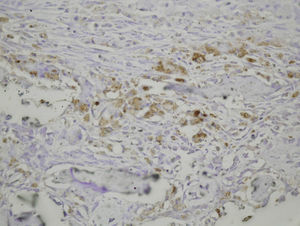
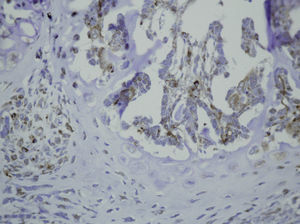
Many studies have been conducted to determine the different effects that reaming or intramedullary nailing have on fracture healing, but there is no evidence in the literature of the effect of intramedullary reaming on osteogenesis. We performed a prospective study to analyze the effect of intramedullary reaming and nailing on the production of growth factors during the process of fracture healing in the femur of rats.
Material and methodsA transverse mid-shaft non-comminuted femur fracture was produced in 64 rats; 34 rats did not receive any treatment, and a standardized surgical procedure was performed on 30 rats, by exposing the left knee, reaming the medullary canal from distal to proximal, and then fixing the fracture with a steel pin. The rats were sacrificed at the 24th hour, 4th, 7th and 15th days after the fracture. The amount of growth factors that appeared in the callus fracture was measured using histopathology studies. The primary categorical variables analyzed were PDGFA, TGF2 and TGFβ-R2. These variables were analyzed in each group at the different sacrifice times.
ResultsThe results of the primary variables of the study, stratified by the time until sacrifice, showed no statistically significant differences.
DiscussionEven if the presence of an intramedullary wire facilitates the fracture repair and the stabilizing the bridge of bone between both edges of the fracture site, no evidence was found that reaming changes the expression of the growth factors studied (PDGFA, TGFβ-R2 and TGFβ2) during the callus formation in rats.
Existen muchos estudios referentes a los diferentes efectos producidos por el fresado intramedular en el callo de fractura, pero no existe evidencia en la literatura del efecto de dicho fresado en la osteogénesis. Realizamos un estudio prospectivo para analizar el efecto del fresado endomedular y enclavado en la producción de factores de crecimiento durante el proceso de consolidación de la fractura en el fémur producida en ratas.
Material y métodosProducimos una fractura diafisaria, transversa, no conminuta de fémur en 64 ratas: 34 ratas no recibieron ningún tratamiento y las otras 30 se trataron mediante un procedimiento quirúrgico estandarizado, consistente en fresado del canal medular de distal a proximal y fijación de la fractura con una aguja de Kirschner. Las ratas fueron sacrificadas a las 24h, 4.°, 7.° y 15.° días después de la fractura. Medimos la cantidad de factores de crecimiento (PDGFA, TGF2 y TGFβ-R2) en el callo de fractura mediante estudio anatomopatológico en los diferentes momentos del sacrificio.
ResultadosLos resultados de las variables primarias del estudio, estratificadas por tiempo hasta el sacrificio, no mostraron diferencias estadísticas significativas.
DiscusiónAunque la presencia de una aguja intramedular facilita la estabilización de la fractura y la formación del callo de fractura, no hemos encontrado ninguna evidencia significativa de que el fresado endomedular produzca cambios en la expresión de los factores de crecimiento estudiados (TGFβ-R2, PDGFA y TGFβ2) durante la formación del callo de fractura de fémur en ratas.
There are multiple growth factors involved in the regulation of bone fracture repairs.1 In the initial stages, macrophages mobilized in the fracture focus generate growth factors, including platelet-derived growth factor (PDGF)2 and transforming growth factor β (TGFβ),3 which stimulate the proliferation of mesenchymal cells and their transformation and differentiation into chondroblasts.4 PDGF, which is also released by damaged endothelial cells and platelets,5 is a potent stimulator of chondroblasts, osteoblasts and their precursors,6 favoring bone production through the regulation of these cells. Tissue regeneration in the inner layer of the periosteum of long bones is of particular importance. These cells can differentiate into chondroblasts and osteoblasts, which makes them particularly important for bone formation after fractures.7
Numerous works have studied the effect of reaming and nailing in the process of fracture consolidation.8 Some of these studies have examined the mechanical properties of nailing,9 the effect of nail rigidity10 and instability criteria after femoral nailing,8 the effects of reaming on blood flow11 and its restoration after reaming,12 as well as comparing the effects of external fixation and intramedullary nailing,13 but the literature contains no evidence regarding the effects of intramedullary reaming and nailing on osteogenesis.
The aim of this study was to evaluate the morphological changes and production of cellular messengers and their evolution in a fracture callus generated in 2 groups of rats: one group treated by intramedullary reaming and another group which received no treatment. The distribution of the growth factors involved in the proliferation and differentiation of mesenchymal cells participating in the process of fracture consolidation was analyzed through immunohistochemistry.
Material and methodsWe performed a pilot study with 64 Sprague-Dawley model rats, with a mean weight of 200g. None of the rats included in the study had been used in previous studies and none of them was excluded. All received the same perioperative treatment; they were exposed to a 12h of light and 12h of darkness at 22°C. The rats were kept in rodent cages housing 2 rats each and received a standard diet and water ad libitum.
Animal care and surgeryThis study followed the international guidelines for the protection of animals and was approved by the Ethics Committee of the Surgery Department of our center.
We created a fracture of the femur in all rats and separated them into 2 groups: 30 rats were treated with an intramedullary nail and 34 rats received no treatment.
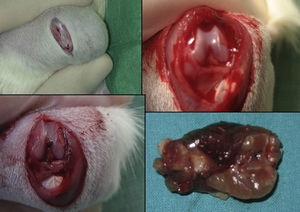
Perioperative anesthesia consisted of 2cc (1cc per 100g) of a solution of 10mg ketamine, 1mg atropine and 10mg diazepam in 5cc of saline solution administered intraperitoneally. A fracture of the middle third of the femur was produced manually in all rats through the principle of a type 1 lever, using a fulcrum between both load areas (hip and knee). This technique allowed us to create a non-comminuted transverse fracture in all animals.
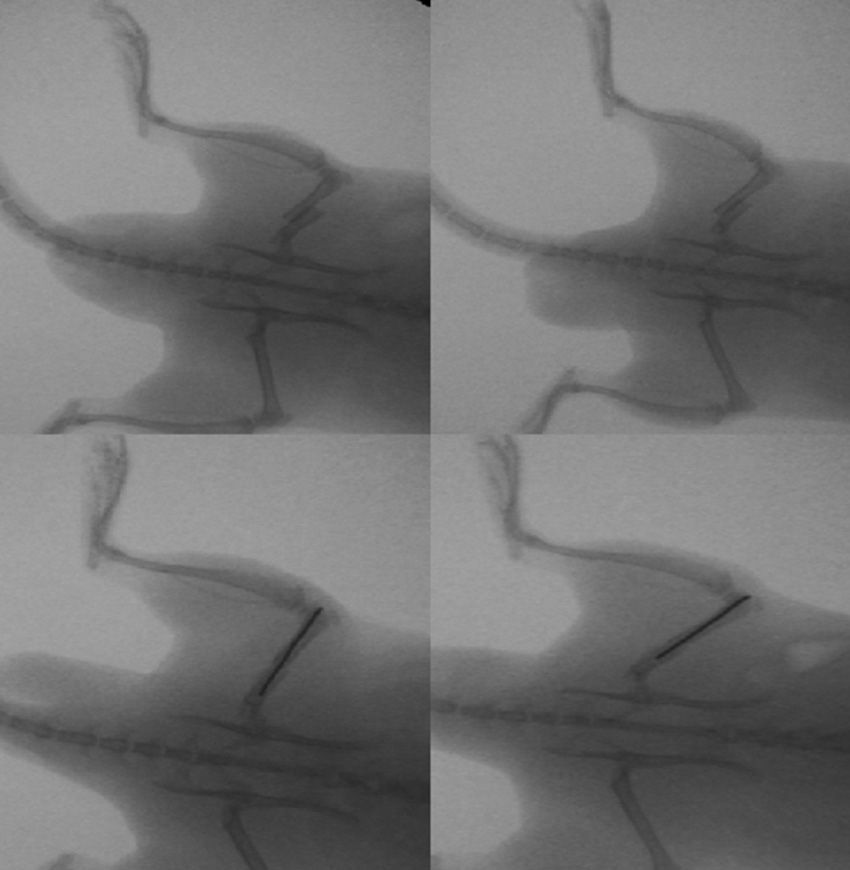
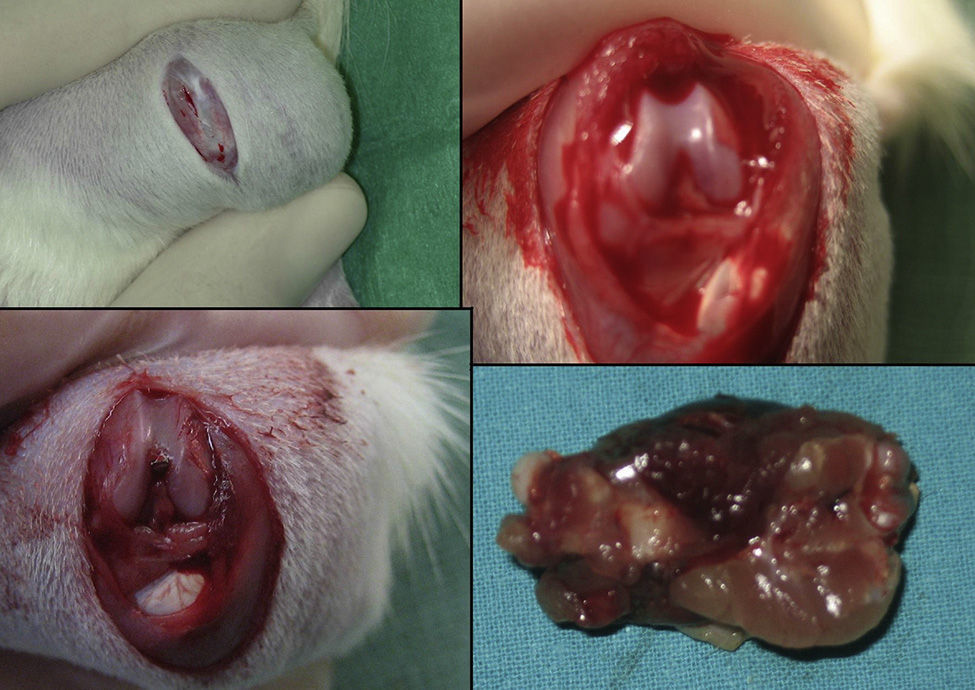
The group with the intramedullary nail (30 rats) underwent a standard surgical procedure. After intraperitoneal anesthesia and local shaving, the left knee was exposed through a midline incision and a medial parapatellar approach, dislocating the patella and exposing the intercondylar notch. We then performed retrograde reaming of the intramedullary canal and definitive fixation with a 1.6mm Kirschner wire. The wound was washed with saline solution and closed with absorbable suture. Load was not prevented after surgery. The animals were observed daily during the first 3 days and weekly thereafter. Amoxicillin was administered in the water for 7 days.
The rats were sacrificed by an ether overdose at 4 different times: at 24h, and on days 4, 7 and 15 after the fracture. In sacrificed rats, extraction of the femur along with the thigh muscle compartments was performed through a lateral approach, so as not to disrupt callus formation. We measured the amount of growth factors in the fracture callus through anatomopathological assessment.
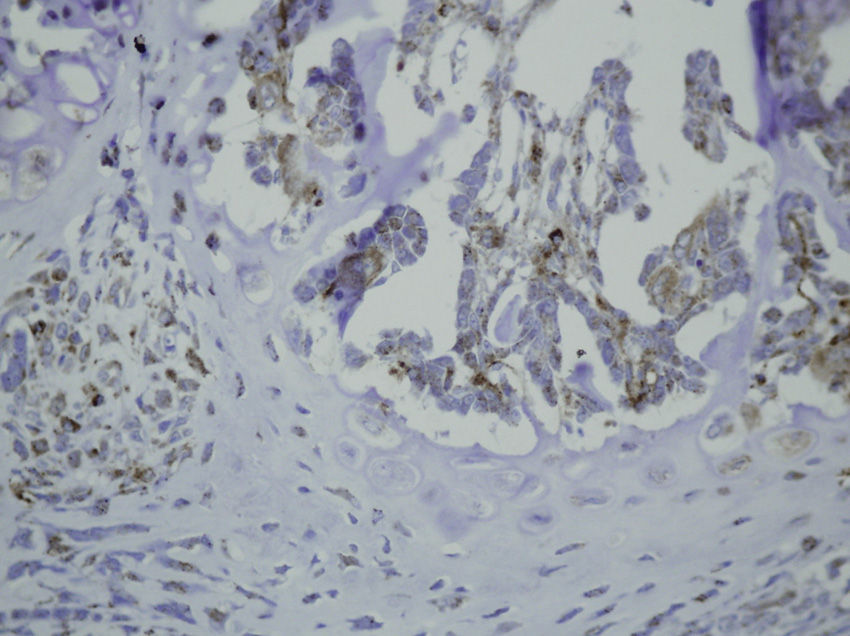
Study variablesThe main categorical variables analyzed were the presence of PDGFA, TGFβ2 and TGFβ-R2. In each group we evaluated these variables at different times of sacrifice (24h, 4, 7 and 15 days after fracture). To do this, we conducted an immunohistochemistry analysis in endothelial cells, mesenchymal cells, osteoblasts, osteocytes, chondroblasts and chondrocytes. We applied a quantitative score depending on the number of antibodies present for each growth factor: not positive (−) only occasionally positive (+/−), less than 10% positive cells (+), 10–50% positive cells (++) and over 50% positive cells (+++).
We analyzed secondary categorical variables such as the extent of necrosis, hemorrhage, inflammatory cells and formation of new vessels and callus. We evaluated the response of each treatment group.
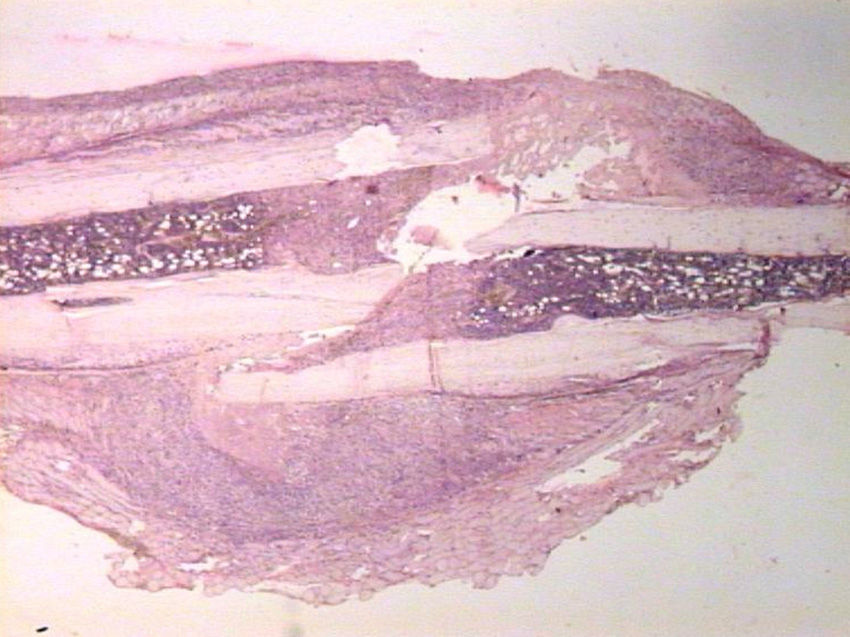
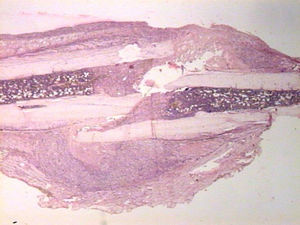
HistologyEach specimen consisted of muscle and bone with adjacent soft tissues fixed in 10% formalin and decalcified in a solution of 10% formic acid. The samples were sectioned along the femoral axis in the sagittal plane and introduced in paraffin. In the nailing group, the Kirschner wire was removed prior to preparation. We made 4μm thick sections which were stained with hematoxylin-eosin and Masson trichrome. Specific preparations for immunohistochemistry were made using a TechMate™ (DakoCytomation) dyeing machine. We used the following polyclonal antibodies and dilutions: TGFβ2 (Santa Cruz Biotechnology®, sc-90; 1:40), PDGFA (N-30) (Santa Cruz Biotechnology®, sc-128; 1:20) and TGFβ-R2 (L-21) (Santa Cruz Biotechnology®, sc-400; 1:40). Binding of primary antibodies was visualized using the peroxidase-antiperoxidase method. After completing the immunoreaction, the sections were stained with hematoxylin.
Statistical analysisThe variables were described by frequency distribution. Comparison between the experimental and control groups for primary categorical outcomes (PDGFA, TGFβ2, TGFβ-R2) was assessed through the chi-square test or Fisher's exact test, if necessary. Furthermore, the relative risk (RR) and 95% confidence interval were also estimated. The same analysis was also carried out stratified by the time of sacrifice (before and after 24h). We used logistic regression analysis in order to evaluate the effect of the interaction between the study group and the dichotomous (or dummy) variable, time of sacrifice.
The positive or negative assessment results were grouped based on the presence or absence of positive cells.
A value of 5% was accepted as significant for all tests. Data processing and analysis were performed using the software package SPSS® v15.0.
ResultsAnimal preparationThere were no perioperative incidences, and no signs of distress were observed during the experimental period. The group operated with internal fixation regained normal gait on the first day after surgery, while the group without internal fixation was able to move on 3 legs during the first days after the fracture. There were no cases of infection or death.
HistologySacrifice within the first 24hThe morphological characteristics of both groups were very similar on the first day after the fracture. We observed areas of necrosis, hemorrhage with hematoma and numerous inflammatory cells, such as neutrophils and macrophages, on the edges of the fracture.
We did not observe formation of new bone and cartilage, although we did observe osteoblasts in the inner layer of the periosteum.
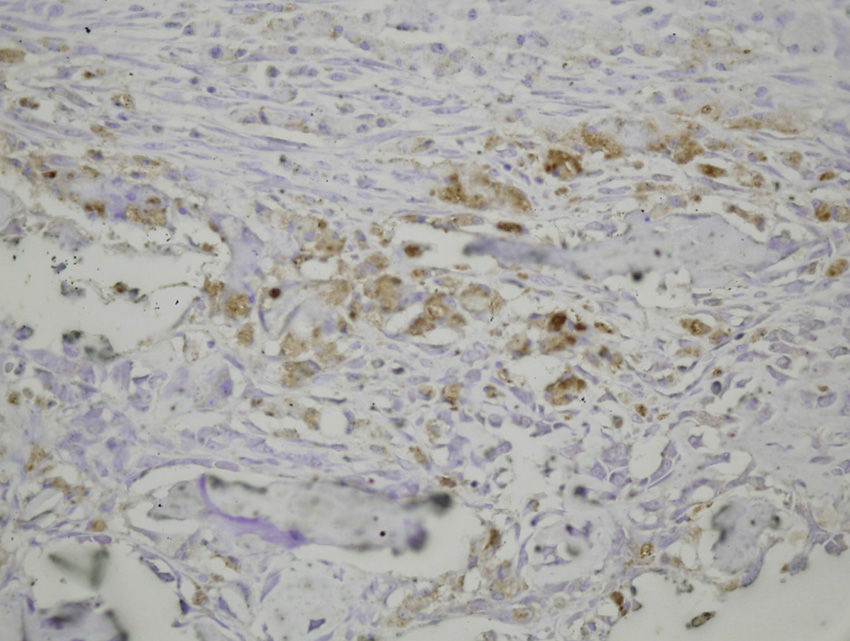
The histological samples obtained in the groups sacrificed at 24h after the fracture presented positive PDGFA immunostaining in mesenchymal cells and endothelium. Immunostaining for TGFβ-R2 was positive in endothelial cells, mesenchymal cells, osteoblasts, and osteocytes. Furthermore, we observed weak TGFβ2 immunostaining in mesenchymal cells and only occasionally in other surrounding tissues and cells.
Sacrifice on the fourth day after fractureWe observed growth of new vessels on the fourth day after the fracture. Periosteal and endosteal callus were present and small islands of chondroblasts and chondrocytes could be identified among abundant osteoblast proliferation. Mesenchymal cells were also numerous. Bone formation seemed more extensive in the group with intramedullary fixation. Subsequently, on the fourth day after fracture, the histological study found that PDGFA staining was positive for endothelial cells and mesenchymal cells, but also for osteoblasts. All cell lines, including endothelial cells, mesenchymal cells, chondroblasts, chondrocytes, osteoblasts and osteocytes presented high positive immunostaining for TGFβ-R2. Staining for TGFβ2 showed no differences with the group sacrificed in the first 24h, with only a slight increase in some mesenchymal cells.
Sacrifice at 1 week after fractureOne week after the fracture, the morphological changes were similar in both groups, although the formation of bone, both in the periosteum and endosteum, was more extensive than at previous times. PDGFA staining was observed in all cell types studied, including endothelial cells, mesenchymal cells, osteoblasts and osteocytes, appearing for the first time in chondroblasts and chondrocytes. Similar to what was observed on the fourth day after fracture, staining for TGFβ-R2 was positive in all cells and more intense in both groups of rats than PDGFA staining. As in previous samples, TGFβ2 staining was generally very weak.
Sacrifice at 15 days after fractureThe morphological changes after 15 days of the fracture did not present any significant changes, although inflammatory infiltrates were not as intense. PDGFA staining did not present differences compared to the group of the seventh day after fracture, with a more intense TGFβ-R2 staining. TGFβ2 staining was equal to previous days.
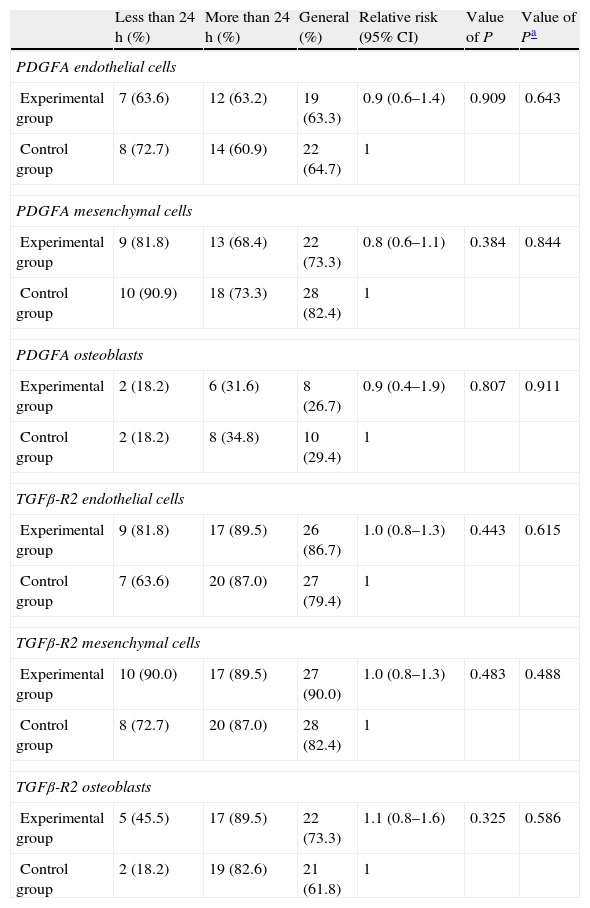
Statistical resultsTable 1 shows the results of the main study variables stratified by time until sacrifice. There was no evidence of statistically significant differences for any variable in the overall sample. No interaction presented statistically significant differences when we examined the differences in the incidence of outcomes of the various variables between the 2 groups according to the time of sacrifice.
Comparison of the incidence of growth factors according to the time variable.
| Less than 24h (%) | More than 24h (%) | General (%) | Relative risk (95% CI) | Value of P | Value of Pa | |
| PDGFA endothelial cells | ||||||
| Experimental group | 7 (63.6) | 12 (63.2) | 19 (63.3) | 0.9 (0.6–1.4) | 0.909 | 0.643 |
| Control group | 8 (72.7) | 14 (60.9) | 22 (64.7) | 1 | ||
| PDGFA mesenchymal cells | ||||||
| Experimental group | 9 (81.8) | 13 (68.4) | 22 (73.3) | 0.8 (0.6–1.1) | 0.384 | 0.844 |
| Control group | 10 (90.9) | 18 (73.3) | 28 (82.4) | 1 | ||
| PDGFA osteoblasts | ||||||
| Experimental group | 2 (18.2) | 6 (31.6) | 8 (26.7) | 0.9 (0.4–1.9) | 0.807 | 0.911 |
| Control group | 2 (18.2) | 8 (34.8) | 10 (29.4) | 1 | ||
| TGFβ-R2 endothelial cells | ||||||
| Experimental group | 9 (81.8) | 17 (89.5) | 26 (86.7) | 1.0 (0.8–1.3) | 0.443 | 0.615 |
| Control group | 7 (63.6) | 20 (87.0) | 27 (79.4) | 1 | ||
| TGFβ-R2 mesenchymal cells | ||||||
| Experimental group | 10 (90.0) | 17 (89.5) | 27 (90.0) | 1.0 (0.8–1.3) | 0.483 | 0.488 |
| Control group | 8 (72.7) | 20 (87.0) | 28 (82.4) | 1 | ||
| TGFβ-R2 osteoblasts | ||||||
| Experimental group | 5 (45.5) | 17 (89.5) | 22 (73.3) | 1.1 (0.8–1.6) | 0.325 | 0.586 |
| Control group | 2 (18.2) | 19 (82.6) | 21 (61.8) | 1 | ||
The ideal fracture in an experimental study should be standard, both in location and in type of fracture, degree of comminution, soft tissue lesion, stability and displacement of fragments.14 The literature describes various fracture models14–17 using different femoral fixation methods and even percutaneous techniques,18 although it is also true that the greatest number of experimental studies are usually performed in tibial fractures.
In our study, we created an experimental model using a simple and reproducible technique which created a fracture manually. Soft tissue lesion with this model was scarce in order to avoid possible tissue hypoperfusion of the thigh compartments that could affect fracture healing. Adequate muscle coverage of the cortical bone was necessary to maximize the osteogenic potential in fractures with intramedullary reaming, perhaps due to the ability of muscles to restore blood flow to the periosteum and the contribution to the differentiation of mesenchymal stem cells into cells with a capacity for osteogenesis in the fracture focus.19
The repair of fractures involves 2 different processes,20 cell proliferation and differentiation, which can be divided into 4 stages based on the characteristics of cells, extracellular matrix and the time of appearance of these cells.21 These stages are: a first stage involving an immediate response to the lesion (from the time of injury until 2 days after the fracture); a second stage involving formation of intramembranous bone (from 2 to 4 days); a third stage where chondrogenesis takes place (from 4 to 10 days), and a fourth stage of endochondral ossification (15 days).
This follows a specific biological response which starts with an acute inflammatory response. After the trauma which caused the fracture, the hematoma forming around the fracture will act as a mold for callus formation. As shown in this study, the acute inflammatory response is initiated within the first 24h and is completed 7 days after the trauma. These inflammatory cells promote angiogenesis.
Mesenchymal cells would participate in a second phase by differentiating into osteogenic cells. Osteochondral formation takes place between the ends of the fracture and the outer area of the periosteum. As fracture healing requires an adequate blood supply, angiogenesis and revascularization take place at the point of fracture. Finally, the cartilaginous callus is resorbed and replaced by a hard, bony callus.22
Nevertheless, this method of healing can be modified by intramedullary reaming.23 It has been shown that intramedullary reaming damages the endosteal blood supply, leading to avascular necrosis of the endosteum. However, reaming also has some positive effects, such as increased blood supply to the periosteum24 and contribution of autograft to the fracture site.25
The present study offered similar results. The earliest event in the process of fracture healing was the presence of an inflammatory infiltrate, including macrophages and proliferation of mesenchymal cells. Both endothelial cells and mesenchymal cells began to express PDGFA, which was released at the fracture site, and induced the proliferation of mesenchymal cells and their differentiation into osteoblasts and chondroblasts. Shortly after the first 24h, osteoblasts were evident in the endosteal surface and periosteal mesenchymal cells began to appear around the edges of the fracture. Then, after 4 days, the formation of intramembranous bone continued with osteoblasts, chondroblasts and chondrocytes.
Resorption is carried out by numerous osteoclasts in bone surfaces adjacent to the necrotic bone, whilst vascular growth becomes evident. Endochondral ossification in the fracture begins after 7 days, and 15 days later a bridge of bone and cartilage is formed on the external surface of the bone. This bridge was found in all cases in rats with intramedullary fixation, but not in the other group, although cartilage formation was observed in both groups.
Even if the fracture has a potential risk of rotational instability, as was the case, we found no cases of nonunion or delayed union in the present study, instead obtaining good consolidation rates compared to other studies.10,26 Many studies have assessed the effect of reaming on intramedullary blood flow and on the fracture callus11,12 and have found highly vascularized areas in the callus despite reaming. We found no differences in callus size or morphology between the reamed and non-reamed fractures.
Previous in vitro and in vivo studies have shown that numerous growth factors and extracellular matrix molecules play an essential role in the repair of fractures. In this study, we analyzed the differences in time patterns of expression of various growth factors when the fracture was reamed and fixed by an intramedullary osteosynthesis, specifically analyzing PDGFA, TGFβ-R2 and TGFβ2.
Some in vitro studies have suggested that TGFβ induces differentiation of periosteal mesenchymal cells into osteoblasts and chondrocytes, but the mechanism by which TGFβ would promote chondrogenesis and osteogenesis is unknown.27 In our study, TGFβ2 staining was positive only occasionally. This may be due to the poor reactivity of the antibody, since the TGFβ-R2 receptor is expressed by all cell types in the bone callus. TGFβ-R2 staining was positive at 24h, mainly in endothelial cells and mesenchymal cells, and after the fourth day it was also positive in chondroblasts, chondrocytes, osteoblasts and osteocytes.
PDGF enhances both the proliferation and osteogenic differentiation of mesenchymal cells.28 In our study, we observed a slightly positive PDGFA staining in endothelial and mesenchymal cells until the seventh day, when we found some positive staining in chondroblasts and osteoblasts.
In summary, our results demonstrate that the repair of rat femoral fractures involves both intramembranous and endochondral repair. These processes are stimulated by various growth factors, which are involved in cell proliferation and differentiation. Nevertheless, even if the presence of intramedullary fixation can facilitate repair of the fracture by stabilizing the bony bridge between the edges of the fracture site, we found no evidence that intramedullary reaming affected the expression of the growth factors studied (PDGFA, TGFβ-R2 and TGFβ2) during the formation of the fracture callus (Figs. 1–5).
Level of evidence ii.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation adhered to the ethical guidelines of the Committee on Responsible Human Experimentation, as well as the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that this work does not reflect any patient data.
Right to privacy and informed consentThe authors declare that this work does not reflect any patient data.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Mingo-Robinet J, Valle-Cruz JA, Ortega-Medina L, Fuentes-Ferrer M, López-Durán Stern L. Efecto del fresado y enclavado endomedular sobre la producción de factores de crecimiento en el callo de fractura de fémur en ratas. Rev Esp Cir Ortop Traumatol. 2013;57:384–390.