 35 cycles of 30″ at 95°C, 30″ at 55–60°C, 30″ at 72°C. The DNA fragments were visualised in 1.7% buffered agarose gels TBE 0.5× containing RealSafe 1× (Durviz, Valencia, Spain), with a digital photo documenter (Alliance 2.7, UVItec, Cambridge, UK). A CFX96 touch thermo-cycler (BioRad, Hercules, CA, USA) was used for quantitative PCR, with a reaction volume of 20μl, with Quantimix Easy kit (Biotools) containing 0.3μM oligonucleotides, and SYBR Green 0.5× (Invitrogen-Life Technologies, Grand Island, NY, USA). The cDNA was amplified for 40 cycles of 30″ at 95°C, 30″ at 60–65°C, 30″ at 72°C, and 2″ at 80°C when fluorescence was detected. The 2−ΔΔCt method was used to calculate the expression of the different mRNA. The oligonucleotides for CD44, COL2A1 and ß-actin specific to human sequences are detailed in Table 1. The oligonucleotides for TGFß, IL-10 and IL-6 have been described previously.12 The data are expressed as mean±SD. ANOVA and t-Student tests were used for the statistical analysis of the results with Prism 5 software (GraphPad, La Jolla, CA, USA); a value of P<.05 was considered statistically significant.
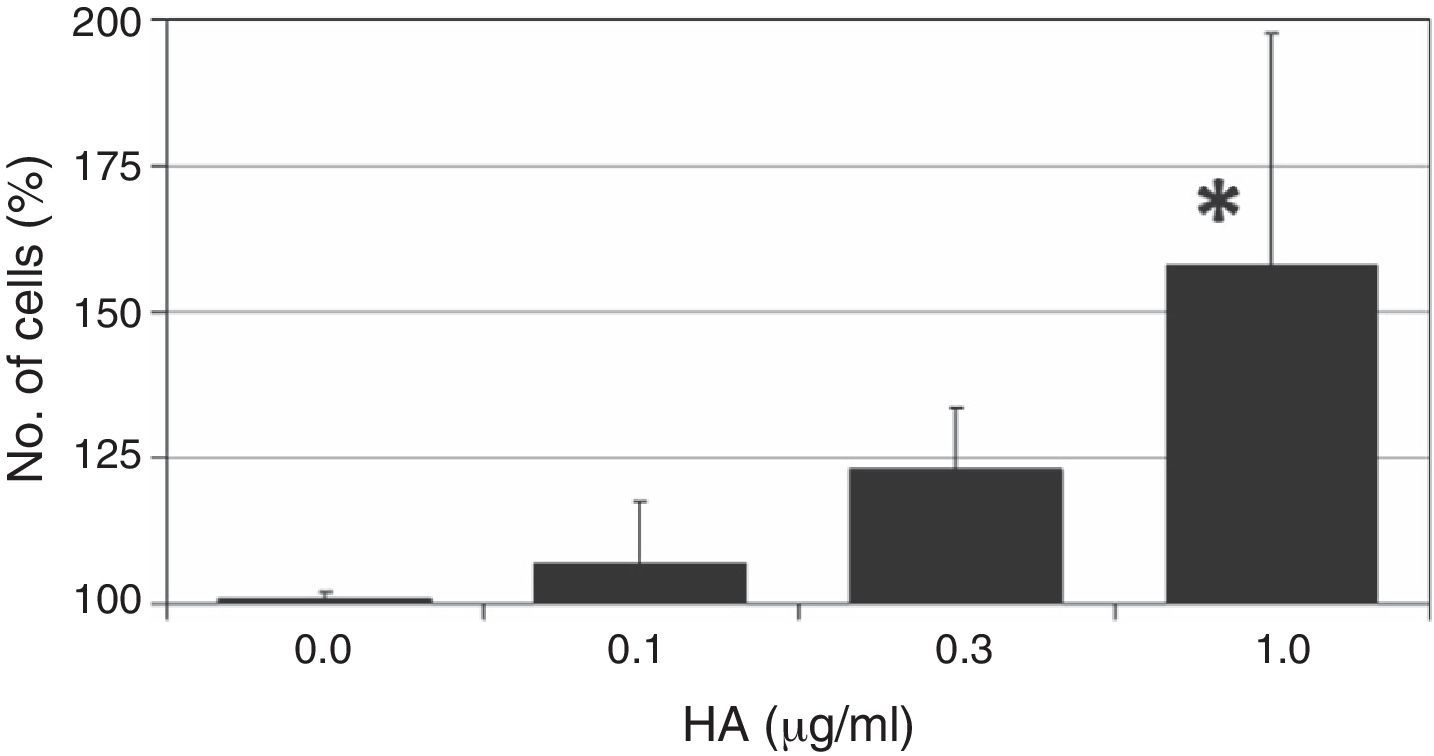
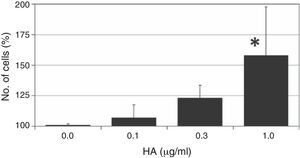
ResultsThe ASC cells were treated with different doses of HA to assess the effect of the polysaccharide on cellular proliferation. The results of this colorimetric test are shown in Fig. 1. As can be observed, the administration of HA to quiescent cells (in 1% FBS) gives rise to an increased number of cells after 7 days of treatment, with a dose-dependent effect, and the maximum effect is observed at the highest concentration of HA used (1mg/ml); this effect is statistically significant with respect to the growth of the control cells (158±39%, P<.05).
The effect of different doses of HA on the proliferation of ASC. Quiescent cells (with 1% FBS) were incubated with different does of HA for 7 days after which the number of cells was determined by a colorimetric test of formazan formation. The values are mean±SD of four independent experiments in triplicate. *P<.05.
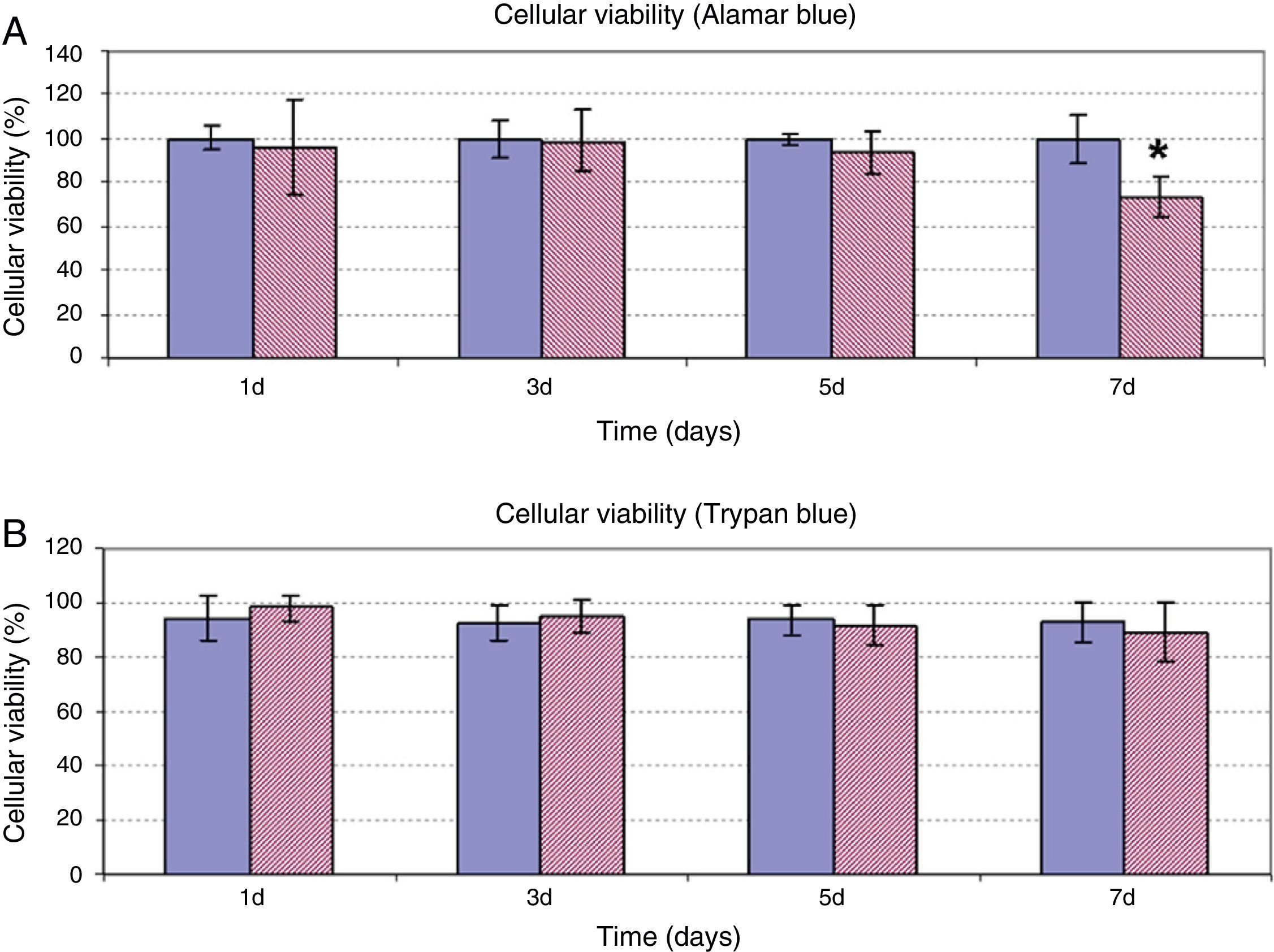
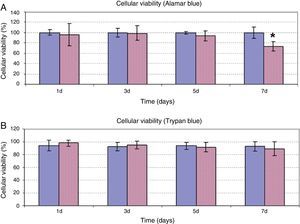
In addition to assessing the effect of HA on cellular proliferation, possible alteration of cellular viability was studied when the ASC were treated at a dose of 5mg/ml for 7 days. As can be observed in Fig. 2, both the results of the determination of cellular viability using Alamar Blue® (Fig. 2A) and of exclusion of Trypan Blue (Fig. 2B) were coincident. In days 1, 3 and 5 of treatment, viability of the ASC treated with HA was observed similar to the viability of the control cells, with values between 80% and 100% in both experiments. Only after 7 days incubation of the ASC with HA, was a significant decrease of viability observed with respect to the control cells in the quantification experiment using Alamar Blue®. However, despite the differences compared to the control, the viability value obtained at that time was 73±9%.
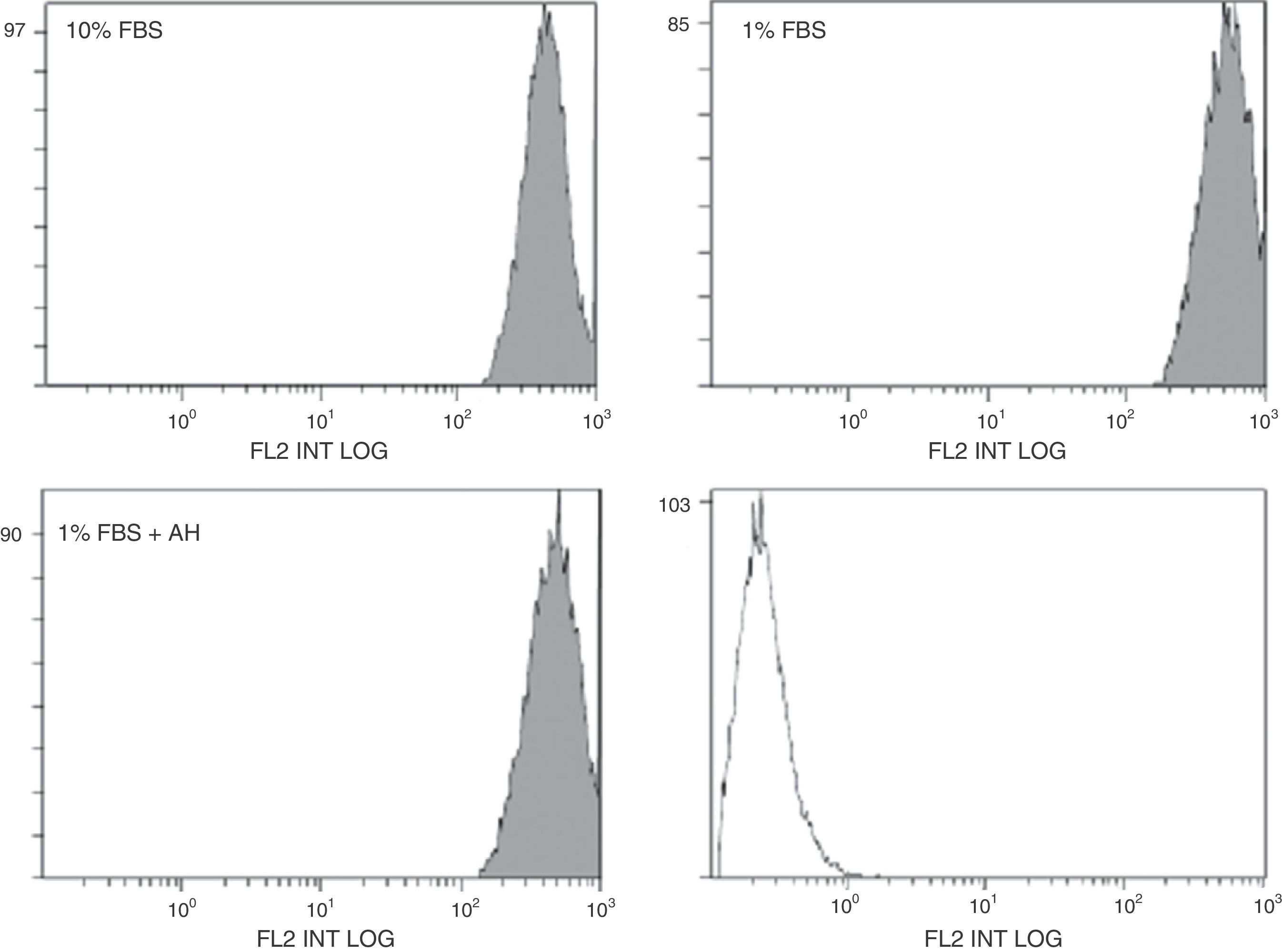
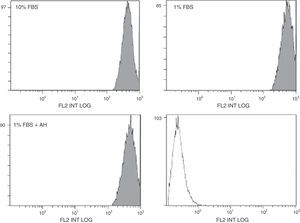
The effects of HA are measured by their membrane receptor, CD44. The stimulating effect of HA on the proliferation of the ASC could be mediated by changes in CD44 expression. Possible modification of CD44 expression in the ASC in the presence of HA was assessed by flow cytometry (protein expression, Fig. 3) and RT-qPCR (mRNA expression, Table 2). In the case of flow cytometry, there seems to be a slight displacement towards the right of the fluorescent signal in the case of the quiescent cells (treated with 1% FBS) compared to those treated with 10% FBS (Fig. 3). This increase in the expression of CD44 protein in the cellular membrane was confirmed by the results obtained with RT-qPCR, where the mRNA expression of CD44 was also greater in the case of the cells treated with 1% FBS compared to those treated with 10% FBS (Table 2). However, treatment with HA on quiescent cells did not appear to significantly affect the expression of this receptor at their protein or their mRNA level, since no significant differences were found using either of the two techniques compared to its control (1% FBS).
Quantification of mRNA expression for CD44 in the ASC by RT-aPCR.
| Sample | Times |
|---|---|
| ASC+10% FBS | 1.0 |
| ASC+1% FBS | 3.18* |
| ASC+1% FBS+1mg/ml AH | 2.25 |
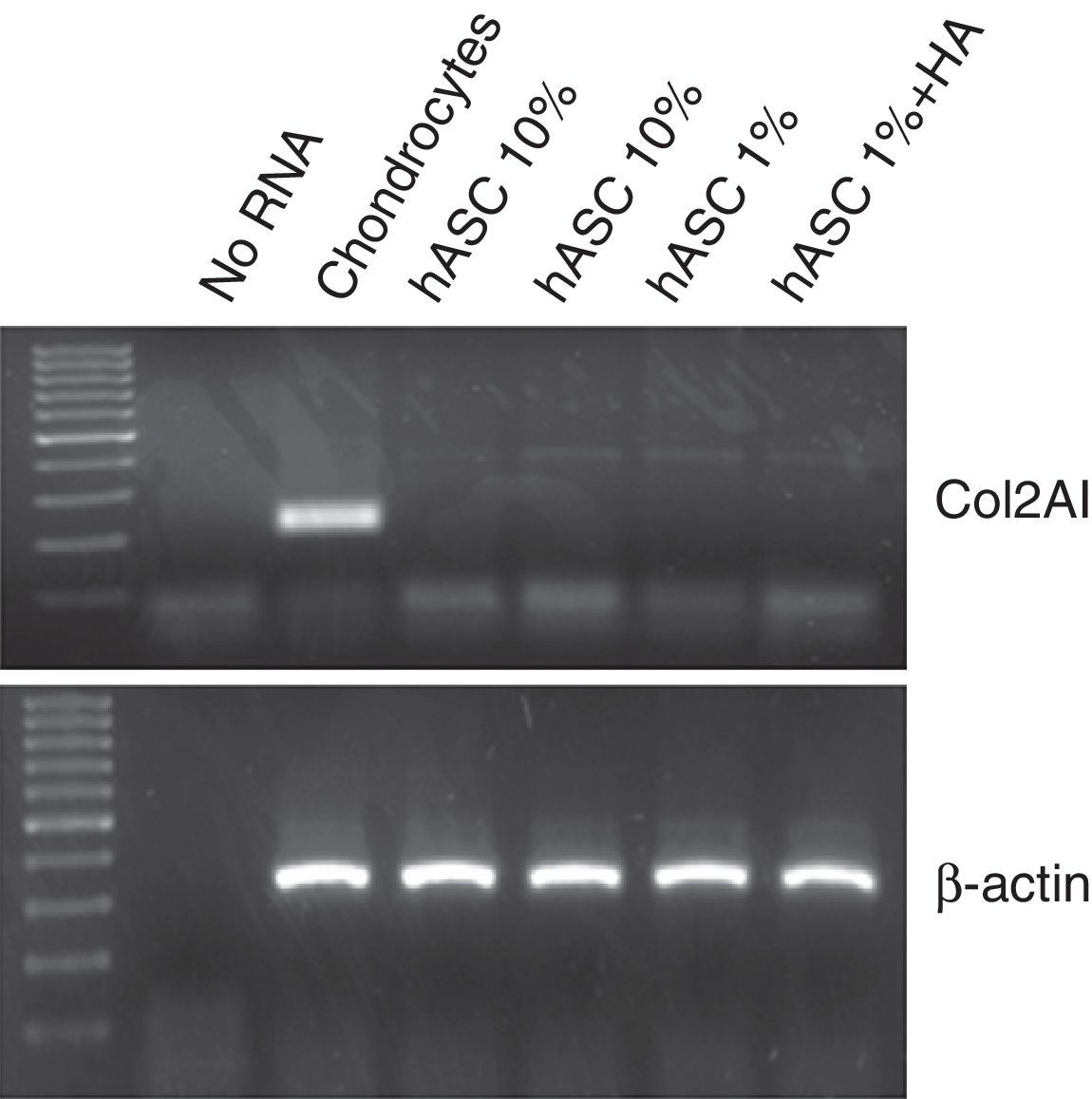
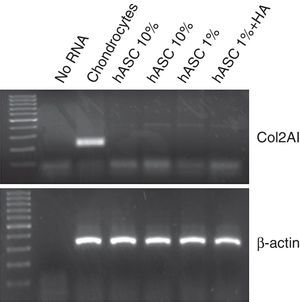
In order to analyse possible chondrogenic differentiation of the ASC after treatment with 1mg/ml of HA, the mRNA expression for type 2 collagen was assessed using RT-PCR. As can be observed in Fig. 4, in none of the conditions in which the ASC were cultured, in proliferation with 10% FBS or in quiescence with 1%FBS±HA for 7 days, could mRNA be detected for type 2 collagen, unlike human chondrocytes used as positive control.
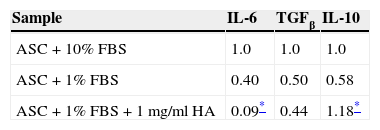
Finally, RT-qPCR was used to evaluate the mRNA expression for IL-6, TGFβ and IL-10. The data obtained from this test are shown in Table 3. In the case of TGFβ no significant differences were observed between the cells treated with HA and the control cells. However, treatment of the ASC with 1mg/ml HA led to a decrease in IL-6 proinflammatory cytokine expression with a result up to four times lower than the expression in the control cells, whereas the IL-10 anti-inflammatory cytokine increased 2-fold with respect to its control.
DiscussionArthrosis is one of the most common diseases of the locomotor system and is associated with deteriorated physical function and quality of life in relation to health, affecting different age groups, most of whom are in the productive stage of life. The search for more effective treatments than those already available is a research priority in the field of traumatology. Tissular engineering is one of the most attractive and promising alternatives towards improving current treatment, in particular the administration of mesenchymal stem cells embedded in hydrogels which provide appropriate support for the in vivo survival of cells. This work attempts to study the behaviour and survival of human mesenchymal cells derived from in vitro adipose tissue in the presence of diverse doses of HA, commonly used as treatment for knee arthrosis, in order to assess the possible administration of ASC vehiculised in HA.
HA has been used for many years as biomaterial, complying with the concepts of biocompatibility and biodegradability which are generally required of these types of materials, and is, in fact, one of the most used compounds in the design of three-dimensional structures for tissular engineering.2,13 However, there are very few data in literature concerning its effect on mesenchymal stem cells in vitro and we only found one recent work which presents some data in this regard. Ding et al. evaluated the effect on cellular proliferation of ASC from infra-patellar fat pad using the XTT test (Roche, Mannheim, Germany) and they concluded that at concentrations between 25% and 75%, HA does not affect the proliferation of these cells. Cellular viability studies were not undertaken in this work, although an increase in the cellular differentiation of ASC was found in the presence of HA after 2 weeks’ cultivation.14 In our study we characterised parameters such as proliferation and cellular toxicity along with other factors which could affect cellular differentiation and the anti-inflammatory usefulness of this biomaterial applied to this specific cell type.
The viability and proliferation results demonstrate that HA is biocompatible with ASC, allowing gradual growth of the cells with a dose-dependent tendency. We also demonstrated that ASC cells present high cellular viability values in cultivation with HA. In fact, the viability values obtained in our study are similar to those obtained by other researchers who also used HA and MSC hydrogels for application in bone regeneration. 15
CD44 is a cellular receptor involved in the mediation of diverse cellular processes which include morphogenesis, inflammation and metastasis, and it is expressed on the surface of many cell types, including ASC. This receptor interacts very actively with HA and has been considered the principal HA receptor in the majority of cell types. By means of flow and RT-qPCR cytometry studies, we have been able to confirm that the expression of this receptor is not affected by treatment with HA at a dose of 1mg/ml; therefore the cells maintain expression of CD44 on their surface membrane in the same conditions as the control cells. The only significant difference that we saw in our study was as a consequence of serum deprivation from the culture medium, on passaging from 10% FBS to 1% FBS, thus increasing DC44 expression. However, treatment with HA was completed using 1% FBS, therefore it can be considered that there are no differences compared to its real control, and that treatment with the polysaccharide does not affect the expression of said receptor on the surface of ASC; this would support there being no differentiation of the ASC under the conditions studied.
One of the advantages presented by mesenchymal cells for tissular engineering applied to the field of chondral regeneration compared to the use of chondrocytes is precisely their capacity to maintain their undifferentiated state despite in vitro culture. During cellular culture in vitro of chondrocytes, changes are produced in the cells which then prevent them redifferentiating. However, both MSC and ASC remain undifferentiated during in vitro culture, thus conserving all their characteristics for their subsequent implantation in vivo.11 It is for this reason that we wanted to verify the degree of cellular differentiation of cultured ASC by quantifying the expression of type 2 collagen. Type 2 collagen is a marker which is commonly used to indicate the differentiation of these types of mesenchymal cells towards a chondrogenic cellular phenotype.16 Neither treatment with HA or the subculture itself of cells in vitro caused phenotypical differentiation of the ASC, which confirms that one of the most appealing properties of these cells is maintained. As we mentioned earlier, the studies performed by Ding et al. do indeed demonstrate a prodifferentiating effect of HA on ASC cultures,14 although the culture conditions were not the same, and the time that the cells were kept in culture was of particular importance: 14 days, compared to the 7 days of our study.
ASC, in addition to presenting the differentiation potential and the self-renewing capacity of MSC, secrete diverse cytokines and growth factors involved in tissue repair processes, with anti-inflammatory, anti-apoptotic and immunomodulatory properties. Numerous studies highlight the paracrine action of these soluble factors as responsible for the beneficial effects of cellular therapy with MSC, rather than attributing them to their differentiation capacity.17 Therefore, we wanted to confirm the effect of HA on the cytokine secretion capacity of ASC compared to the control cells by determining the IL6, IL10 and TGFβ levels. IL-6 acts as a pro-inflammatory factor, especially in knee osteoarthritis,18 whereas IL10 and TGFβ are considered inflammatory factors.19 In our study we were able to verify how the anti-inflammatory role is fully demonstrated by a very significant increase in IL10 production, whereas there is a considerable decrease of IL6 expression by the ASC treated with HA for 7 days.
On all of these grounds, and taking into account the results of the tests we performed, we can confirm the biocompatibility of HA and ASC, and the anti-inflammatory potential of their combined administration. These encouraging results mean that HA can be considered an ideal vehicle for the intra-articular administration of mesenchymal stem cells from adipose tissue.
Evidence levelEvidence level IV.
Ethical responsibilitiesProtection of individuals and animalsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflicts of interest to declare.
This work was completed thanks to funding from the Hyalgan grant from the Spanish Society of Orthopaedic and Traumatology Surgery (SECOT) 2012. Fernando de Miguel has a Miguel Servet II Researcher contract (CPII13/00006) from the Carlos III Health Institute.
Please cite this article as: Moreno A, Martínez A, Olmedillas S, Bello S, de Miguel F. Efecto del ácido hialurónico sobre células madre mesenquimales derivadas de tejido adiposo. Evaluación biológica in vitro. Rev Esp Cir Ortop Traumatol. 2015:59:215–221.