Orthopedic and trauma surgical procedures (OTS) can lead to significant blood losses and acute postoperative anemia, which in many cases requires allogeneic blood transfusions (ABT). The clinical, economic and logistical disadvantages of ABT have promoted the development of multidisciplinary and multimodal programs generically known as Patient Blood Management (PBM) programs, which have as their objective to reduce or eliminate the need for ABT and improve clinical outcomes. These programs are supported by the implementation of four groups of perioperative measures: (1) use of restrictive transfusion criteria; (2) stimulation of erythropoiesis; (3) reduction of bleeding; and (4) autologous blood transfusion. In this article, a review is presented of the effectiveness, safety and recommendations of applicable strategies in OTS, as well as the barriers and requirements to the development and implementation of PBM programs in this surgical specialty.
Los procedimientos de cirugía ortopédica y traumatológica (COT) pueden ocasionar pérdidas significativas de sangre y anemia postoperatoria aguda, que en muchos casos requiere transfusión de sangre alogénica (TSA). Las desventajas clínicas, económicas y logísticas de la TSA han promovido el desarrollo de programas multidisciplinares y multimodales, genéricamente conocidos como programas de Patient Blood Management (PBM), cuyo objetivo es el de reducir o eliminar la necesidad de TSA y mejorar el resultado clínico. Estos programas se apoyan en la aplicación de cuatro grupos de medidas perioperatorias: (1) uso de criterios restrictivos de transfusión; (2) estimulación de la eritropoyesis; (3) reducción del sangrado; y (4) transfusión de sangre autóloga. En este artículo, revisamos la eficacia, seguridad y recomendaciones de las estrategias aplicables en COT, así como los condicionantes para el desarrollo e implementación de los programas de PBM en esta especialidad.
Orthopedic and traumatology surgical procedures (OTS), such as knee (TKP) and hip arthroplasties (THP), instrumented spinal operations or repairs of hip fractures can produce, on average, up to 2L of blood loss resulting in acute post-operative anemia, which in many cases requires allogenic blood transfusion (ABT).1–3 However, there are several factors which suggest that it might be advisable to transfuse less: human blood is a limited resource; the costs of the preparation, distribution and administration of blood components are high; transfusion has adverse effects such as: acute hemolytic reactions due to identification errors, the transmission of infectious diseases, acute lung damage, circulatory overload, immunomodulation, amongst others; and the different provisions of current legislation.4
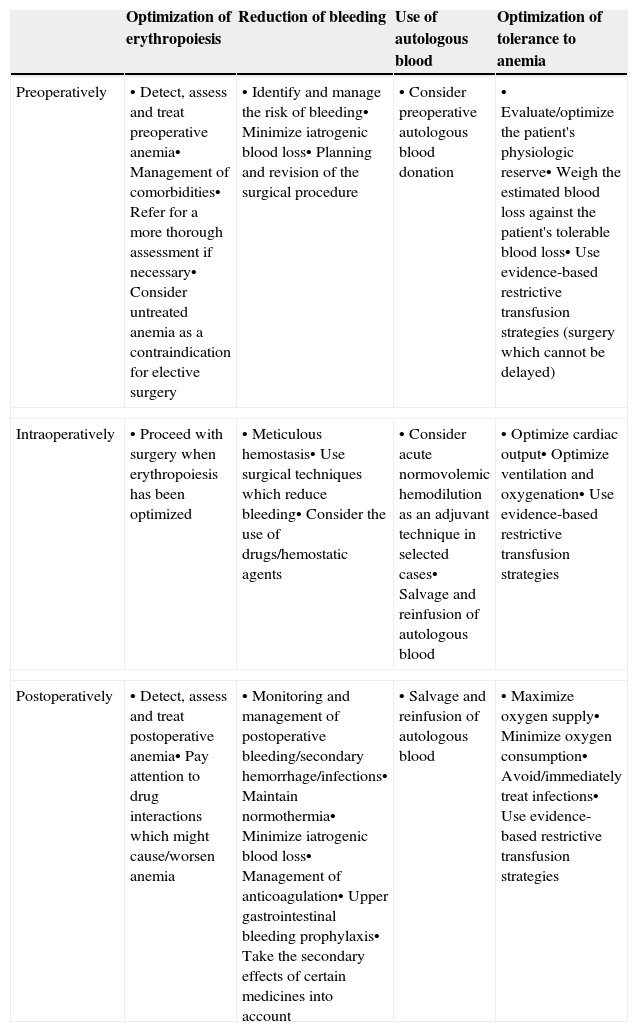
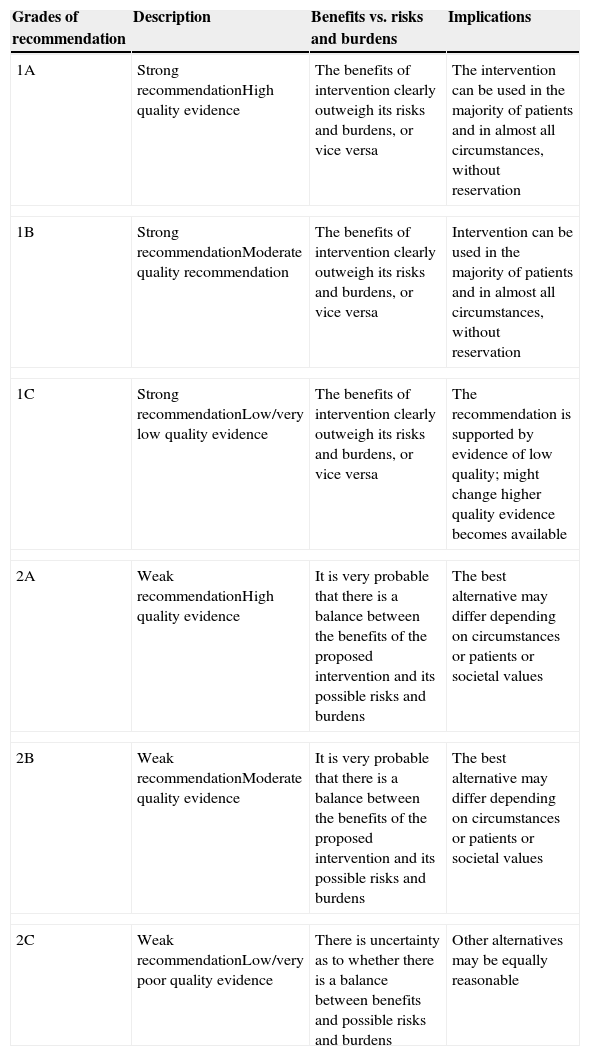
These clinical, financial and logistical disadvantages of ABT have prompted recommendations for its restricted use, especially in order to avoid unnecessary transfusion, and for the development of multidisciplinary and multimodal programs for the integral management of these patients which are known generically as Patient Blood Management (PBM) programs, whose ultimate objective is to reduce or eliminate the need for ABT and improve clinical results. These programs are supported by the application of four groups of perioperative measures: (1) optimization of tolerance of normovolemic anemia to enable the use of restrictive transfusion criteria; (2) erythropoiesis stimulation; (3) correction of hemostasis and reduction of bleeding; and (4) the use of autologous blood5 (Table 1). The efficacy and safety of, and recommendations for most of these measures are included in the update of the Seville Consensus Document on Alternatives to Transfusion (DS 2013)6 and the European Guideline to the Management of Severe Perioperative Bleeding (ESA Guideline),7 drawn up using the GRADE methodology (Grading of Recommendations Assessment, Development and Evaluation). GRADE makes graded recommendations which can be strong [1] or weak [2], positive or negative, and supported by high [A], moderate [B] or low/very low quality [C] evidence (Table 2).6 When a strong recommendation is made, the terminology “we recommend…” is used, when a weak recommendation is made, a less conclusive statement is used such as “we suggest …”.
Summary of peri-operative strategies to address the implementation of a patient blood (amendment to Ref. 5).
| Optimization of erythropoiesis | Reduction of bleeding | Use of autologous blood | Optimization of tolerance to anemia | |
|---|---|---|---|---|
| Preoperatively | • Detect, assess and treat preoperative anemia• Management of comorbidities• Refer for a more thorough assessment if necessary• Consider untreated anemia as a contraindication for elective surgery | • Identify and manage the risk of bleeding• Minimize iatrogenic blood loss• Planning and revision of the surgical procedure | • Consider preoperative autologous blood donation | • Evaluate/optimize the patient's physiologic reserve• Weigh the estimated blood loss against the patient's tolerable blood loss• Use evidence-based restrictive transfusion strategies (surgery which cannot be delayed) |
| Intraoperatively | • Proceed with surgery when erythropoiesis has been optimized | • Meticulous hemostasis• Use surgical techniques which reduce bleeding• Consider the use of drugs/hemostatic agents | • Consider acute normovolemic hemodilution as an adjuvant technique in selected cases• Salvage and reinfusion of autologous blood | • Optimize cardiac output• Optimize ventilation and oxygenation• Use evidence-based restrictive transfusion strategies |
| Postoperatively | • Detect, assess and treat postoperative anemia• Pay attention to drug interactions which might cause/worsen anemia | • Monitoring and management of postoperative bleeding/secondary hemorrhage/infections• Maintain normothermia• Minimize iatrogenic blood loss• Management of anticoagulation• Upper gastrointestinal bleeding prophylaxis• Take the secondary effects of certain medicines into account | • Salvage and reinfusion of autologous blood | • Maximize oxygen supply• Minimize oxygen consumption• Avoid/immediately treat infections• Use evidence-based restrictive transfusion strategies |
Grades of recommendation according to GRADE methodology.
| Grades of recommendation | Description | Benefits vs. risks and burdens | Implications |
|---|---|---|---|
| 1A | Strong recommendationHigh quality evidence | The benefits of intervention clearly outweigh its risks and burdens, or vice versa | The intervention can be used in the majority of patients and in almost all circumstances, without reservation |
| 1B | Strong recommendationModerate quality recommendation | The benefits of intervention clearly outweigh its risks and burdens, or vice versa | Intervention can be used in the majority of patients and in almost all circumstances, without reservation |
| 1C | Strong recommendationLow/very low quality evidence | The benefits of intervention clearly outweigh its risks and burdens, or vice versa | The recommendation is supported by evidence of low quality; might change higher quality evidence becomes available |
| 2A | Weak recommendationHigh quality evidence | It is very probable that there is a balance between the benefits of the proposed intervention and its possible risks and burdens | The best alternative may differ depending on circumstances or patients or societal values |
| 2B | Weak recommendationModerate quality evidence | It is very probable that there is a balance between the benefits of the proposed intervention and its possible risks and burdens | The best alternative may differ depending on circumstances or patients or societal values |
| 2C | Weak recommendationLow/very poor quality evidence | There is uncertainty as to whether there is a balance between benefits and possible risks and burdens | Other alternatives may be equally reasonable |
Establishing clearly defined transfusion criteria which can be applied uniformly during the entire hospital stay is the cornerstone of any PBM program (Table 3). In recent years, various clinical practice guidelines have been recommending the use of “restrictive” transfusion criteria rather than “liberal” criteria. Amongst these, the AABB (formerly the American Association of Blood Banks)8 makes the following recommendations:
- •
Recommendation 1: The AABB recommends adhering to a restrictive transfusion strategy (7–8g/dL) in hospitalized, stable patients (GRADE 1A).
- •
Recommendation 2: The AABB suggests adhering to a restrictive strategy in hospitalized patients with preexisting cardiovascular disease and considering transfusion for patients with symptoms or a hemoglobin level of 8g/dL or less (GRADE 2B).
- •
Recommendation 3: The AABB cannot recommend for or against a liberal or a restrictive threshold for hospitalized, hemodynamically stable patients with acute coronary syndrome.
- •
Recommendation 4: The AABB suggests that transfusion decisions be influenced by symptoms as well as Hb concentration (GRADE 2C).
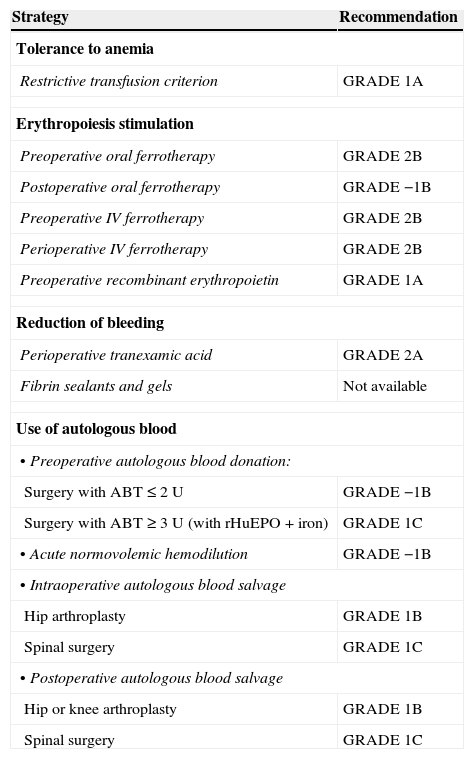
Grades of recommendation (GRADE) of the different strategies included in a Patient Blood Management program.6,7
| Strategy | Recommendation |
|---|---|
| Tolerance to anemia | |
| Restrictive transfusion criterion | GRADE 1A |
| Erythropoiesis stimulation | |
| Preoperative oral ferrotherapy | GRADE 2B |
| Postoperative oral ferrotherapy | GRADE −1B |
| Preoperative IV ferrotherapy | GRADE 2B |
| Perioperative IV ferrotherapy | GRADE 2B |
| Preoperative recombinant erythropoietin | GRADE 1A |
| Reduction of bleeding | |
| Perioperative tranexamic acid | GRADE 2A |
| Fibrin sealants and gels | Not available |
| Use of autologous blood | |
| • Preoperative autologous blood donation: | |
| Surgery with ABT≤2 U | GRADE −1B |
| Surgery with ABT≥3 U (with rHuEPO+iron) | GRADE 1C |
| • Acute normovolemic hemodilution | GRADE −1B |
| • Intraoperative autologous blood salvage | |
| Hip arthroplasty | GRADE 1B |
| Spinal surgery | GRADE 1C |
| • Postoperative autologous blood salvage | |
| Hip or knee arthroplasty | GRADE 1B |
| Spinal surgery | GRADE 1C |
ABT, allogenic blood transfusion; rHuEPO, recombinant human erythropoietin.
These recommendations help us to decide “when to consider the need to transfuse” whereas the recommendations of the DS 2013,6 which complement those of the AABB, provide us with guidelines as to “how much to transfuse”:
- •
Recommendation 1: In critically ill, polytraumatized and/or surgical patients, without cardiac and/or central nervous dysfunction, we recommend transfusion of packed red cells maintaining hemoglobin concentrations between 7 and 9g/dL, to reduce the transfusion rate (GRADE 1A).
- •
Recommendation 2: In critically ill, polytraumatized and/or surgical patients, with cardiac and/or central nervous system dysfunction, we recommend transfusion of packed red cells maintaining hemoglobin concentrations between 8 and 10g/dL, to reduce the transfusion rate (GRADE 1A).
These recommendations fully apply to OTS patients (Table 3), and clinical trials to date have demonstrated that the use of restrictive criteria (generally Hb <8g/dL) reduces the requirement for ABT and the incidence of postoperative infection (relative risk 0.70 [95% CI, 0.54–0.91]), with no significant differences in terms of morbidity, mortality and functional status of the patients, compared to using liberal criteria (generally Hb <10g/dL).9
Therefore, it has become common practice to use restrictive criteria in our OTS departments. Already in 2004, a survey to which 59 hospitals responded revealed that transfusions are usually given at <8g/dL (58%) or <9g/dL (25%), although certain clinical circumstances (e.g., age, cardiac, respiratory, renal or liver disease, bleeding or previous Hb) could affect the need for a transfusion. However most traumatologists still consider that the “minimum transfusion” should be two units of packed red blood cells10; this idea is erroneous and must be corrected since, whenever possible, units of packed red blood cells should be administered one at a time, and the patient should be reassessed clinically and/or tested between each unit.
In any case, it should be remembered that implementing restrictive transfusion criteria, although effective in reducing both the number of transfused patients and the volume of component administered (and the costs of this treatment) (Table 4), is not always sufficient and other measures should be implemented. It has been demonstrated recently that, despite the application of restrictive criteria, the outcome for critically ill patients who are transfused is poorer than for those who are not.11
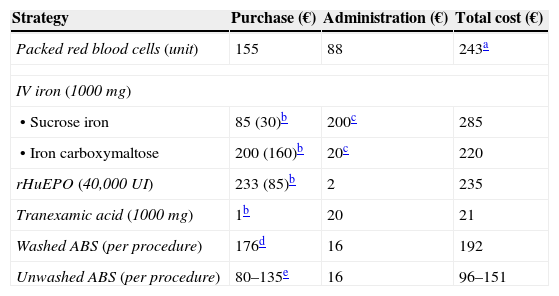
Estimated costs of some strategies included in a Patient Blood Management program.15,16,42,53
| Strategy | Purchase (€) | Administration (€) | Total cost (€) |
|---|---|---|---|
| Packed red blood cells (unit) | 155 | 88 | 243a |
| IV iron (1000 mg) | |||
| • Sucrose iron | 85 (30)b | 200c | 285 |
| • Iron carboxymaltose | 200 (160)b | 20c | 220 |
| rHuEPO (40,000UI) | 233 (85)b | 2 | 235 |
| Tranexamic acid (1000mg) | 1b | 20 | 21 |
| Washed ABS (per procedure) | 176d | 16 | 192 |
| Unwashed ABS (per procedure) | 80–135e | 16 | 96–151 |
Does not take the cost of complications into account (e.g., prolonged hospital stay in transfused patients: 320€/day). With regard to allogenic units, the total close of autologous units would increase according to the amount of unused units, because these cannot be transfused as allogenic to other patients.
Laboratory sales price, although the real purchase prices, in brackets, are usually lower. rHuEPO, recombinant human erythropoietin.
According to WHO criteria, preoperative anemia is present in a good many OTS patients, and up to 30% present a suboptimal Hb level (Hb <13g/dL).1,2 Preoperative anemia is shown to have an independent association with increased morbidity and mortality and a longer hospital stay, along with the risk of ABT, which in turn is associated with increased perioperative morbidity and mortality.1,12,13 Despite this, it is still not receiving due attention and in many cases no treatment is started to correct or improve it and patients are receiving ABT as necessary. Postoperative anemia is even more common and should be corrected, not necessarily by ABT. For this reason, perioperative erythropoiesis stimulation is a fundamental pillar of the PBM program.
Diagnosis and treatment of preoperative anemiaPatients scheduled for major OTS should be checked for preoperative anemia at least 30 days prior to surgery, to make a differential diagnosis and start appropriate treatment, if necessary (GRADE 1C).6,14 In the event of unexpected anemia, elective surgery should be postponed until it has been duly classified and treated.
The presence of anemia is generally defined as an Hb <13g/dL in males or <12g/dL in females, but perhaps we require a different definition in OTS patients. Women have less circulating volume than men, whereas blood loss in these surgical procedures is similar for both sexes. In the Austrian multicenter study (18 hospitals, 1401 THP, 1296 TKP), the relative perioperative loss of erythrocytic mass was 37% in THP (women 39%, men 34%) and 35.2% in TKP (women 44%, men 34%). This was reflected in the requirements for allogenic and autologous transfusion: 43% in THP (women 52%, men 30%) and 41% in TKP (women 45%, men 31%).2 It had already been observed in the OSTHEO study that, for the same level of preoperative Hb (e.g., 13g/dL), the rate of ABT in TKP and THP was greater in women (34%) than in men (22%).1
Therefore, the definition of anemia for female OTS patients should be at least the same as for male patients; i.e., Hb <13g/dL. In order to avoid conflicting with the usual definitions, we should change the term “preoperative anemia” to “suboptimal preoperative Hb level” when it is <13g/dL. Consequently, the objective of preoperative treatment should be to optimize Hb to a level of at least 13g/dL and to minimize the risk of transfusion.
In the case of postoperative anemia, the objective of treatment will be to achieve Hb levels which avoid ABT, and to correct the anemia in the shortest time possible, to enable functional recovery and to improve quality of life. In addition, attention should be paid during this period to drug interactions which could cause or aggravate the anemia.5
An important, and often forgotten, aspect is the diagnosis of hematinic deficiencies without anemia (iron, vitamin B12, folate deficiency), as it is of paramount importance to correct these deficiencies in order to optimize preoperative Hb levels, especially in patients undergoing treatment with erythropoiesis-stimulating agents, and to hasten recovery from postoperative anemia (GRADE 1C).14
FerrotherapyIndications. The ESA Guideline recommends generally treating iron deficiencies by administering oral or intravenous iron (GRADE 1 B),7 but this recommendation has to be adjusted according to the severity of the anemia, the type of surgery and the time available in which to treat it. In the case of preoperative ferropenic anemia, wherever possible and as long as there is sufficient time, oral iron should be considered (e.g., ferrous sulfate) due to its low cost and ease of administration (GRADE 2B) (Table 3).6
However, on occasion, either because of poor absorption, poor tolerance or insufficient time before surgery, the use of intravenous iron is fully justified (IVFe). In which case, the medullary response and repletion of iron stores will be more rapid (1–2 weeks) (GRADE 2B) (Table 3).6 In anemic patients scheduled for OTS, the preoperative administration of IVFe significantly increased the Hb, corrected the anemia in the majority of the patients and reduced the need for ABT, although the costs of the treatment were lower using iron carboxymaltose rather than iron sucrose (Table 4).15
Conversely, available evidence shows that the oral administration of iron in the post-operative period does not improve Hb levels or reduce the need for ABT and is not recommended (GRADE −1B) (Table 3).6 Whereas IVFe does appear to be effective in achieving these objectives (GRADE 2B).16,17
Posology. Although there are numerous forms of oral iron on the market (ferrous salts, amino-chelated compounds, iron–protein complexes, iron compound in liposomes, etc.), the benchmark drug is still ferrous sulfate and the usual daily dose is generally 100mg of elemental iron.
When IVFe is used, the objective is to cover the patient's iron deficiency, avoiding giving them too much. The dose of IVFe can be calculated using Ganzoni's formula [iron deficiency (mg)=weight (kg)×(targetHb−actualHb) (g/dL)×2.4+500] or a simplified dosage guideline can be used (200mg of IVFe per g/dL of Hb that we want to recover+500mg to replenish stores).
Safety. The adverse effects which are usually of most concern of IVFe preparations are hypersensitivity and a possible increase in infections. The European Medicines Agency (EMA) maintains that, when correctly indicated and administered at the appropriate dose, the benefits of these preparations outweigh the risks, the incidence of serious allergic reactions is low and there is no difference in safety between the different iron preparations available in Europe (iron gluconate, iron sucrose, low molecular weight iron dextran, iron carboxymaltose, iron isomaltoside), although the quality of evidence to support the latter consideration is low.18 For this reason, the EMA recommends that IVFe is only administered in an environment with the facilities and trained personnel to perform cardiopulmonary resuscitation, there is no need to give a test dose and they recommend that all patients are monitored for at least 30min after infusion.18
With regard to the increased risk of infection, Litton et al.19 published a meta-analysis of 72 clinical trials which included a total of 10,000 patients to evaluate the efficacy and safety of IVFe. They concluded that these preparations improve Hb levels and reduce ABT rates, but that they increase the relative risk of infection. It is very questionable for the authors to establish a causal relationship between IVFe and an increased risk of infection when: (1) in the majority of studies included this was not a predefined “objective variable”; (2) there is great heterogeneity in the studies included in terms of dosages and duration of treatment, types of iron, study population, etc.; (3) it was not possible to demonstrate a dose–effect relationship between the administration of IVFe and the greater incidence of infections or mortality; and (4) the studies which did include the definition criteria for this variable do not demonstrate an increased rate of infection in patients treated with IVFe.
Finally, IVFe does not have a direct influence on erythropoiesis, so its administration does not cause supraphysiological Hb levels (as can occur with erythropoietin) and, therefore, it does not increase the risk of thromboembolic disease. It might even reduce the risk as the thrombocytosis induced by the iron deficiency decreases.20
Contraindications. The use of IVFe is contraindicated in cases of hypersensitivity to the active substance, anemia which is not attributed to an iron deficiency, or traces of iron overload or problems with its use. And, in patients with allergies or liver failure, and pregnant women (2nd and 3rd trimester), the benefits and risks of administering IVFe need to be carefully assessed.18
- –
Recombinant human erythropoietin
Indications and posology. 30% of scheduled OTS patients present a preoperative Hb of less than 13g/dL.1,12 Recombinant human erythropoietin (rHuEPO) has been approved for use in patients with Hb levels between 10 and 13g/dL and adequate levels of iron (ferritin ≥100ng/mL), with two administration protocols: 4 doses of 600UI/kg/week s.c., starting three weeks before surgery, or 15 doses of 300UI/kg/day s.c., starting 10 days before surgery, the day of the operation and the four days following surgery. Both protocols achieve a significant reduction in the risk of ABT (GRADE 1A) (Table 3), but the minimum effective dose of rHuEPO in order to achieve the target Hb (generally 14g/dL) and to reduce ABT in these patients6 is not known. Nevertheless, Rosencher et al.21 observed that the majority of OTS patients achieved this target Hb with one or two doses of 40,000UI rHuEPO, rather than the 4 doses recommended by the manufacturer, which enables a significant reduction in costs (Table 4). Although the administration of rHuEPO with oral iron significantly reduces transfusion requirements,22 in general, better results are achieved by the administration of IVFe in monotherapy, or in combination with one or two doses of 40,000UI rHuEPO when the desired response has not been achieved or in patients with inflammation.23,24
It is very often the case that we do not have several weeks in which to treat anemia, either because the surgery is non-elective or emergency, or because the patient has been assessed only a few days before the date set for the operation. In these cases, peri- or postoperative treatment for anemia is still a possibility. In OTS to repair a fractured hip and for knee or hip arthroplasty, perioperative administration of rHuEPO (40,000UI) plus a restrictive transfusion criterion (Hb <8g/dL), reduced the need for ABT and contributed to a more rapid correction of postoperative anemia (GRADE 2B).6,25
Safety. With regard to rHuEPO in OTS, a recent meta-analysis confirmed its efficacy in reducing ABT with no differences in thromboembolic risk compared to the placebo, although the authors argue that the majority of patients received thromboprophylaxis and presented a low cardiovascular and thromboembolic risk.26 In any case, it is important to point out that for an optimal response to rHuEPO (reducing the dosage and potential adverse effects), iron, folic acid and vitamin B12 deficiencies should be ruled out before starting therapy. We also consider it prudent to avoid intentionally exceeding 14g/dL (target Hb), except in special cases such as Jehovah's Witnesses or surgery on patients which involves more blood loss (revision surgery).
Contraindications. The administration of rHuEPO is contraindicated in patients with uncontrolled hypertension, a history of venous or arterial thromboembolic disease, and patients who for some reason cannot take appropriate antithrombotic prophylaxis treatment or who have curable neoplasia.
Reduction of perioperative bleedingThe second pillar of PBM groups together the perioperative actions aimed at “correcting hemostasis and minimizing hemorrhage and blood loss”. Reducing perioperative bleeding in OTS is fully justified and can be achieved by6,7:
- (1)
The use of standardized questionnaires to evaluate a history of bleeding, thrombosis and consumption of drugs which interfere with hemostasis (GRADE 1C).
- (2)
Appropriate management of antiaggregants and anticoagulants, although stopping aspirin treatment is not recommended (GRADE 1B).
- (3)
Limiting blood taking for diagnostic purposes.
- (4)
Maintaining normothermia.
- (5)
The use of controlled hypotension (induced or permissive; maintaining normovolemia, but avoiding excess administration of fluids).
- (6)
Appropriate positioning of the patient to prevent venous statis.
- (7)
Monitoring hemostasis using point-of-care systems (Thromboelastography [TEG]/Thromboelastometry [ROTEM]) (GRADE 1C).
- (8)
Performing careful surgical hemostasis and, when possible, minimally invasive surgery.
These measures can be complemented with the use of drugs which might encourage clot formation, ensure its stability or delay its lysis and the use of drains with minimum suction pressure, which we will analyze below.
- –
Antifibrinolytic drugs
Antifibrinolytics, especially tranexamic acid (TXA), are the most commonly used drugs to reduce blood loss. However, their use in OTS procedures is controversial as they are considered of “very high thrombotic risk”, and it is suggested that antithrombotic prophylaxis should be maintained for ≥35 days after surgery.27
The efficacy and safety of using tranexamic acid (TXA) and epsilon aminocaproic acid (EACA) was analyzed in the DS 20136; these are synthetic lysine analogs which inhibit the conversion of plasminogen to plasmin. TXA is 10 times stronger than EACA and the maximum benefit and greatest safety of this drug is achieved in diseases or circumstances where increased bleeding predominates or there are signs of increased bleeding due to primary or secondary hyperfibrinolysis. The role of aprotinin in reducing blood loss and transfusion requirements28,29 was not analyzed in the DS 2013 as it had been removed from the market.
Indications. According to the latest version of 2012 of the authorized product datasheet,30 the specific indications for using TXA on surgical patients include ear, nose and throat, chest and abdominal surgery and other important surgical interventions such as cardiovascular surgery. OTS is not included nominally in the indications for TXA.
Off-label use. In Spain TXA is being used in OTS for an indication which has not been expressly approved and therefore must comply with CHAPTER III of RD 1015/2009 “Access to medicines in conditions other than those authorized”, Article 13 “Requirements for access to medicines in conditions other than those authorized in Spain” which states that “the use of medicines authorized under conditions other than those set out in their product datasheet shall be exceptional and limited to situations where there are no authorized therapeutic alternatives for a particular patient, respecting, where appropriate, the established restrictions linked to prescribing and/or dispensing the medicine and the therapeutic care protocol of the healthcare center. The doctor responsible for the treatment shall duly justify the need for the use of the medicine in the clinical history and inform the patient of the benefits and potential risks, and obtain consent in line with Law 41/2002, of 14 November”. The Spanish Medicines and Health Products Agency (AEMPS) concludes that, although the use of medicines which have already been authorized for conditions other than those set out in their product datasheet is not subject to approval by AEMPS on a case-by-case basis, the Agency might issue recommendations that would have to be taken into account when drawing up the health-care centers’ therapeutic care protocols.31
Dosage. On analysis of the studies which were revised in order to draw up DS 2013,6 a great variety and availability of guidelines and doses of TXA were found. The following doses were most frequently used:
- •
TKP and THP: an initial dose of 10–15mg/kg perioperatively followed or not followed by an infusion of 1mg/kg/h for 4–6h or a repeat of the initial dose in the postoperative period. However, some authors recommend set intravenous doses of 1–2g TXA to prevent calcium errors and excessive doses.32
- •
Spinal: an initial dose of 20–100mg/kg, followed by an infusion of 10mg/kg/h for 4–6h.
Safety and secondary effects. The efficacy and very low cost of TXA (1000 mg=1€) appear to be one of the main drivers for its gradually increasing use in TKP and THP, despite the fact that orthopedic surgery is not nominally included in the specific indications for TXA on the product's revised datasheet.30 An added driver is the impact of the results of the CRASH-2 study on more than 20,000 patients with traumatic bleeding, which found a decrease in mortality from bleeding (4.9% compared to 5.7%; P=.0077), with no increase in thromboembolic episodes (0.3% compared to 0.5%; P=.096), but without reducing the requirements for ABT.33 The great impact of this publication has lead many practitioners to consider that the use of TXA in patients with early-onset coagulopathy with secondary hyperfibrinolysis associated with traumatic bleeding can be applied to elective orthopedic surgery patients, whose clinical context is very different.34
There is uncertainty, therefore, as to the safety of administering TXA to orthopedic surgery patients, and the risk factors of thromboembolic disease should be considered before it is used. According to the product datasheet,30 in patients with a family or personal history of thromboembolic diseases, TXA should only be given if there is a clear indication, after consulting a doctor with experience in hemostasis and under strict supervision. Similarly, TXA should be administered with caution to patients who are taking oral contraceptives or hormone replacement therapy, estrogens in particular, due to the increased risk of thrombosis, and the dose should be adjusted according to the level of serum creatinine in cases of mild to moderate renal failure (often elderly OTS patients). Finally, it should be remembered that its use is contraindicated in cases of severe renal failure (risk of accumulation); and where there is a history of seizures.
With regard to adverse effects, cases of seizure have been reported in association with treatment with TXA, with a clear relationship with the dose given, as has been documented in cardiac surgery.32
Furthermore, it is well known that both surgery and ABT are risk factors for hypercoagulability. As TXA significantly reduces the ABT rate, a reduced rate of thrombotic events would be expected. This reduced rate has not been demonstrated, and the studies undertaken do not have enough statistical power to do so. With these data a hypothesis can be made that the administration of TXA in TKP and THP would increase the risk of thrombotic complications, as has been seen in hip fracture,35 this increased risk being compensated by the benefit offered by the reduction in ABT.34
Evidence-based recommendations. In various randomized studies, it has been found that TXA reduces bleeding and the number of ABTs in these patients, although variably, without an apparent increase in the incidence of thrombotic events or mortality.32,35 However, given that the number of patients included is insufficient to asses low-incidence adverse effects, there are doubts as to the safety of TXA in spinal surgery and lower limb arthroplasty, but especially in hip fracture surgery.36 Its topical administration could be safer although studies are required to confirm this, as there are no pharmacokinetic studies to demonstrate plasma concentrations in blood when it is administered via this route, and even so, it remains an indication which has not been approved.30
According to the ESA Guideline, its generalized use cannot be recommended, and an individualized indication needs to be made after ruling out the presence of risk factors (advanced age, female patient, history of thrombosis, hip fracture, cancer, etc.) (GRADE 2A).7 In fact, 20–30% of OTS patients present some contraindication for the use of TXA.37 Therefore, both the DS 2013 and the ESA Guideline only “suggest” their use in selected cases of orthopedic surgery to reduce bleeding and/or the transfusion rate (GRADE 2A) (Table 3), highlighting that more safety studies are needed before establishing a definitive recommendation for these drugs, whereas they are not recommended in hip fracture.6,7
- –
Fibrin sealants and gels
There is a group of local, autologous or commercial, hemostatic agents which in some cases can help to reduce bleeding during surgery. With regard to efficacy and safety in scheduled OTS, a recent meta-analysis of 8 randomized and controlled studies including 641 TKP patients, the use of fibrin sealant significantly reduced postoperative drainage volume (−350mL), ABT rate (RR=0.47, 95% CI=0.35–0.63) and the range of mobility was improved, but there was no significant reduction of total blood loss, and no significant differences were found in the incidence of fever, infection or hematoma amongst the study groups.38 These results appear to indicate that the use of fibrin sealant as hemostatic therapy in prosthetic surgery patients is effective and safe, although more data is needed to make a firm recommendation for its use (Table 3).
Nevertheless, in recent years we have witnessed an increased use of platelet-rich autologous plasma (PRAP), not only as a base for preparing fibrin sealants but also in many other OTS applications and other specialties. In this regard, AEMPS drew up a report deciding to classify PRAP as a medicinal product for human use and showing the framework for its autologous use in Spain, including the rules for prescribing it, which is limited to physicians, odontologists and podiatrists, solely and primarily responsible for correctly collecting (if collecting PRAP using open technique), applying and monitoring this medicine.39
Post operative drainsThe use of postoperative drainage systems is common practice in lower limb arthroplasty, especially TKP. The theoretical advantage to using these drains is a reduction in the appearance of hematomas (associated with increased pain, altered wound healing and postoperative infections) and compression of vital structures, as both can increase hospital stay and delay rehabilitation.
However, there are doubts as to whether they are effective in achieving these objectives,40 or whether high or low vacuum drains should be used.41,42 Therefore, it could be argued that if postoperative drains are to be used, a low vacuum recovery/re-infusion system could be beneficial for the patient if a significant volume of blood is drained.
Use of autologous bloodPreoperative autologous blood donation (PABD), acute normovolemic hemodilution (ANH) and perioperative autologous blood salvage (PABS) are included under this heading.
- –
Preoperative autologous blood donation
Preoperative ABD is a means of autotransfusion which consists in taking one or more units of the patient's own blood in the days or weeks prior to surgery, to be used during surgery and/or in the immediate postoperative period. In adult patients undergoing major orthopedic surgery (knee, hip or spine), preoperative ABD has been widely used and has been demonstrated to reduce the absolute risk of ABT by up to 20%, although 40% of the units stored were not used. At present, preoperative ABD would be indicated in patients undergoing elective orthopedic procedures whose risk of transfusion exceeds 30–50% and whose Hb is generally <14.5g/dL and/or in patients for whom it is difficult to find compatible allogenic blood or who refuse ABT. Therefore, the DS 3013 does not recommend the routine use of preoperative ABD to reduce the transfusion rate, in OTS procedures which generally require ≤2 units of ABT (GRADE −1B) (Table 3).6 However, they do recommend it for those who might need ≥3 units (e.g., change of hip prosthesis or extensive spinal instrumentation), preferably together with coadjuvant treatment with iron and/or rHuEPO (GRADE 1C) (Table 3).6,7,43 Furthermore, preoperative ABD should only be implemented in patients who have a guaranteed date for surgery, to prevent the units’ expiring.6
- –
Acute normovolemic hemodilution
ANH consists of the extraction and anticoagulation of an amount of blood and its simultaneous replacement with crystalloids and/or colloids to maintain normovolemia. It is used in OTS with moderate/severe hemorrhage and is performed after induction of anesthesia and before the hemorrhagic stage of the surgery.
Most studies do not show a significant reduction in the risk of exposure to ABT, although the use of ANG is associated with a decreased risk of post-operative infection and thrombotic events in OTS.44 Therefore, despites its low cost, the DS 2013 does not recommend routine use of ANH as a single blood-saving technique (GRADE −1B) (Table 3), except in hospitals where it is not possible to implement other alternatives to ABT.6
- –
Perioperative autologous blood salvage
ABS is an autotransfusion technique which, because part of the blood shed during and/or after surgery is returned to the patient, reduces the number of patients receiving ABT and/or the volume of ABT in different elective or emergency surgical procedures where there is significant bleeding. In the intraoperative period, ABS is performed using devices which aspirate, anti-coagulate, flush and concentrate the blood which is lost into the surgical field, returning it in the form of packed red cells in saline solution. In the postoperative period, ABS consists of the collection and reinfusion of blood from the postoperative drains. When salvage has not taken place intra-operatively, postoperative salvage is usually undertaken using devices which recover and re-infuse filtered, unwashed whole blood.
In OTS, intraoperative or perioperative ABS would be indicated in procedures where blood loss of more than 1500mL is anticipated: revision of hip arthroplasty (GRADE 1B), and correction of scoliosis, within a multidisciplinary program (GRADE 1C), whereas its use in tumor surgery is controversial (GRADE 2C) (Table 3).6 While postoperative ABS, with reinfusion of filtered, unwashed blood is limited to scheduled OTS patients who are expected to lose between 500 and 1500mL of blood postoperatively, such as after knee or hip arthroscopy (GRADE 1B) or spinal arthrodesis (GRADE 1C) (Table 3).6,45–47 In these operations, postoperative ABS is a unique technique to reduce blood loss, maintain high Hb levels and reduce the use of ABT. However it is not recommended in hip fracture repair surgery (as it is of no value).6
Safety of ABS. With regard to the safety of postoperative ABS, despite these good clinical results, reinfusing unwashed blood from drains in OTS is still surrounded in a degree of controversy, in terms of both its quality and safety (hematimetric quality, free hemoglobin, grease and cement particles, activated coagulation and fibrinolysis factors, inflammatory mediators, etc.), and its efficacy in different groups of patients.48 However, the results of numerous studies appear to support the idea that postoperative ABS with unwashed blood is safe, and there are also data which point toward an immunostimulant effect.48 In this regard, in an assessment of 2190 reinfusions after elective TKP or THP in 31 Dutch hospitals, significant adverse effects of only 1% were recorded (vagal reaction, hypotension, fever or chills); this incidence is lower than that published for ABT.49
These data coincide with the results of different randomized, observational clinical studies in different types of surgery in which intra- and/or postoperative ABS did not increase morbi-mortality or the length of hospital stay, although some isolated cases of adverse events have been described, in particular coagulopathy, when large volumes of salvaged and processed blood were reinfused.6
Another aspect to note is the possible interaction of local infiltration analgesia (LIA) with postoperative blood salvage; two important techniques in the postoperative treatment of OTS patients. Although the combination of these techniques has been questioned recently, in 6 studies of TKP or THP with LIA (200–490mg of ropivacaine), reinfusion of the blood from the drain did not produce neurological toxicity symptoms due to local anesthetic in any of the patients.50 In a more recent study on TKP patients who had been given peri-articular ropivacaine (567mg) plus continuous intra-articular and subfascial infusion (192mg/24h), Thomassen et al.51 determined the concentrations of ropivacaine, free and attached to α1-glycoprotein, in blood salvaged from drains and made an estimation of the possible effect of its reinfusion on the patients’ plasma levels. The salvaged volume was 600mL (303–869), with a concentration of ropivacaine of 33mg/L,18–42 the free fraction was 70%. According to these data, the concentrations of free ropivacaine after reinfusion would be 0.18mg/L (0.08–0.40mg/L); concentrations which would be below the systemic toxicity threshold of this drug (0.56mg/L [0.34–0.85]).52 Therefore, the available data show that postoperative ABS with filtered blood can be safely combined with the use of LIA, administering a slow reinfusion over 30–60min or more.
Contraindications. In general, the contraindications for the use of blood salvaged perioperatively are determined, on the one hand, by the characteristics of this blood, and on the other, by the particular conditions of the patient. Amongst them we would highlight: renal failure, altered liver function, coagulation disorders, patients with red cell alterations, the use of local hemostatics irrigation of the field with inappropriate solutions, HIV, Hepatitis B- and C-seropositive patients, the patient's refusal of the technique, septic or neoplastic pathology.48 However, given the hematological and biochemical characteristics of unwashed salvaged blood, infusing a volume greater than 1000–1500 ML is not recommended.48
Daily clinical practice. From this perspective, we could say that postoperative ABS with filtered blood offers the following benefits:
- 1.
It does not interfere with the standard surgical procedure.
- 2.
It does not require equipment, software or supply or vacuum sources; the pack usually contains everything necessary for collecting and reinfusing the drained blood.
- 3.
It is easy for the surgeon and nursing staff to use, and minimal training is required to handle it.
- 4.
It is accepted by patients (including Jehovah's Witnesses, adding a continuity line between the drain and the patient).
- 5.
It provides compatible blood immediately and almost in the same amount as is collected by the drains (approx. 1 unit/patient), without the disadvantages of allogenic blood.
- 6.
It has an Hb concentration which is similar to that of the patient; although unlike stored blood, it has optimal oxygenation capacity.
- 7.
It is clinically safe and saves on units of packed allogenic red blood cells.
- 8.
And finally, but no less important, its use is compatible with almost any other blood saving strategy.
Cost-effectiveness of ABS. In this regard, although there are few cost-effectiveness studies and those that there are have been undertaken using very different methodologies, in general, it is accepted that the procedure would be cost-effective if it is possible to salvage at least the equivalent of 1.5–2 units of packed red blood cells in autologous washed blood salvage, or the equivalent to 1 unit of packed red blood cells in autologous filtered blood salvage (Table 4).6,53
ConclusionsRevision of the efficacy of the methods for optimizing the use of blood products (perioperative iron, erythropoiesis-stimulating agents, predonation, perioperative autologous blood salvage, antifibrinolytics, etc.) shows that their use reflects a general trend toward improved clinical results (a lower incidence of ABT, postoperative complications and mortality, shorter hospital stay and improved quality of life).54 Nonetheless, the available scientific evidence comes from very heterogenous studies of their design and quality, and this significantly influences the strength of recommendations on the use of these strategies, which has been acknowledged in different consensus documents and clinical practice guides (Table 3).6,7 Furthermore, this evidence comes predominantly from studies evaluating the efficacy and/or safety of a specific strategy (along with the application of restrictive transfusion criteria in the most recent of them). Finally, it should be taken into account that, although a given technique might be effective, the objective of performing major surgical procedures, without using ABT and without exposing the patient to the risk of complications, could be better realized by integrating several of these strategies within a defined algorithm.
Therefore, the introduction of the concept of PBM implies a paradigm shift. The PBM approach is multidisciplinary and multimodal and focuses on identifying and providing the necessary provision for continuity of care for patients, where communication and coordination between the different disciplines might not only reduce the probability of a patient requiring ABT, but also improve their clinical result and reduce treatment costs.55
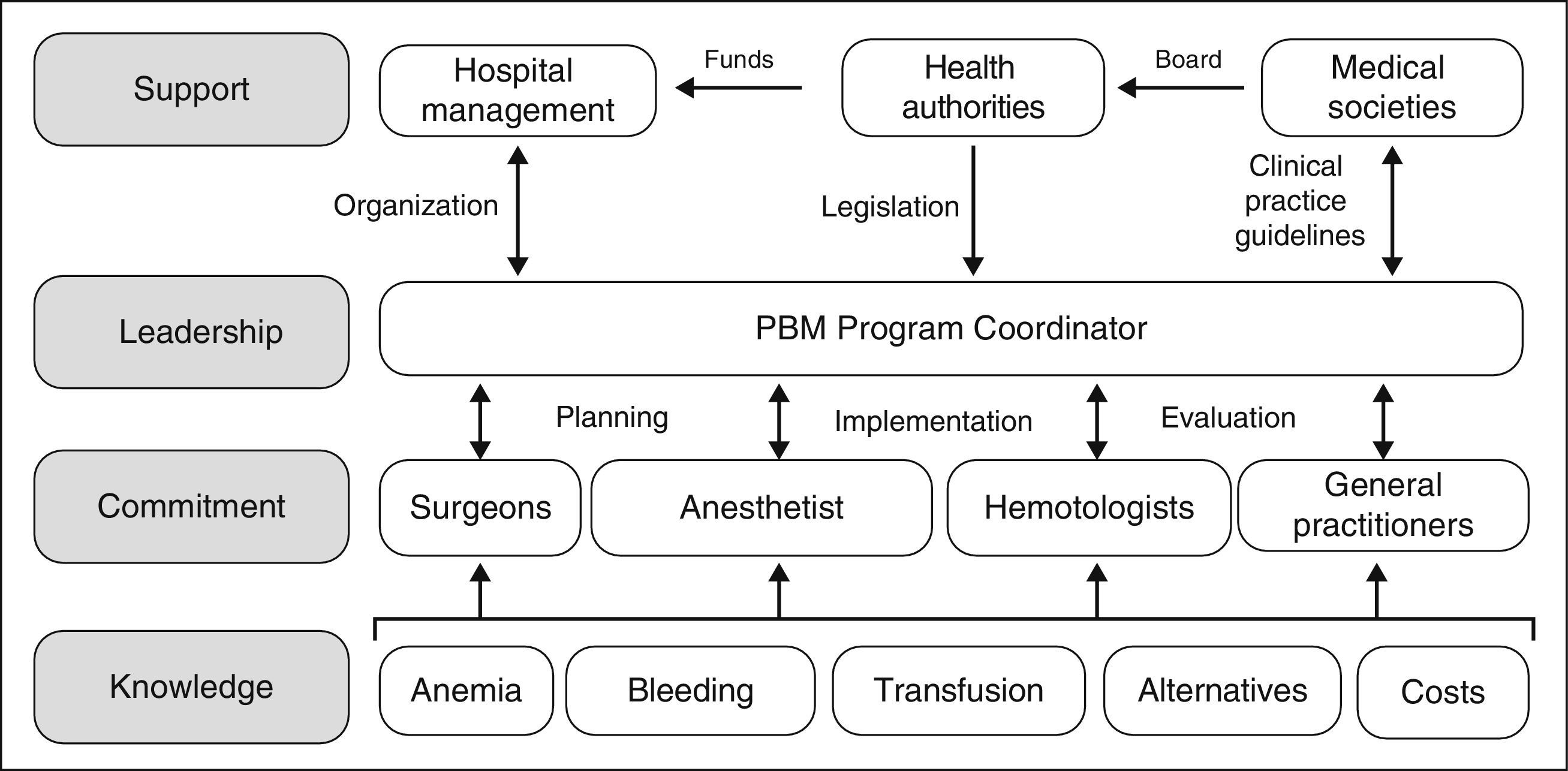
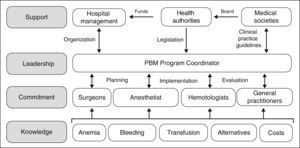
However, although the benefits of PBM programs both for the patient and the healthcare system appear indisputable, they are not easy to implement, as several obstacles need to be overcome. The development and implementation of PBM programs involve planning and forecasting; they will not come about spontaneously just because they are desired and neither will they simply evolve because, frankly, it is just too easy to ask for a unit of packed red blood cells from the blood bank. As shown in Figure 1, the practitioners involved need to be provided with specific knowledge, to ensure their commitment to the program. A Coordinator is required to put the PBM program into practice, and they must also interact with doctors and nurses in order to plan, implement and audit the program. Moreover, the Coordinator will require the support of the hospital management (to provide the organizational aspects), the health authorities (to provide the necessary funding, although over time the PBM will produce significant saving, and to provide a regulatory framework for these activities) and of the scientific societies in the area of healthcare (to offer advice to the health authorities and to develop clinical practice guidelines).
Level of evidenceEvidence level V.
Ethical responsibilitiesProtection of human beings and animalsThe authors declare that no experiments were performed on humans or animals for this investigation
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis article has no sources of funding.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Canillas F, Gómez-Ramírez S, García-Erce JA, Pavía-Molina J, Gómez-Luque A, Muñoz M. “Patient blood management” en cirugía ortopédica. Rev Esp Cir Ortop Traumatol. 2015;59:137–149.