Preoperative bone mass index has shown to be an important factor in peri-prosthetic bone remodelling in short follow-up studies.
Material and methodsBone density scans (DXA) were used to perform a 10-year follow-up study of 39 patients with a unilateral, uncemented hip replacement. Bone mass index measurements were made at 6 months, 1 year, 3 years, 5 years, and 10 years after surgery. Pearson coefficient was used to quantify correlations between preoperative bone mass density (BMD) and peri-prosthetic BMD in the 7 Gruen zones at 6 months, 1 year, 3 years, 5 years, and 10 years.
ResultsPre-operative BMD was a good predictor of peri-prosthetic BMD 1 year after surgery in zones 1, 2, 4, 5 and 6 (Pearson index from 0.61 to 0.75). Three years after surgery it has good predictive power in zones 1, 4 and 5 (0.71–0.61), although in zones 3 and 7 low correlation was observed 1 year after surgery (0.51 and 0.57, respectively). At the end of the follow-up, low correlation was observed in the 7 Gruen zones. Sex and BMI were found to not have a statistically significant influence on peri-prosthetic bone remodelling.
ConclusionAlthough preoperative BMD seems to be an important factor in peri-prosthetic remodelling 1 year after hip replacement, it loses its predictive power progressively, until not being a major factor in peri-prosthetic remodelling 10 years after surgery.
El índice de masa ósea preoperatoria ha demostrado ser un factor importante en la remodelación ósea periprotésica en estudios a corto plazo.
Material y métodosSe utilizó DEXA para realizar un estudio de seguimiento de 10 años a 39 pacientes con una artroplastia de cadera no cementada unilateral. Las mediciones de densidad de masa ósea (DMO) se realizaron a los 6 meses, un año, 3 años, 5 años y 10 años después de la cirugía. El coeficiente de correlación de Pearson se utilizó para cuantificar las correlaciones entre DMO preoperatoria y la densidad mineral ósea periprotésica en las 7 zonas de Gruen a los 6 meses, un año, 3 años, 5 años y 10 años.
ResultadosLa DMO preoperatoria fue un buen predictor de DMO periprotésica un año después de la cirugía en las zonas 1, 2, 4, 5 y 6 (índice de Pearson 0,61-0,75). Tres años después de la cirugía mantiene un buen poder predictivo en las zonas 1, 4 y 5 (0,71-0,61), aunque en las zonas 3 y 7 se observó baja correlación un año después de la cirugía (0,51 y 0,57 respectivamente). Al final del seguimiento se evidenció baja correlación en las 7 zonas de Gruen. El sexo y el IMC no tuvieron una influencia estadísticamente significativa en la remodelación ósea periprotésica.
ConclusiónAunque la DMO preoperatoria parece ser un factor importante en la remodelación periprotésica un año después de la implantación de una artroplastia, este factor va perdiendo progresivamente poder predictivo; no siendo un factor determinante en la remodelación periprotésica 10 años después de la cirugía.
Bone remodelling is a commonly observed phenomenon following hip arthroplasty with all prosthetic designs. The extent of this remodelling may vary depending on factors related to the patient (gender, weight, preoperative bone mass) as well as the implant (geometry, size, material, surface coating). It is generally accepted that the majority of remodelling changes around the femoral stem take place in the first years after the surgical intervention,1,2 whilst changes after this point reflect the biomechanical response of the bone according to Wolff's law and changes associated to ageing.
The study of multifactorial causes determining periprosthetic bone remodelling considers preoperative bone mass as a vitally important factor, since non-cemented arthroplasty techniques are based on an adequate osseointegration of the implant. Coinciding with the design of biomechanically optimised implants and bioactive coatings which promote bone growth, the indication for this type of implants has expanded to include older patients with a foreseeably worse bone quality.3,4
Dual energy X-ray absorptiometry has been extensively used to quantify the extension of periprosthetic bone loss around joint implants. This method has been proven reliable and accurate, and is considered ideal for repeated examinations in serial monitoring due to its precision and low radiation dosage.5,6
For these reasons, the technique has been used in the study of various implants which have shown different remodelling patterns: the first generation of non-cemented stems showed losses around 45% of the bone mass in proximal areas,7 whilst the second generation of anatomical stems managed to reduce this loss to levels around 20–25%.8 Customised implants, tailor-made for each patient, showed losses of around 10–15% at the third year of follow-up.9
In order to know the influence of preoperative bone mass on periprosthetic femoral remodelling, a prospective study was designed to analyse 39 patients undergoing implantation of a model ABG-II total hip arthroplasty due to unilateral primary coxopathy (avascular necrosis or osteoarthritis). Determinations were carried out through dual energy X-ray absorptiometry with a monitoring period of 10 years following the intervention. The purpose of the study was to determine and quantify the changes in bone mineral density in the 7 Gruen zones throughout the monitoring period, analysing the influence of preoperative bone mass on periprosthetic bone remodelling. The study also analysed the influence of gender and body mass index.
Material and methodA controlled, prospective study was designed to assess the influence of preoperative bone mass density (BMD) on periprosthetic remodelling caused by implantation of a model ABG-II non-cemented stem. The sample size was calculated according to mean comparison tables to obtain a statistical power of 95% and an alpha risk of 0.05.
The inclusion criteria were the following: firstly, patients with an indication for implantation of a model ABG-II stem according to their disease and bone quality (osteoarthritis or avascular necrosis, with no signs of osteoporosis), secondly, the coxopathy had to be unilateral and, lastly, patients could not have undergone reinterventions in the hip being studied, either due to replacements or periprosthetic fractures. All the patients signed an informed consent form to participate in the study which had been approved by the Ethics Committee of the centre. The group under study was comprised of 39 patients (25 males and 14 females), with a mean body mass index of 30.2kg/m2 (range: 22–50.3kg/m2) and a mean age of 68 years (range: 51–82 years). All were intervened at Hospital Universitario Miguel Servet between 1 February 2000 and 1 February 2001. All the patients completed the monitoring period satisfactorily.
The implant used was a model ABG-II (Stryker, Howmedica) stem and a model ABG-II non-cemented cup coated with hydroxyapatite. The ABG-II stem is anatomical, non-cemented, with a metaphyseal fixation by pressure adjustment and manufactured with a titanium alloy (TM12Z6F2). It has a hydroxyapatite coating with a width of 70μm limited to the metaphyseal area, with a purity of 99.99% and a scale-shaped design on the anterior and posterior aspects which transforms shear forces into compression forces, thus increasing its stability. The tail of the implant is thin and smooth to avoid contact with the diaphyseal endosteum.
All the patients were intervened through a posterolateral approach route by the same team of 4 surgeons. The femoral canal was prepared by diaphyseal drilling 1mm larger than the tail of the stem. During the postoperative period, all patients received antithrombotic prophylaxis with low molecular weight heparin and antibiotic prophylaxis for 48h after the surgical intervention. Patients maintained partial support for 6 weeks, when the ipsilateral cane was removed and full load was authorised. Subsequently, gluteal strengthening exercises were prescribed, following the current physiotherapeutic recovery protocol of our service at the time of the procedures.
Clinical monitoring was performed through the Merle D’Aubigné scale. We also conducted annual radiographic studies, which did not observe any cases of radiolucency or osteolytic images.
The densitometry study was carried out by dividing the proximal femur into 30mm×30mm boxes representing the 7 Gruen zones, both in the operated and contralateral femurs. The densitometry device employed for all measurements was a model LUNAR DPX enCORE (General Electrics Healthcare), together with metal exclusion software.
In order to ensure the reliability of the measurements, it was considered vitally important to establish a protocol to place patients on the examination table of the densitometer, as some authors have reported variations in the determination of BMD around −5%.9 Patients were placed in the supine position on the densitometer table, with knees in extension and the entire limb stabilised in a neutral position by means of a rigid device equipped with Velcro straps.
Densitometry determinations began in the preoperative period. Additional determinations were carried out on the intervened femur at 6 months, 1, 3, 5 and 10 years after the intervention. The preoperative bone mineral density value was taken as a reference to establish densitometry variations, in order to offset the decrease in bone stock caused by the surgical intervention, which has been observed to reach up to 10%.10
Comparison of percentages was carried out by means of the Chi-squared test, whilst comparison of means was done by means of the Student t test with a significance level of 0.025 to analyse the evolution of BMD in the intervened femur. Correlations were quantified using the Pearson correlation coefficient.
ResultsAt the end of the follow-up period, the radiographic images proved that all the stems were stable and no reactive lines or radiolucency were observed.
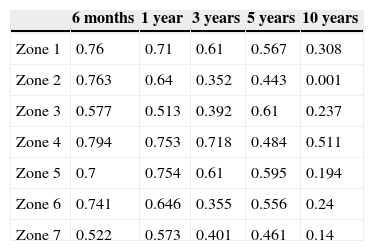
By studying the relationship between the initial BMD and BMD throughout the follow-up period it was possible to appreciate that the initial value maintained a good correlation and was considered a good predictive factor during the first year in zones 1, 2, 4 and 6 (Pearson correlation coefficients between 0.61 and 0.75). At 3 years after the surgery, there was still a good predictive power in zones 1, 4 and 5 (0.71–0.61). A low correlation was observed in zones 3 and 7 from the determination at 1 year after the intervention (0.51 and 0.57, respectively). At the end of the monitoring period, a low correlation was observed for the 7 Gruen zones. The results are shown in Table 1.
Pearson correlation coefficient showing the predictive capacity of preoperative bone mass to estimate BMD throughout monitoring in each Gruen zone.
| 6 months | 1 year | 3 years | 5 years | 10 years | |
|---|---|---|---|---|---|
| Zone 1 | 0.76 | 0.71 | 0.61 | 0.567 | 0.308 |
| Zone 2 | 0.763 | 0.64 | 0.352 | 0.443 | 0.001 |
| Zone 3 | 0.577 | 0.513 | 0.392 | 0.61 | 0.237 |
| Zone 4 | 0.794 | 0.753 | 0.718 | 0.484 | 0.511 |
| Zone 5 | 0.7 | 0.754 | 0.61 | 0.595 | 0.194 |
| Zone 6 | 0.741 | 0.646 | 0.355 | 0.556 | 0.24 |
| Zone 7 | 0.522 | 0.573 | 0.401 | 0.461 | 0.14 |
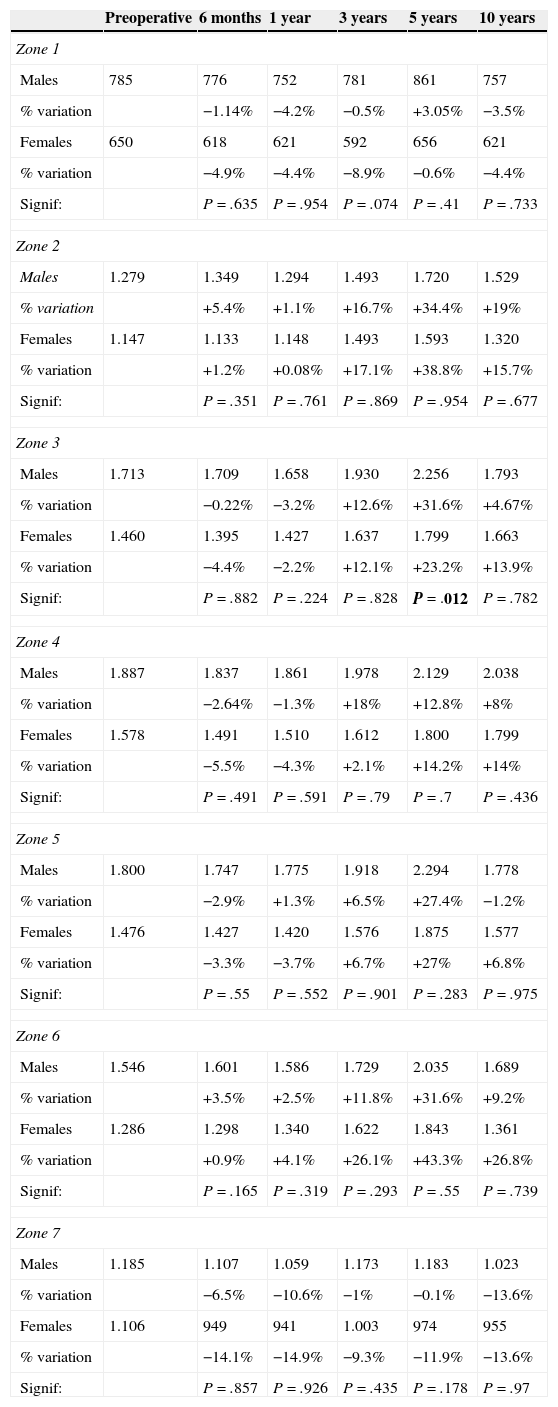
In order to study the influence of body mass index on bone remodelling, the sample was divided into 2 groups: group 1 with a body mass index below or equal to 30 (19 patients), and group 2 with a body mass index over 30 (20 patients). No statistically significant influence was observed between this factor and periprosthetic bone remodelling. Similar data were obtained in the analysis of gender as a factor influencing bone remodelling, which did not record any statistically significant differences, as reflected in Table 2.
Comparison of BMD in mg/cm2 in both genders in the intervened femur in the different Gruen zones.
| Preoperative | 6 months | 1 year | 3 years | 5 years | 10 years | |
|---|---|---|---|---|---|---|
| Zone 1 | ||||||
| Males | 785 | 776 | 752 | 781 | 861 | 757 |
| % variation | −1.14% | −4.2% | −0.5% | +3.05% | −3.5% | |
| Females | 650 | 618 | 621 | 592 | 656 | 621 |
| % variation | −4.9% | −4.4% | −8.9% | −0.6% | −4.4% | |
| Signif: | P=.635 | P=.954 | P=.074 | P=.41 | P=.733 | |
| Zone 2 | ||||||
| Males | 1.279 | 1.349 | 1.294 | 1.493 | 1.720 | 1.529 |
| % variation | +5.4% | +1.1% | +16.7% | +34.4% | +19% | |
| Females | 1.147 | 1.133 | 1.148 | 1.493 | 1.593 | 1.320 |
| % variation | +1.2% | +0.08% | +17.1% | +38.8% | +15.7% | |
| Signif: | P=.351 | P=.761 | P=.869 | P=.954 | P=.677 | |
| Zone 3 | ||||||
| Males | 1.713 | 1.709 | 1.658 | 1.930 | 2.256 | 1.793 |
| % variation | −0.22% | −3.2% | +12.6% | +31.6% | +4.67% | |
| Females | 1.460 | 1.395 | 1.427 | 1.637 | 1.799 | 1.663 |
| % variation | −4.4% | −2.2% | +12.1% | +23.2% | +13.9% | |
| Signif: | P=.882 | P=.224 | P=.828 | P=.012 | P=.782 | |
| Zone 4 | ||||||
| Males | 1.887 | 1.837 | 1.861 | 1.978 | 2.129 | 2.038 |
| % variation | −2.64% | −1.3% | +18% | +12.8% | +8% | |
| Females | 1.578 | 1.491 | 1.510 | 1.612 | 1.800 | 1.799 |
| % variation | −5.5% | −4.3% | +2.1% | +14.2% | +14% | |
| Signif: | P=.491 | P=.591 | P=.79 | P=.7 | P=.436 | |
| Zone 5 | ||||||
| Males | 1.800 | 1.747 | 1.775 | 1.918 | 2.294 | 1.778 |
| % variation | −2.9% | +1.3% | +6.5% | +27.4% | −1.2% | |
| Females | 1.476 | 1.427 | 1.420 | 1.576 | 1.875 | 1.577 |
| % variation | −3.3% | −3.7% | +6.7% | +27% | +6.8% | |
| Signif: | P=.55 | P=.552 | P=.901 | P=.283 | P=.975 | |
| Zone 6 | ||||||
| Males | 1.546 | 1.601 | 1.586 | 1.729 | 2.035 | 1.689 |
| % variation | +3.5% | +2.5% | +11.8% | +31.6% | +9.2% | |
| Females | 1.286 | 1.298 | 1.340 | 1.622 | 1.843 | 1.361 |
| % variation | +0.9% | +4.1% | +26.1% | +43.3% | +26.8% | |
| Signif: | P=.165 | P=.319 | P=.293 | P=.55 | P=.739 | |
| Zone 7 | ||||||
| Males | 1.185 | 1.107 | 1.059 | 1.173 | 1.183 | 1.023 |
| % variation | −6.5% | −10.6% | −1% | −0.1% | −13.6% | |
| Females | 1.106 | 949 | 941 | 1.003 | 974 | 955 |
| % variation | −14.1% | −14.9% | −9.3% | −11.9% | −13.6% | |
| Signif: | P=.857 | P=.926 | P=.435 | P=.178 | P=.97 | |
Lastly, it is worth noting that there were no medical complications during monitoring which could have been responsible for variations in bone mineral density.
DiscussionBone remodelling after a hip arthroplasty has a multifactorial origin.1,2,7 It is possible to detect a rapid loss of bone during the first 6 months, which may go from 20% to 50% depending on the implant and study methodology. This bone loss is affected by different factors, such as surgical aggression, rest in the immediate postoperative period and partial weight load. The bone mass figures are affected by surgical drilling and preparation of the proximal femur, causing an immediate decrease of the bone stock, by up to 10%.11
Postoperative rest and partial weight load cannot fully explain some of the bone mass losses taking place in proximal areas of the femur. The surgical technique has a significant influence on the changes taking place in the early period. The preparation of the metaphysis and the pressure fitting of the implant can cause microfractures in the cancellous bone, producing new reductions of bone mass detected during the first 6 months of monitoring. The amount and extension of these changes are due to an unequal surgical aggression on different areas of the proximal femur.
Femoral preparation in the medial and distal areas causes a significant disruption of endosteal circulation due to drilling and can produce bone necrosis in the most internal part of the cortex.12 On the other hand, on the calcaneus, the surgical exposure of the femoral neck at the moment of osteotomy damages periosteal vascularisation. In addition, filing also affects endosteal circulation. As a result, at the end of the process this area may have suffered denervation and a significant loss of vascularisation, causing a variable degree of bone necrosis and resorption. The sum of biological and mechanical factors may explain the reduction of bone mass in this area, reaching up to 24% during the first 6 months in some studies,13 and which is gradually regained in controls carried out 18 months after the intervention.14
It is generally accepted that most of the remodelling becomes stabilised at the end of the first year after the intervention, when the bone density seems to reach a plateau in all areas around the prosthesis.15,16 After this point, the changes reflect the biomechanical response of the bone according to Wolff's law.
Other studies conducted with quantitative computed tomography (CT)17 have observed a progressive decrease in BMD in the metaphysis, with losses around 13.8% in the major trochanter and 17.8% in the minor trochanter at 5 years, along with greater involvement of cancellous bone than cortical bone. No correlation has been observed with factors such as gender, body mass index and affected side. This study concluded that the most relevant factors in the reduction of periprosthetic bone mass are age, osteolysis and the stress-shielding effect.
Regarding the influence of other factors such as body mass index and gender on bone remodelling, several studies17–21 have reported similar results to those obtained in this work, which has not found any significant difference in remodelling when taking these factors as study determinants.
Regarding the predictive power of the initial bone mass to determine BMD throughout the monitoring period, various authors have reached the conclusion that preoperative BMD is an important factor in periprosthetic bone remodelling,22–24 a fact that has been verified in this study only during the first year of monitoring, thus indicating that further, long-term studies would be necessary to evaluate the predictive capacity of initial BMD for periprosthetic remodelling. Other authors maintain that preoperative BMD is a minor factor, with no influence on other events such as stem collapse25 and periprosthetic fractures,26 arguing that the stability of press-fit components is based on cortical contact, rather than purely metaphyseal bone transfer.27
We are aware of various limitations in this study: firstly, the number of subjects included could be insufficient and a larger number of patients could provide greater value to the statistical analysis. Secondly, the monitoring period could be insufficient, since it is precisely in older patients that the changes due to long-term bone remodelling may have a greater effect and cause more significant consequences for implant fixation.
In conclusion, we consider that preoperative bone mass has not shown any correlation with periprosthetic bone mass at the end of the follow-up period. Other factors assessed in this study, such as gender and body mass index, did not show any measurable influence.
Level of evidenceLevel of evidence III.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation did not require experiments on humans or animals.
Confidentiality of dataThe authors declare that this work does not reflect any patient data.
Right to privacy and informed consentThe authors declare having obtained written informed consent from patients and/or subjects referred to in the work. This document is held by the corresponding author.
Conflict of interestThe authors have no conflict of interests to declare and have not received any kind of financing to carry out this work.
Please cite this article as: Aguilar Ezquerra A, Panisello Sebastiá JJ, Mateo Agudo J. Influencia de la masa ósea preoperatoria en la remodelación femoral periprotésica tras la implantación de una prótesis ABG-II: resultados tras 10 años de seguimiento. Rev Esp Cir Ortop Traumatol. 2015;60:53–58.