Slipped capital femoral epiphysis (SCFE) is characterised by displacement of the capital femoral epiphysis from the metaphysis through the physis. The term is confusing, because the metaphysis moves upward and outward while the epiphysis remains in the acetabulum. The SCFE is considered stable when the child is able to walk with or without crutches, and it is considered unstable when the child cannot walk with or without crutches. Patients with SCFE present with pain in the groin, knee and limp. The current treatment of stable SCFE is in situ stabilisation with a single screw.
La epifisiolisis de la cabeza femoral (ECF) se describe como el desplazamiento de la epífisis (cabeza femoral) respecto a la metáfisis (cuello) a través de la fisis. El término es confuso ya que es la metáfisis la que se desplaza en dirección anterosuperior mientras que la epífisis no se mueve y mantiene su posición respecto al acetábulo. La ECF se considera estable cuando el paciente es capaz de caminar e inestable cuando no puede hacerlo ni siquiera con ayuda de bastones. Los pacientes con ECF son adolescentes que presentan dolor en la región inguinal y/o en la rodilla asociado a cojera. El tratamiento de elección en las estables es la fijación in situ con un tornillo.
Slipped capital femoral epiphysis (SCFE) is the most frequent hip pathology in adolescents. It normally affects overweight patients who come to consultation for lameness and pain in the hip and/or knee region. In SCFE, the metaphysis slips in the anterosuperior direction with respect to the epiphysis due to a lack of restraint in the growth plate.1 On rare occasions, it can produce displacement of the metaphysis in the posterior-inferior direction, when it is called valgus SCFE.2
EpidemiologyThe incidence of SCFE fluctuates from geographical region to region and varies widely, from 0.2 per 100,000 inhabitants in Japan up to 10 per 100,000 inhabitants in the United States.1 Its incidence varies significantly among the different races, being more frequent in groups with a higher body mass index (BMI). In Caucasians, the frequency is lower (about 1 per 100,000), while it reaches 4.5 in inhabitants of the Pacific Islands and 2.2 in individuals that have African antecedents.3,4
Obesity is a factor present in 51–77% of the patients.5,6 Approximately 50% of the patients are found above the 90th percentile for weight5 and approximately 70% are above the 80th percentile.7
It is more frequent in the peripuberal period. In an observational study with over 1600 patients, Loder et al.5 showed that SCFE is produced at 12±1.5 years in girls and at 13±1.7 years in boys. When diagnosed, approximately 80% of the children had an age of 12–15 years for boys and of 10–13 for girls.8
There is also a high rate of bilaterality. Most series reflect an incidence of between 18% and 50%. Recent studies with long-term follow-up described incidences of up to 63% of the cases.9 This variability in reports may depend on the radiographic method used for diagnosis, on race and even on the treatment for the first hip affected. Hurley et al.10 demonstrated that the prevalence of bilateral affectation reached 36% of the patients treated with in situ screw fixation, while it was only 7% in the cases treated orthopaedically with plaster immobilisation.
Clinically, only half of the patients with bilateral SCFE initially showed bilateral affectation. The greatest risk of the initially healthy contralateral hip becoming affected occurs in the first 18 months after the first slip.5
The age of presentation is less in children who initially suffered unilateral SCFE and later developed bilateral SCFE than in those who did not develop bilateral SCFE. Various studies have evaluated the risk factors associated with bilaterality. Herrera Soto et al.11 concluded that skeletal maturity was not a risk factor for bilaterality and recommended contralateral fixation in cases of severe unstable SCFE. Riad et al.12 observed that chronological age is a predictive factor for contralateral SCFE, recommending that all girls under 10 years of age and boys under 12 with unilateral SCFE should consider contralateral fixation. In children older than this, treatment should be evaluated on the basis of personal conditions (endocrine disorders, etc.). These authors also recommended contralateral fixation of the non-affected hip for patients with severe unstable SCFE.
AetiopathogenyThe aetiology is unknown in most patients. Three aetiopathogenic factors have been described: biomechanical, biochemical and genetic factors. These combine with one another, causing a weak physis that finally fails to maintain hip structure. The mechanical and traumatic factors are related to the acute or severe forms. The hormone factors are associated with specific joint morphological features and with an intrinsic degenerative pathology present in the chronic forms. Cases of genetic inheritance related to type II collagen alterations have also been described. The combination of these factors provokes a weakness in the physis area and an increase of the stress forces through the physis.13 The physeal disruption is produced in the proliferative and hypertrophic layer of the growth plate.14 Histopathologically, the number of cells decreases and the characteristic columnar chondrocytes disappears, leading to disorganised growth plate placement. Alterations in the extracellular matrix have also been described.14
Obesity stands out among the biomechanical factors.5–7,15 Most patients present overweight, which tends to increase the shearing forces on the physis. In addition, obesity is associated with less femoral anteversion (normal femoral head anteversion in an adolescent is approximately 10.6°, while it only reaches 0.4° in those that are obese); this lowers the biomechanical efficacy of the hip.15
Another mechanical factor that affects SCFE is greater physeal obliquity. Patients with SCFE present an increase of 8–11° in the vertical physis orientation in the affected hip, while this orientation is 4–5° in the healthy hip.
Likewise, excess acetabular coverage heightens the stress forces on the physis and favours its displacement. Traumatisms can increase the ease of slipping, although this is a controversial factor.
There are also biochemical and hormone factors involved in the development of SCFE, which typically appears during the peripuberal period.5,8 At that time, growth hormone levels increase, causing a rise in the chondrocytic proliferation rate and in the height of the hypertrophic area. These structural changes lower physeal resistance and favour slipping.15 Among the hormonal alterations, hypothyroidism, panhypopituitarism, growth hormone alterations and hypogonadism. Oestrogens reduce physeal height and raise its resistance, while testosterone lower physeal resistance and permit displacement. This would explain its predominance in males, in patients treated with GH and in those with endocrine alterations of the testosterone/oestrogen ratio.
Among genetic factors, we find the alterations in type II collagen. A decrease in type II collagen and proteoglycan expression has been confirmed, which affects the amount, distribution and organisation of the growth plate components. Histology and electron microscopy show deficiencies and anomalies in the support network of physeal collagen and proteoglycan. There is also a decrease in cell number compared to matrix amount, while the chondrocytes are smaller than those in the control population. These changes may constitute a cause of or be associated with SCFE.16
ClassificationThe traditional classification of SCFE includes pre-slip and acute, chronic and acute-on-chronic slip. This classification is based on the clinical history, symptom duration, physical examination and radiographs.
There are several stages:
- 1
Pre-slip. Clinically, this stage is characterised by weakness, limping and thigh or knee pain that increases with sports activity. The internal rotation of the hip and the support of the affected hip decrease. Radiographically, there is osteopenia in the proximal femur or the hemipelvis, rarefaction of the metaphysis, and thickening and irregularity in the proximal femoral physis.
- 2
Acute slip (10–15%). This is characterised by an abrupt slip through the physis and clinical development of less than 3 weeks. The patients come to consultation due to acute pain in the hip, the medial face of the thigh or knee affected. The pain can be so intense that it makes weight loading impossible. Clinically, it presents with a shortening and external rotation of the member, as well as restricted mobility, especially in internal rotation. There may be a low-force trauma antecedent. In 90% of patients with acute SCFE, there is a history of 1–3 months duration, with moderate prodromal symptoms (hip, thigh or knee pain, limping) before the acute episode.17,18
- 3
Chronic slip (85%). This is the most common type of SCFE; it is characterised by the presence of over 3 weeks of symptoms, which is why delayed diagnosis is frequent. Patients feel intermittent pain in the inguinal region, in the medial side of the thigh, the calf and the knee associated with lameness. In half of the cases, the initial symptom is knee pain19 with antalgic gait, loss of internal hip rotation, abduction and flexion.20 In this stage, the Drehmann sign appears: as the hip is flexed, the limb spontaneously rotates externally and abducts.
- 4
Acute-on-chronic SCFE. These are patients with chronic symptoms whose lameness and pain become aggravated abruptly, preventing walking and standing.
The traditional classification is based on the memory of the child and parents and can be imprecise. For that reason, other systems based on physeal stability arose.
The clinical classification is based on walking capacity and physeal stability. A case is considered stable SCFE when patients are capable of walking, with or without crutches, and unstable SCFE when they cannot walk, with or without crutches. This classification has prognostic value for the development of osteonecrosis, with up to 50% of osteonecrosis incidence in unstable SCFE. This is due to the vascular damage caused in the initial displacement, in comparison with stable SCFE cases where necrosis is non-existent.
There is also a classification related with the presence or absence of joint effusion in the echography. The case is called unstable if there is joint effusion and stable when effusion is absent.
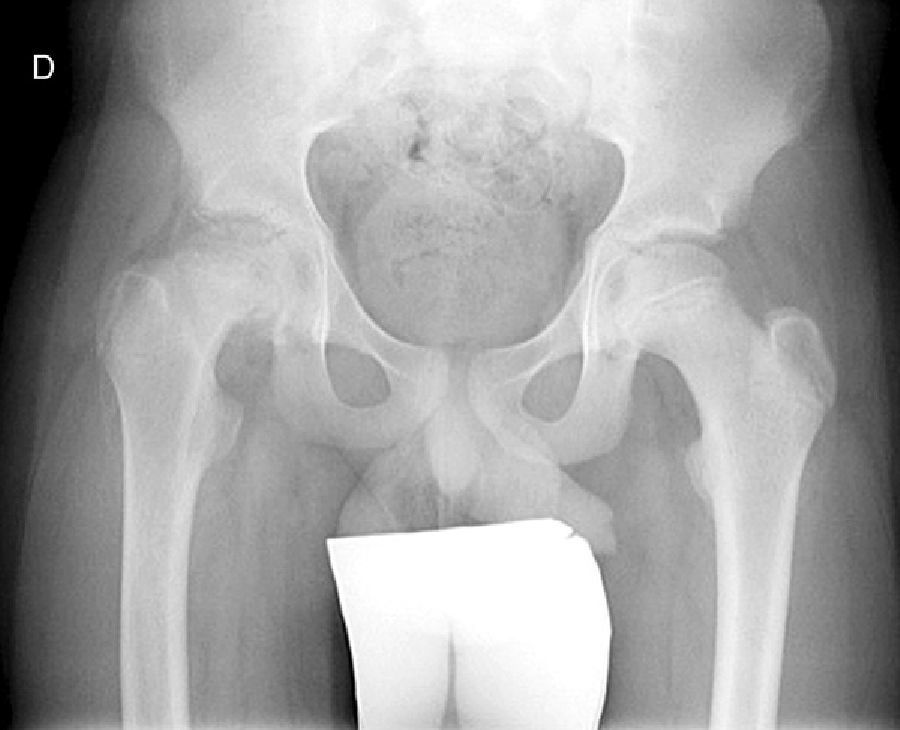
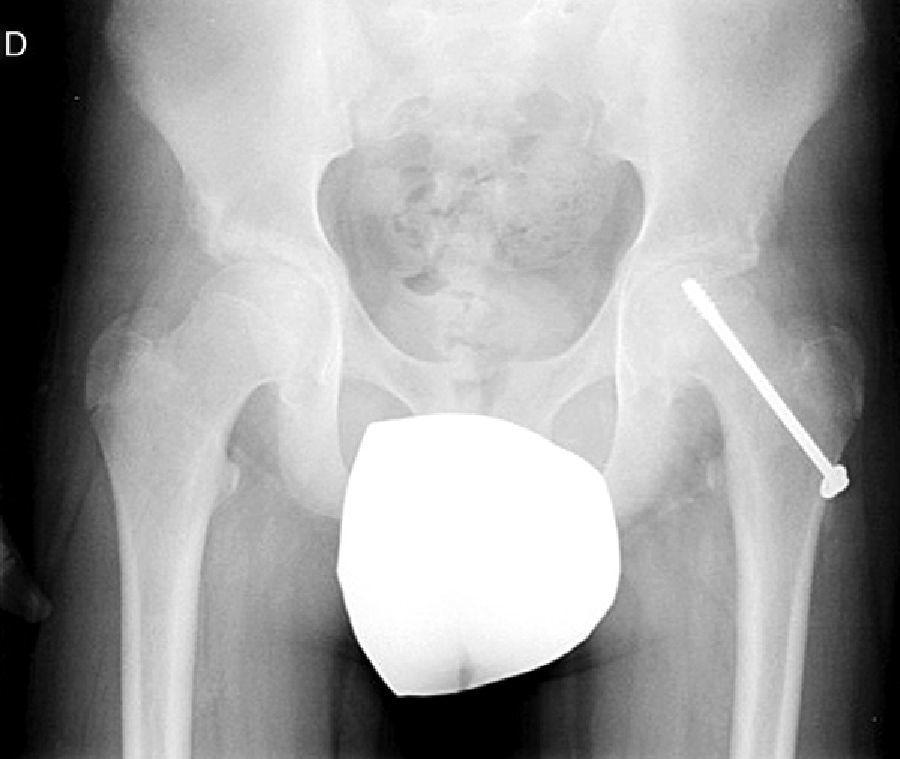
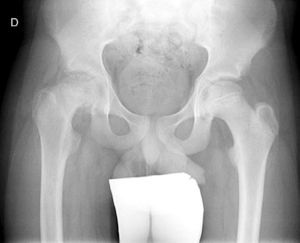
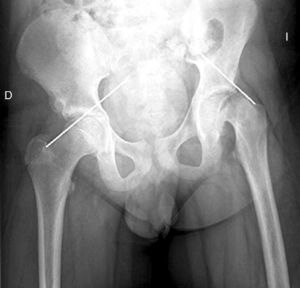
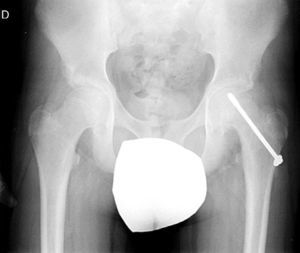
Diagnosis by imagingAxial and anteroposterior radiographs of both hips make it possible to confirm the diagnosis. It is important to take radiographs of both hips because of the high incidence of bilaterality. In the axial and anteroposterior radiographs of both hips, we can see an anterosuperior slip of the proximal metaphysis of the femur (femoral neck) with respect to the epiphysis (femoral head). The name blanch sign of Steel is given to the double radiographic density created by the epiphysis, which slips posteriorly and becomes superimposed on the medial section of the metaphysis (Fig. 1). The Klein line is a line drawn in the anterosuperior segment of the femoral neck in the anteroposterior radiograph and which cuts the epiphysis. In SCFE cases, the femoral epiphysis remains below this line (Fig. 2).
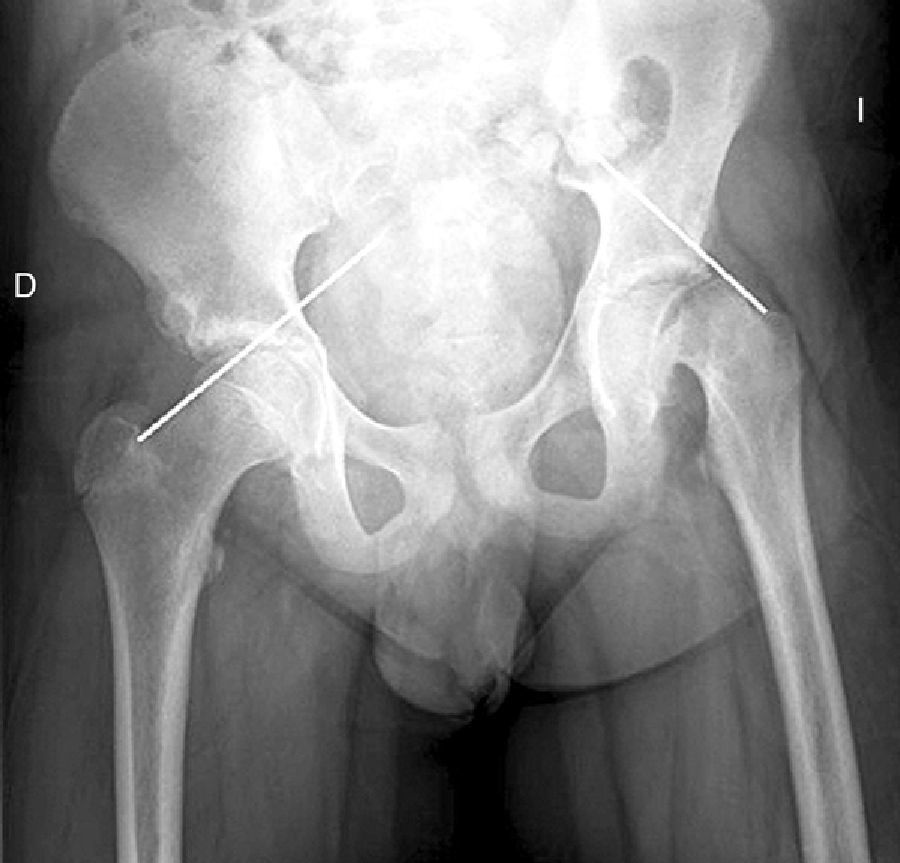
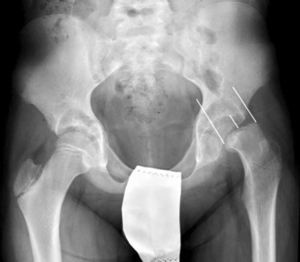
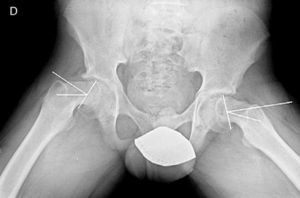
There are 2 ways to measure SCFE severity. The displacement of the epiphysis with respect to the metaphysis is the percent of femoral head displacement with respect to the neck. This slippage can be slight (<33%), moderate (33–50%) and severe (>50%) (Fig. 3). Although this method is commonly used, it has significant inter-/intra-observer variability; it is also influenced by patient position. Southwick described the epiphyseal–diaphyseal angle in the anteroposterior and axial radiograph of both hips. A line is drawn through the physeal surface of the epiphysis and a right angle is drawn from that line. Next, a line is drawn parallel to the femur diaphysis. The angle formed by these last 2 lines is the measurement of posterior slip. The angle obtained in the healthy hip is subtracted from the angle in the SCFE, yielding the degree of slip, which is classified as mild (<30%), moderate (30–50%) and severe (>50%) (Fig. 4).
We can also see changes in the metaphyseal region (remodelling, reabsorption of the anterosuperior section of the metaphysis, new bone formation in the posterior-inferior area of the metaphysis, etc.). Loder21 assessed the correlation between the radiographic changes observed in the metaphysis, SCFE severity, symptom duration and other demographic parameters. They observed that the metaphyseal changes occurred in the upper area (76%), lower (56%), anterior (80%) and posterior area (84%). These changes were more evident in older patients, in those with greater BMI and when the symptoms had a longer duration.
TreatmentThe treatment objectives are preventing slip progression and avoiding possible complications. The seriousness of the SCFE is related with symptom duration and early treatment is indicated to stop slip progression.
Stable epiphysiolysisInitial treatment for patients with stable SCFE is performed setting with screws or needles, using bone graft or by luxation and osteoplasty of femoral neck remodelling.
Screw fixation on a radiolucent traction table is the most accepted treatment method.22,23 Among its advantages are the percutaneous placement, high success rate and low complication rate. There are discrepancies in the literature about the use of 1 or of 2 screws for stabilisation. Karol et al.,24 using lambs, showed that the stability obtained with 2 screws increased the rigidity of the synthesis by 33% compared with the simple screw. Likewise, they concluded that single-screw stability was enough to prevent the deformity from progressing. The ideal screw position is in the centre of the epiphysis, perpendicular to the physis in the anteroposterior and lateral projections. Miyanji et al.,25 in a study using pigs, concluded that there were no statistically significant differences between using partial or full thread screws and that full thread screws provided no biomechanical benefits. Other authors26 have recently indicated that using full thread or 32mm thread screws yielded greater mechanical stability at the level of the femoral neck, recommending their use in SCFE.
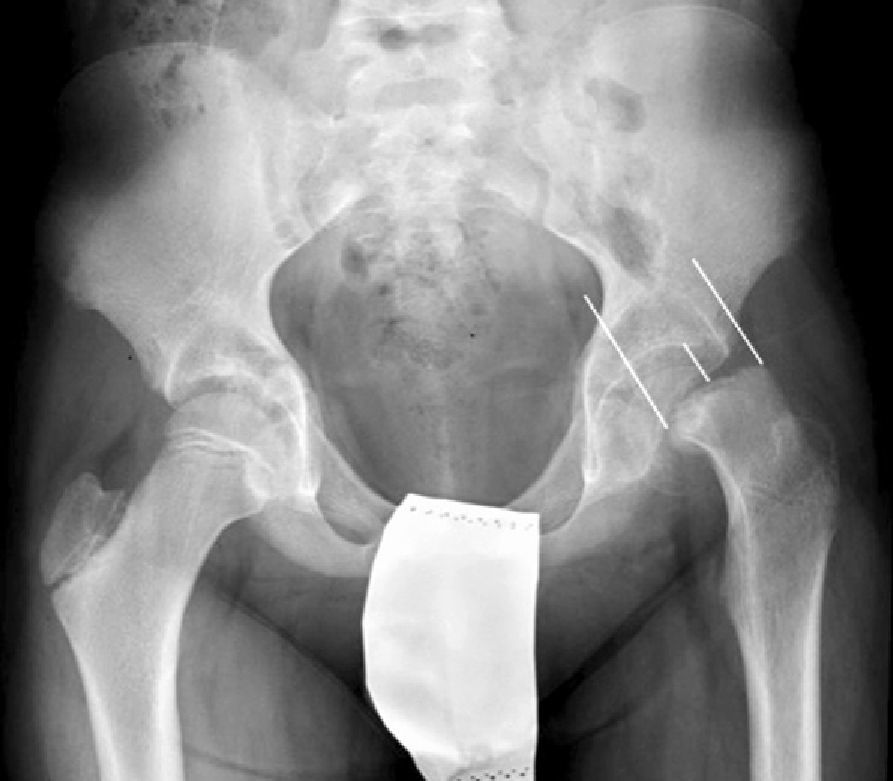
To avoid inserting the screw in the joint voluntarily, the “approach-withdraw phenomenon” is performed.27,28 It consists of rotating the limb from the maximum internal rotation to the maximum external rotation and checking in the fluoroscopy images that the screw does not protrude. During the first part of the rotation, the screw tip seems to move closer to the subchondral bone (approach) and then seems to move away from it (withdrawal). The moment of change indicates the true screw position (Fig. 5).
Epiphysiodesis with bone graft is used less than osteosynthesis with screws. This second technique consists of introducing multiple corticocancellous strips of bone graft through the physeal neck, reaching the epiphysis until epiphysiodesis is achieved. Adamczyk et al.29 published their results after 50 years of experience; they obtained high progressive slip rates (13% in unstable SCFE cases and 6% in stable SCFE), greater blood loss, greater anaesthesia duration and larger surgical scars.
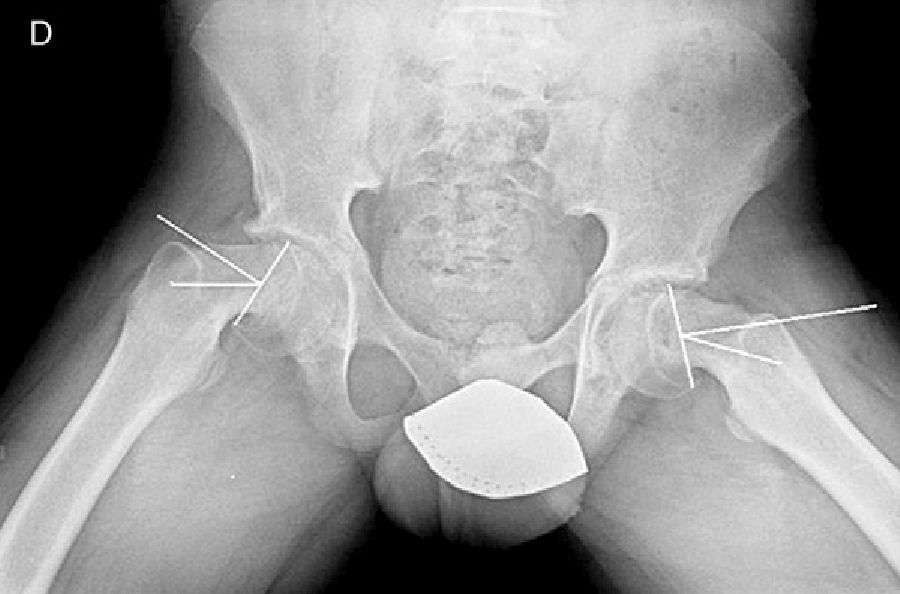
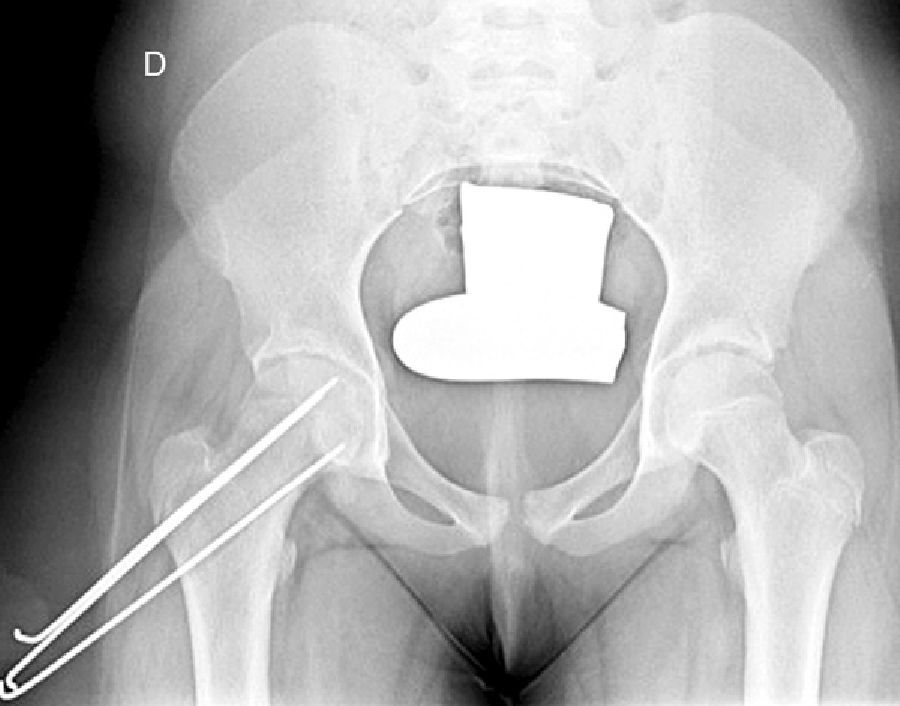
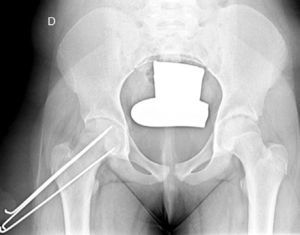
In situ multiple pin fixation (Fig. 7) is an alternative to screw fixation. O’Brien and Fahey30 observed that a remodelling potential existed in the proximal femur section in SCFE patients using multiple pins. This potential is of interest in the youngest patients, given that it improves mobility and reduces the discrepancy in limb length associated with epiphysiodesis. Among the disadvantages of this technique are inadequate fixation, lateral epiphyseal vessel damage, involuntary protrusion and chondrolysis from pin penetration. Seller et al.31 assessed prophylactic stabilisation with Kirschner wires (pins) for unstable SCFE and the consequent disruption of growth and potential for femoral head remodelling; they concluded that fixation with pins produces less physeal growth alteration and remodelling in the non-affected contralateral hip. Sibinski et al.,32 in a retrospective study with 61 hips treated using Kirschner wires, obtained good results without finding avascular necrosis. For that reason, they recommended this treatment in young patients, skeletally immature and with potential for femoral head growth. Other authors33 recommend bilateral synthesis with Kirschner wires. In unstable SCFE cases, Parsch et al.34 described treatment through open reduction, evacuation of the haematoma after ultrasonograph identification and posterior stabilisation using Kirschner wires. They obtained good results and low femoral head avascular necrosis (4.7%) (Fig. 6).
Ever since Ganz et al.35 published good results using joint luxation in adult patients with femoroacetabular impingement, interest in open SCFE treatment has grown. Surgical luxation with remodelling osteoplasty of the femoral neck makes it possible to correct the joint deformity, femoral retroversion and anterior acetabular impingement.36 Consequently, head-neck union morphology is improved, returning the concave contour of the femoral neck. Beck et al.37 assessed 19 patients with a follow-up of at least 4 years, finding clinical improvement in 13 hips.
Many osteotomies have been described for late treatment of femoral retroversion.
Intertrochanteric osteotomy, praised by Southwick,38 corrects the retroversion deformity, improves mobility and reduces osteoarthritis incidence. The osteotomy performed most is that described by Imhäuser,39 in which flexion, internal rotation and abduction of the distal fragment of the femur is performed; however, it is a difficult technique that is not free from complications. That explains why it has been modified on numerous occasions, the Hungria–Kramer–Sugioka modification40 being the most well known. Cuneiform osteotomy, proposed by Dunn,41 is indicated in patients with moderate or severe (>30%) SCFE. In this technique, through external approach, an anterior based metaphyseal–diaphyseal wedge is extracted, which is stabilised using 3 pins; however, it is associated with a high rate of complications, especially osteonecrosis and chondrolysis.
New surgical techniques and diagnostic advances make it possible to recommend urgent surgery instead of delayed reconstructive surgery, although we lack long-term comparative results at present.42
Unstable epiphysiolysisThe treatment is similar to that presented for stable SCFE, although its management is more controversial among the different authors (moment for the surgery, urgent or elective; need for reduction; evacuation of the intra-articular effusion; and so forth).
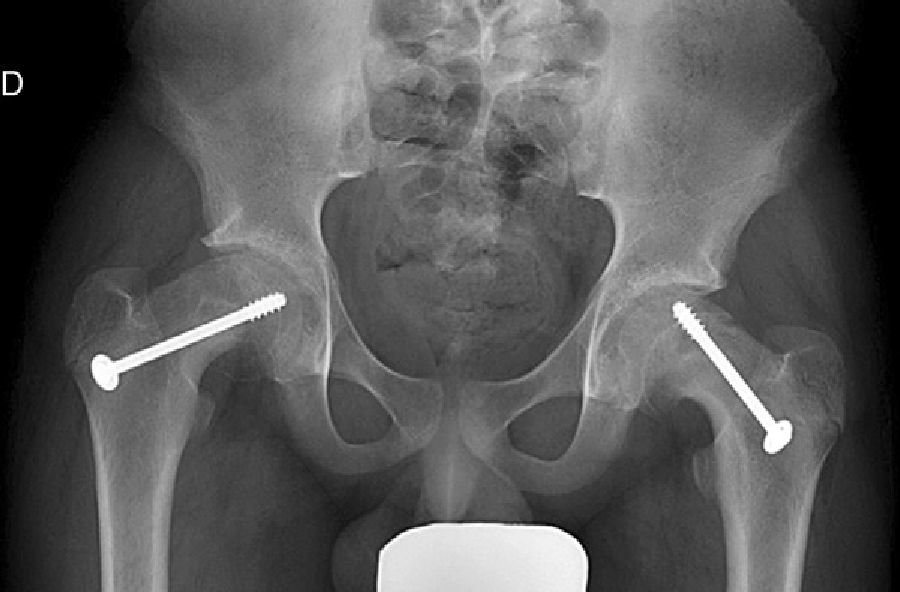
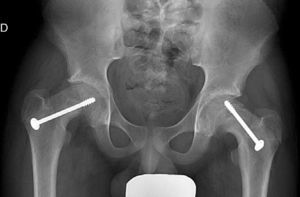
Mooney et al.43 carried out a survey among POSNA membership to assess SCFE management. Among the respondents, 57% recommended urgent treatment (less than 8h), 31% chose preferential treatment and 12% recommended elective reduction. Incidental reduction was the method preferred by 84%, while 11.8% opted for formal (complete) manipulative reduction. Capsular decompression was rejected by 64.6% of those surveyed as part of unstable SCFE treatment and a screw was used by 57.4%, while double-screw fixation was the best for 40.3%. The risk of a contralateral SCFE in a patient with unilateral SCFE is 2.335 times greater than the risk of an initial SCFE.44 Some authors therefore suggest prophylactic contralateral fixation, taking into consideration other parameters (age, sex, endocrine status, preferences of the patient-family, etc.). Despite the scientific evidence for prophylactic contralateral fixation, most surgeons who responded to the survey rejected this treatment43 (Figs. 7 and 8).
A survey among the members of the European Paediatric Orthopaedic Society (EPOS)45 on proximal femoral epiphysiolysis (PFE) management has been published. The treatment preferred by most surgeons was in situ screw fixation. Open reduction and screw fixation was used in 5–10% of the PFE cases with severe slipping. Most of the surgeons trusted the load safety in stable PFE cases, allowing total load without any evidence of complications. Likewise, they stated that there was controversy about other aspects that needed multi-centre studies for a consensus.
In 91 patients with unstable SCFE, Peterson et al.46 observed that closed reduction in the first 24h was associated with 7% of necrosis and, when the reduction was delayed more than 24h, the rate rose to 20%. These results support urgent complete manipulative reduction without preoperative traction. Gonzalez Moran et al.47 later suggested that the drop in osteonecrosis observed in their work could be related to the traction 2 weeks prior to surgery.
Beck et al.48 assessed the effects of increasing intra-articular pressure on the femoral head. To do so, they injected intra-articular saline solution in 11 patients, who then received a surgical luxation to treat a femoroacetabular impingement. The researchers later assessed the blood flow in the femoral head using a laser Doppler flowmetry; after intracapsular injection of 20ml of saline solution, the blood flow showed a loss of signal and joint aspiration improved perfusion. They concluded that urgent decompression of intracapsular haematoma could improve femoral head perfusion.
Kibiloski et al.49 studied the effect of early load in SCFE treatment and recommended avoiding load in the immediate postoperative period, regardless of the synthesis method used. As for the use of single or double screws, controversy still exists about the most appropriate method. Two-screw fixation can increase the risk of necrosis and chondrolysis, without providing greater stability than that from a single screw.24 Kishan et al.,50 in an experimental study using pigs, compared using 1 or 2 screws, as well as different screw placements. They concluded that stability is greater with 2 screws in unstable SCFE cases with respect to using a single screw, although greater risk of involuntary articular penetration is associated. Likewise, they emphasised that different 3-D placement of the screws had no influence in the evolution.
Recent studies (such as those by Zide et al.51 and Popejoy et al.52) have attempted to estimate the risk of contralateral hip slipping and the need for prophylactic fixation. They are based on the application of the Oxford scale modification described by Stasikelis et al.,53 which assesses radiologically the skeletal maturity in 5 areas: triradiate cartilage, iliac, proximal femoral epiphysis, greater trochanter and lesser trochanter. The patients with values between 0 and 2 (more skeletally immature) had a greater risk of contralateral hip displacement. Of the patients who presented an open triradiate, 89% developed contralateral hip slip. This scale provides good intra-interobserver correlation and makes it easier to decide on prophylactic contralateral hip fixation when there is open triradiate cartilage.
ComplicationsOsteonecrosisWithout a doubt, this is the most severe complication and is usually associated with unstable SCFE. Among its causes figure sudden reduction attempts, pin placement in the posterior-superior quadrant of the epiphysis and cuneiform osteotomies. Tokmakova et al.54 described osteonecrosis in 21 of 36 hips (58%) in patients with unstable SCFE and zero cases in 204 hips with stable SCFE. They thus concluded that osteonecrosis is associated with unstable SCFE, intense slipping and more pins used in synthesis.
We should suspect osteonecrosis in patients with pain in the groin, thigh or knee. Upon exploration, there is a loss of mobility, especially in internal rotation, and hip pain. Radiographically, a femoral head collapse can be seen. Osteonecrosis treatment includes relieving weight load with a walking stick, physiotherapy to increase mobility, analgesics and (if applicable) implant removal.
ChondrolysisThe aetiology of chondrolysis may be secondary to an involuntary penetration of the implants (pins or screws) in the femoral head, although the existence of some autoimmune factor might contribute to the chondrolysis. Within the factors associated to chondrolysis, significant ones are involuntary pin penetration, treatment with plaster hip spica, intertrochanteric osteotomy and advanced SCFE. Chondrolysis incidence varies from 1.8% to 55%, depending on the series, with an overall incidence of 7%.55
Patients usually consult because of lameness and pain in the groin region, thigh or knee. Clinically, they have decreased mobility range, which affects internal rotation above all. Simple radiography confirms the diagnosis, with a reduction (>50%) of the joint space with respect to the healthy side or, when the affectation is bilateral, a joint space smaller than 3mm. Patients that develop chondrolysis have a better long-term prognosis than those that develop osteonecrosis.56
Femoroacetabular impingementRab et al.4 described the concept of “impingement” in their study on hip mobility in SCFE. It is characterised by deformity in the femoral neck and head region from physeal slipping and from femoral head retroversion. This anterior deformity produces a cam effect that damages the joint cartilage and the labrum progressively, provoking intense pain with internal flexion and rotation of the hip.35,57 Many authors have described the changes produced in articular cartilage in patients with SCFE. Beck et al.37 established a classification of damage in the articular cartilage and labrum, which range from chondromalacia to the chondral full-thickness defects. Sink et al.58 studied 39 hips, with stable symptomatic SCFE, through surgical luxation and observed changes in the labrum in 34 hips and changes in the cartilage in 32; they concluded that chondromalacia and articular cartilage damage exist in hips affected by SCFE. Articular luxation makes it possible to diagnose this damage. The treatment for femoroacetabular impingement treatment is remodelling osteoplasty of the femoral neck,37 which consists of surgical luxation of the femoral head and resection of the bony metaphyseal fragment that provokes the impingement.
Natural evolution without treatmentThe risk of progression is difficult to determine and natural evolution is unpredictable. In Sweden, Orderberg et al.59 studied the evolution of patients between 20 and 60 years old after SCFE diagnosis, observing that there were hardly any social or work repercussions but that risk of deformity progression existed in patients with open physis. Carney et al.60 later assessed the results of 36 hips treated symptomatically with bed rest, walking sticks or without any treatment. After the initial diagnosis, 6 hips (17%) presented additional slip; in 5 of these the slip was severe. Eleven hips had acute-over-chronic SCFE and all of them progressed towards a significant slip that required surgical stabilisation.
With respect to the risk of developing osteoarthritis, the severity of non-treated SCFE correlates with long-term prognosis. In the studies of Oram61 and Carney and Weinstein,62 hips with moderate or severe SCFE had a greater risk of developing functionally limiting osteoarthritis. Hips with stable SCFE showed a positive evolution, as long as the slip was minimal.
Natural evolution with treatmentMost patients who present mild-moderate SCFE did not develop necrosis or chondrolysis and the long-term results with in situ fixation are usually good and excellent. Patients with severe SCFE and those presenting osteonecrosis develop osteoarthritis at early ages. The osteonecrosis that appears in patients with SCFE differs from that which presents in other paediatric hip problems, in that SCFE occurs at an age in which most of the acetabular development is complete and an adaptation to the femoral head deformity is impossible.63
Level of evidenceLevel of evidence V.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments were performed in humans or animals for this research.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Martínez-Álvarez S, et al. Epifisiolisis de la cabeza femoral. Rev Esp Cir Ortop Traumatol. 2012;56:506–14.