To evaluate the in vivo anti-staphylococcal bactericidal activity of farnesol on Ti6Al4V surfaces.
Material and methodsAn experimental model of infection in biomaterials was developed by inoculation of Staphylococcus aureus ATCC 29213 into the canal of both femurs of 15 Wistar rats. A Ti6Al4V pin impregnated with 30mM of farnesol was inserted into study femur, and a Ti6Al4V control was inserted into the control femur. To evaluate the bactericidal efficacy, a comparison was made between the median of the colony forming units recovered after inoculation in the study group and the control group for different times of euthanasia and inoculum size.
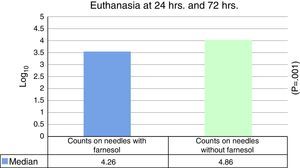
ResultsThe median expressed as Log10 CFU counts obtained with farnesol titanium pin was 4.26, and in control group, it was 4.86, which was statistically significant (P=.001) on applying the Student t test for related samples.
The median reduction obtained in farnesol pins relative to the control was 74%.
ConclusionsTreatment with farnesol 30mM on Ti6Al4V pins appears to decrease the rate of colonisation by S. aureus.
Evaluar in vivo la actividad bactericida antiestafilocócica del farnesol sobre superficies de Ti6Al4V.
Material y métodosSe desarrolló un modelo experimental de infecciones en biomateriales inoculando Staphylococcus aureus ATCC 29213 en los fémures de 15 ratas wistar. Seguidamente se insertó una aguja de Ti6Al4V impregnada con farnesol 30mM en el fémur estudio y una aguja control en el fémur control. Para valorar la eficacia bactericida se compararon las medianas de unidades formadoras de colonias recuperadas después de la inoculación en el grupo estudio y en el grupo control, para diferentes tiempos de eutanasia y tamaño de inóculos.
ResultadosLa mediana expresada en Log10 de los recuentos de UFC obtenidos en agujas de titanio con farnesol fue de 4,26 y en agujas sin farnesol, controles, fue de 4,86. Esta diferencia, al aplicar la prueba de t de Student para muestras relacionadas, resultó ser estadísticamente significativa (p=0,001). La reducción mediana obtenida en las agujas con farnesol respecto a las agujas control fue del 74%.
ConclusionesEl tratamiento con farnesol de agujas de Ti6Al4V, a una concentración de 30mM, parece disminuir la tasa de colonización por Staphylococcus aureus en dichas agujas.
The infections in biomaterials implanted in live beings are normally associated with the formation of a biofilm that is difficult to eradicate. In the majority of cases it is necessary to remove the infected implant. All of this gives rise to a considerable increase in morbidity, mortality and healthcare costs.
Pathogenic bacteria have developed many defence mechanisms against antibacterial agents, so that resistance against old and new pharmaceutical products is increasing.
Due to the gradual increase in the antibiotic resistance of these bacteria, researchers have studied different organic molecules with antibacterial capacity. In this context attention has focused strongly on natural products such as plant-derived compounds including the essential oils.
Farnesol (C15H26O) is a natural organic compound, an acyclic sesquiterpene alcohol that is found in many essential oils such as citronella oil.1 It intervenes in the quorum sensing of Candida, blocking the formation of a biofilm as well as the production of other virulence factors by this fungus.2 Farnesol affects the growth of a large number of bacteria and fungi, such as Staphylococcus aureus,2,3Streptococcus mutans4 and Fusarium graminearum,5 which underlines its potential use as an antimicrobial agent.6
Bhattacharyya et al. stated that farnesol penetrates the biofilm, accumulating in the cell membrane, where it increases the porosity of the same by its mechanism of action.7 This increase in cell membrane permeability to different substances may increase the absorption of antibiotics if they are used together with farnesol. This would mean that a lower dose of antibiotic would be necessary, and this in turn would reduce the possible appearance of resistence. i.e., farnesol would increase the susceptibility of bacteria to antibiotics and other antimicrobial compounds.8
In a recent paper Unnuntana et al. give an in vitro demonstration of the capacity of farnesol to inhibit the formation of methicillin-sensitive biofilms of S. aureus at concentrations of 30mM on titanium discs.9
The aim of this work is to analyse whether treatment with farnesol of the surface of Ti6Al4V needles prior to their implantation in rat femurs reduces the rate of S. aureus colonisation on the said needles and in the femur containing them.
Material and methodsOsteosynthesis material: Ti6Al4VThe Ti6Al4V alloy was supplied by Kirschner Maschinenbau GmbH (Unterschneidheim, Germany) in the form of 1.2mm×150mm wire, which was cut using a chisel into 1.2mm×20mm pieces.
The needle-cleaning protocol prior to their implantation was as follows: Derquim DSF at 2%, sonication, immersion in distilled water at 60°C during 15min, 10min in acetone at 70% and finally a Pasteur oven during 30min at 40°C.
The needles were divided into 2 groups:
- a)
Those which were to be used as control needles were not treated with any other process.
- b)
The needles which were used to study the bactericide effect of farnesol on Ti6Al4V alloy were subjected to the following process as well as those described above:
- 1)
Immersion in a piranha solution with 5ml H2SO4 concentrate at 5ml and 30% H2O2% during one hour.
- 2)
Washing with water and ethanol in an ultrasound bath during 10min with each liquid.
- 3)
The needles were then immersed in a farnesol solution (30mM) during 24h.
- 4)
Lastly they were dried for 2–3h on sterile absorbent paper in a Pasteur oven at 50°C.
- 1)
The pathogen used was S. aureus, of the ATCC 29213 (American Type Culture Collection) strain. The bacteria were allowed to multiply during 18–24h in a stove at 37°C in tryptic soy broth (TSB) (BBL, Becton Dickinson and Company, Sparks, USA). It was then diluted to achieve the desired final concentration of bacteria.
Experimental animalAll of the in vivo trials conducted for this work were previously approved by the Ethics Committee of Extremadura University (study number: 161/2009).
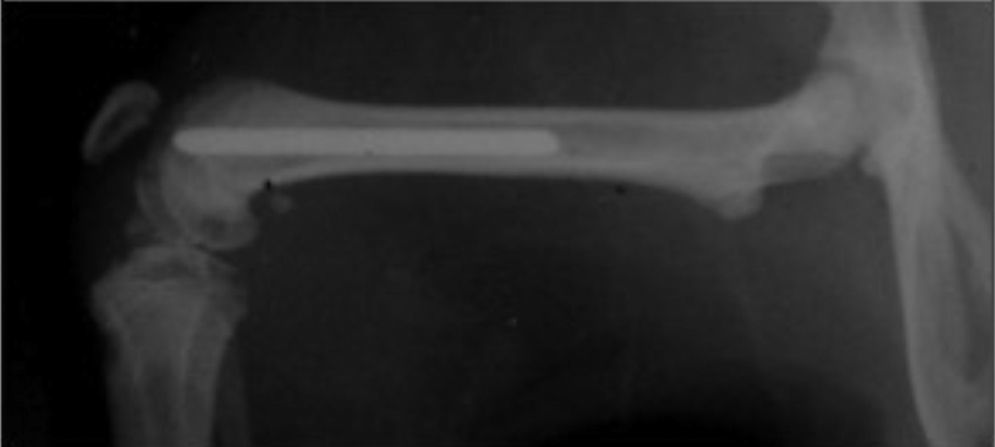
In this study 15 male Wistar rats of similar weight (300–350g) were operated. With no preoperative antibiotic prophylaxis a Ti6Al4V titanium needle measuring 1.2mm×20mm was implanted at random in both femurs of the rats (Fig. 1). One needle had been treated with farnesol and the other had not, so that one leg with the farnesol-treated needle functioned as the study leg and the other with the needle without farsenol acted as the control leg.
The euthanasia of the rats took place at two stages, at 24h (7 rats) and at 72h after the operation (8 rats).
Surgical technique, euthanasia, sample collection and processingThe anaesthetic solution was composed of 50% ketamine, 40% diazepam and 10% atropine. A dose of 0.004ml/kg weight was administered intraperitoneally. A 1cm internal approach to the patella was used with external luxation of the patella. An orifice was created in the intercondyle region by manual puncture with the needle of a number 20 Abbocath catheter, moving downward from the metaphyseal zone until reaching 3.5cm in the femoral diaphysis, thereby creating an intramedullar channel while always respecting the cortical ones. The diameter of this channel was then increased using Abbocath needles gradually larger in calibre (numbers 18 and 16). Then with the aid of a microsyringe inserted into the depth of the canal each leg was inoculated with 10μL of different inoculates of S. aureus ATCC 29213 containing an approximate number of bacteria from 300 to 1300. The same numbers of bacteria were always inoculated into the study and control legs. Finally, and following randomisation, the Ti6Al4V titanium measuring 1.2mm×20mm were inserted into the cavities created, with farnesol in the study leg and no farnesol in the control leg.
Once the operation ended the rats were stabled in independent cages until their euthanasia, with routine feeding and care.
The rats were sacrificed at the planned time (24–72h after the operation). Euthanasia was by the intracardiac injection of 0.5ml ClK/100g bodyweight, after sedation of the animal with the anaesthetic solution described above. At the same time a sample of blood was taken by cardiac puncture for haemoculture and to evaluate the existence of any possible bacteraemia.
Immediately afterwards and under strict aseptic conditions in the operating theatre both femurs were removed by decoupling them from the knees and hips with the minimum possible amount of muscle tissue. The precaution was always taken of using sterile surgical material for each leg. Once the femurs had been extracted they were fractured using gouge forceps to obtain the intramedullar needle. The femurs as well as the needles were collected in tubes separated by sterile PBS and marked to identify whether they were from the right or left leg, together with the number of bacteria they had been inoculated with.
For microbiological analysis the bone samples were fragmented to carry out the bacterial dilutions, while no previous process was required for the needles.
The bacterial dilutions were carried out by sonication during 15min. In an ultrasound bath (Ultrasons P Selecta, Madrid, Spain), to remove the bacteria which adhered strongly to the samples. Once the bacteria had been re-suspended in the medium, serialised dilutions were performed and were then sown in common agar plates. These plates were incubated for 18–24h at 37°C in a stove. The Colony-Forming Units (CFU) which had grown in the different dilutions of each sample were then counted using a magnifying glass after this time, and these data were recorded. To obtain a more exact number of CFU per sample the average of those which had grown in the series of sample dilutions was calculated.
Statistical analysisStatistical analysis was undertaken using the SPSS 20 program for Mac (SPSS, Chicago, IL, USA). All results were expressed as measurements with a central tendency, such as the average and median, together with dispersion measurements such as variance. The differences in the counts of colonies recovered following inoculation between the different study groups were evaluated using the parametric Student t-test and Wilcoxon's non-parametric test of signed ranges for related samples. Probability values of less than 0.05 were considered statistically significant.
ResultsThe Kolmogorov–Smirnov and Shapiro–Wilk normality tests were first applied to the different study variables, giving a result that was not statistically significant for all of the variables except for the Log count variable for the control leg. i.e., all of the variables except this one followed a normal distribution, so that parametric as well as non-parametric tests were used for statistical analysis.
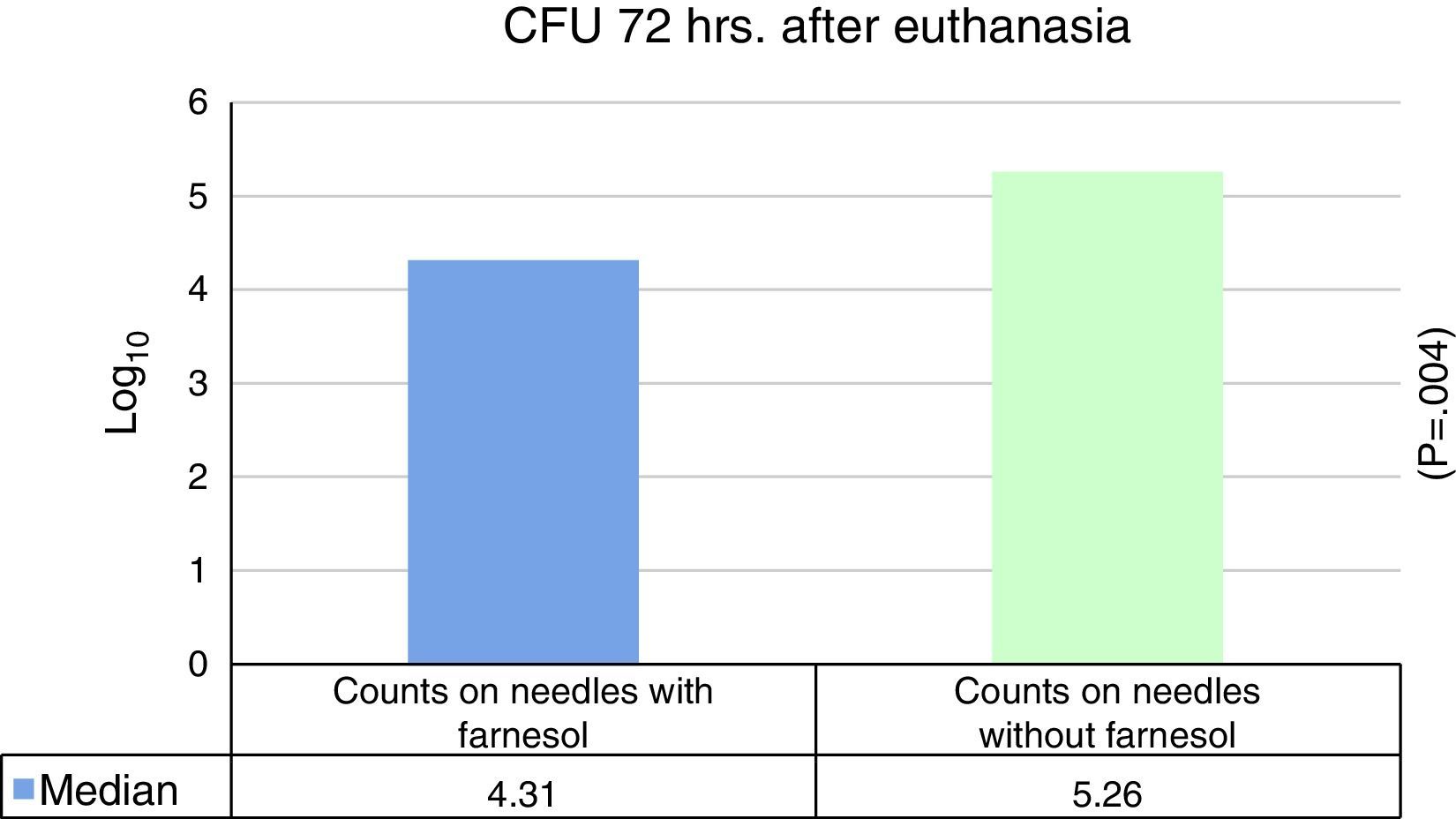
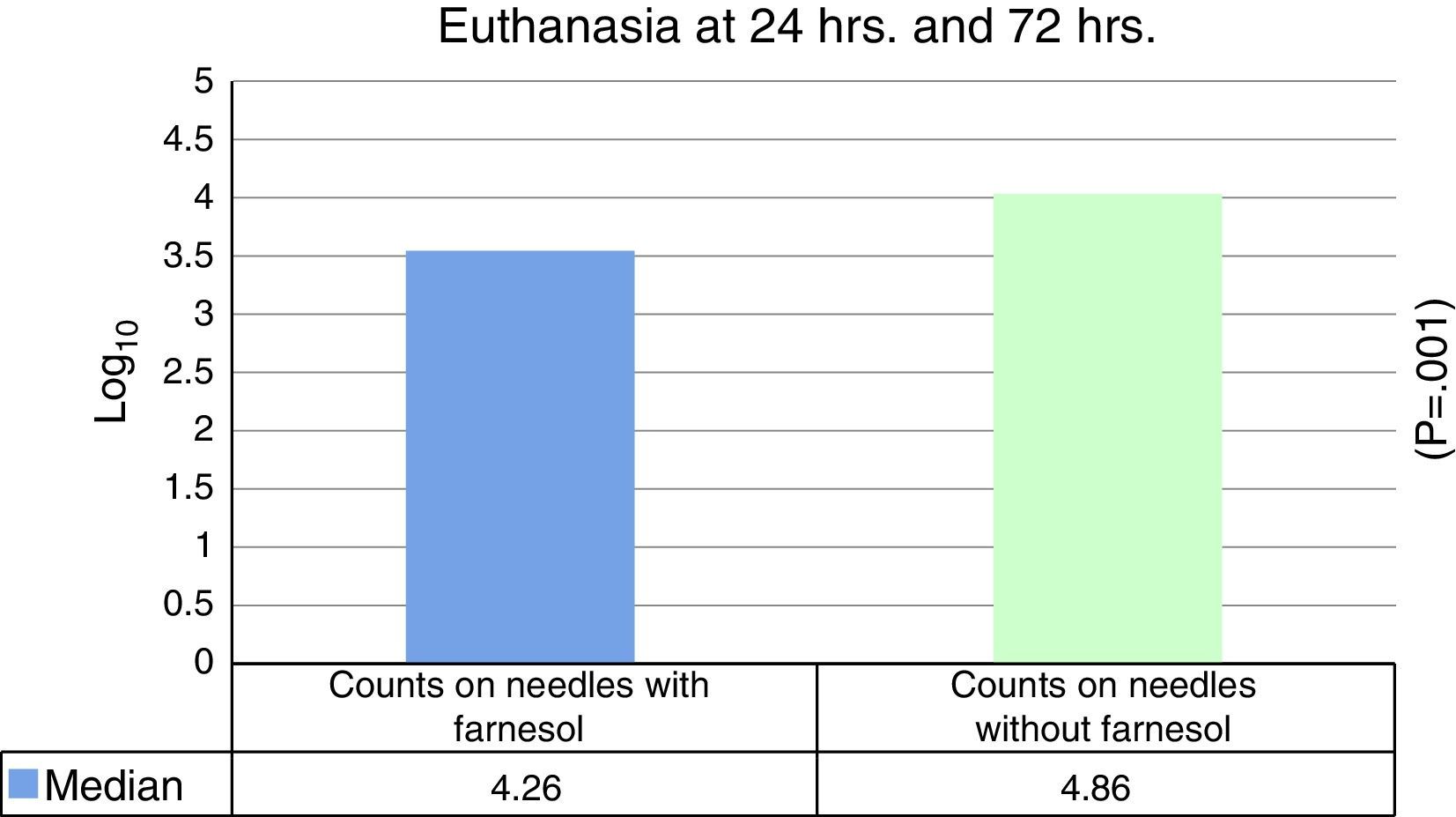
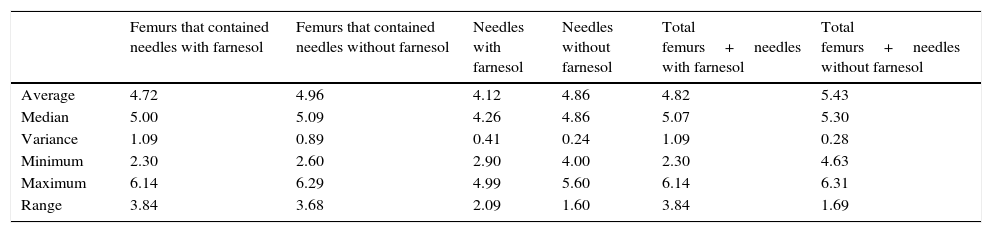
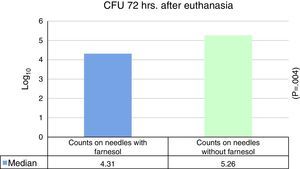
No statistically significant differences were found for euthanasia at 24h in the recounts of farnesol-impregnated needles in comparison with the control needles. This was also the case for recounts of femurs that had contained farnesol-impregnated needles with those that had contained control needles (Table 1). For euthanasia at 72h the only statistically significant difference found (Student's t-test) (P=.004) was in the median expressed as Log10 of the CFU counts in farnesol-impregnated titanium needles (4.31) compared to those of the farnesol-free or control needles (5.26). There was an 88% reduction in the farnesol-treated needles compared to the control ones (Fig. 2; Table 2). When the overall results were analysed without taking euthanasia time into account the median expressed in Log10 of the CFU counts obtained in titanium needles with farnesol was 4.26, and in the farnesol-free control needles the corresponding figure was 4.86. When Student's t-test for related samples was applied this difference was found to be statistically significant (P=.001). The median reduction obtained in the needles with farnesol compared to the control needles was 74% (Fig. 3; Table 3).
Summary of the descriptive statistics obtained in the recovered counts of needles and femurs expressed in Log10 CFU for euthanasia at 24h.
| Femurs that contained needles with farnesol | Femurs that contained needles without farnesol | Needles with farnesol | Needles without farnesol | Total femurs+needles with farnesol | Total femurs+needles without farnesol | |
|---|---|---|---|---|---|---|
| Average | 4.47 | 4.43 | 3.79 | 4.63 | 4.59 | 5.02 |
| Median | 4.62 | 4.64 | 3.70 | 4.61 | 4.71 | 5.19 |
| Variance | 0.81 | 1.12 | 0.89 | 0.10 | 0.77 | 0.11 |
| Minimum | 3.26 | 2.60 | 2.90 | 4.12 | 3.41 | 4.63 |
| Maximum | 5.43 | 5.20 | 4.90 | 5.06 | 5.54 | 5.30 |
| Range | 2.18 | 2.60 | 1.99 | 0.94 | 2.13 | 0.67 |
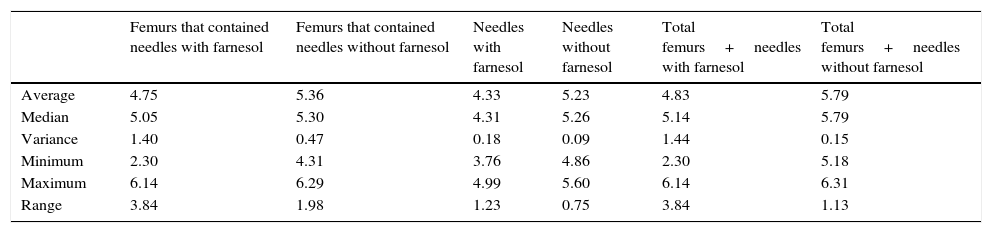
Summary of the descriptive statistics obtained in the recovered counts of needles and femurs expressed in Log10 CFU for euthanasia at 72h.
| Femurs that contained needles with farnesol | Femurs that contained needles without farnesol | Needles with farnesol | Needles without farnesol | Total femurs+needles with farnesol | Total femurs+needles without farnesol | |
|---|---|---|---|---|---|---|
| Average | 4.75 | 5.36 | 4.33 | 5.23 | 4.83 | 5.79 |
| Median | 5.05 | 5.30 | 4.31 | 5.26 | 5.14 | 5.79 |
| Variance | 1.40 | 0.47 | 0.18 | 0.09 | 1.44 | 0.15 |
| Minimum | 2.30 | 4.31 | 3.76 | 4.86 | 2.30 | 5.18 |
| Maximum | 6.14 | 6.29 | 4.99 | 5.60 | 6.14 | 6.31 |
| Range | 3.84 | 1.98 | 1.23 | 0.75 | 3.84 | 1.13 |
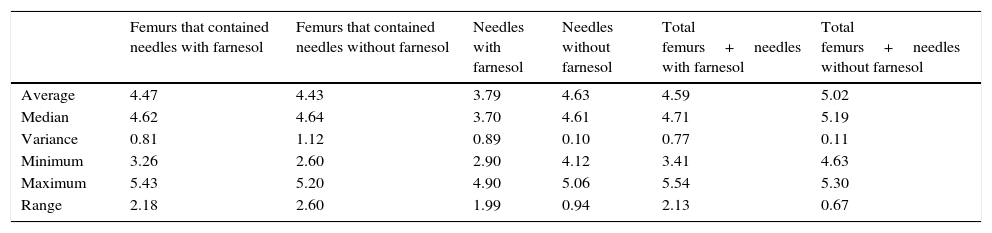
Summary of the descriptive statistics obtained in the recovered counts of needles and femurs expressed in Log10 CFU for euthanasia at 48h and 72h.
| Femurs that contained needles with farnesol | Femurs that contained needles without farnesol | Needles with farnesol | Needles without farnesol | Total femurs+needles with farnesol | Total femurs+needles without farnesol | |
|---|---|---|---|---|---|---|
| Average | 4.72 | 4.96 | 4.12 | 4.86 | 4.82 | 5.43 |
| Median | 5.00 | 5.09 | 4.26 | 4.86 | 5.07 | 5.30 |
| Variance | 1.09 | 0.89 | 0.41 | 0.24 | 1.09 | 0.28 |
| Minimum | 2.30 | 2.60 | 2.90 | 4.00 | 2.30 | 4.63 |
| Maximum | 6.14 | 6.29 | 4.99 | 5.60 | 6.14 | 6.31 |
| Range | 3.84 | 3.68 | 2.09 | 1.60 | 3.84 | 1.69 |
New antibiotic-coated biomaterials are being researched; nevertheless, their use for the prevention of infection in biomaterials is controversial, as the concentration of bactericide agents placed in these biomaterials may cause local or systemic toxicity, while the prolonged release of antibiotics at sub-inhibitory levels may lead to the appearance of new strains of resistant microorganisms.10 There is therefore a critical need for the development of new antimicrobial compounds with inhibitory power that do not increase resistance. In this context the natural substances used in traditional medicine may play an important role.
Farnesol is an antifungal and antibacterial sesquiterpene alcohol that is found in the essential oils of certain citrus fruits. Farnesol was first found to inhibit intercellular communication in Candida albicans11 fungus. It has also been shown to inhibit the filamentation process and quorum sensing system in fungi and certain bacteria.2,7 Farnesol does not seem to have any systemic or mutagenic toxic effects in vitro or in vivo.2
Due to these properties, research groups have examined its effect on biofilms of S. aureus in vitro. Unnuntana et al. demonstrated the capacity of concentrations of 30mM farnesol to inhibit the formation of biofilms of methicillin-sensitive S. aureus on titanium discs.9 The Jabra-Rizk and Gomes groups showed that lower concentrations in the range of 200–300μM still have an antibacterial effect on biofilms of S. Aureus, which they attribute to the disruption of its cell membrane.3,11
We found no in vivo studies in the literature of the influence of farnesol in the prevention of the colonisation of titanium implants by S. aureus. Basing ourselves on the article by Unnuntana et al., we designed this work in rats using Ti6Al4V needles impregnated with a farnesol solution (30mM).
Several experimental models of osteomyelitis have been developed using different animals, including rats,12–14 rabbits,15 dogs,16 pigs,17 etc. In the majority of osteomyelitis models, including those in rats, different agents and strategies to facilitate bone infection are used: sclerosing agents such as Morrhuate Sodium13 or its derivative arachidonic acid18 have been used, as has thermal bone necrosis created by electrocauterisation19 or a drill to open the intramedullar canal in the bone. We did not use a sclerosing agent or drill in our experimental model to perforate the femur, and nor did we use bone wax to cap the hole in the canal to try to isolate the effect of the titanium as a foreign body which favours osteomyelitis.
On analysing the results obtained we found no statistically significant differences between the median bacterial counts in the femurs which contained the farnesol-coated needles and those which held the control needles (control femurs). Nevertheless, we did find statistically significant differences in the bacterial counts obtained in farnesol-coated needles and their controls 72h after inoculation.
No statistically significant reduction in bacterial counts between farnesol-coated needles and the controls were found for euthanasia at 24h. This may be due to the small sample size of the subgroups, and in fact when both (24h and 72h) subgroups are analysed together statistically significant differences are obtained.
The actual mechanism due to which farnesol has an antibacterial effect is unknown, although several theories have been accepted. The main mechanism of action of this substance seems to be disruption of the cellular membrane of S. aureus. Given the lipidic nature of this small molecule, it is relatively easy for farnesol to accumulate in the cellular membrane and cause it to fragment. This has been shown by the increase in liberation of potassium ions from the cell membrane.2,3 This increase in potassium ions occurs 10s after the addition of farnesol, indicating that farnesol is able to damage the membrane of S. aureus very quickly.3 Farnesol also reduces S. aureus fibrin matrix formation by inhibiting coagulase,2,7,13,20 and it also inhibits the mevalonate pathway, hindering the appropriate production and repair of cell membrane.8,20 Farnesol therefore increases the permeability of bacterial cell membranes to exogenic chemical components including antibiotics in a non-specific way. This would make it possible to increase the efficacy of antimicrobial agents against bacteria as well as S. aureus biofilm. Some in vitro studies have shown the capacity of farnesol to increase the susceptibility of microorganisms to antimicrobial agents, thereby indicating its possible use as an adjuvant drug.5 Brehm-Stecher et al.8 described an increase in the susceptibility of S. aureus to ciprofloxacin, clindamycin, erythromycin, gentamycin, tetracyclin and vancomycin, as well as increased susceptibility of E. coli to polymyxin B, when these drugs are combined with farnesol.
Local toxicity and possible harmful effects on osteointegration are always a concern when any agent is used directly on bone. Unnuntana et al.9 investigated the effects of farnesol in vitro on osteoblasts on titanium discs. These authors demonstrated that concentrations of farnesol of from 3 to 30mM had irreversible negative effects on preosteoblasts, so that they merged and formed conglomerates but did not spread over the titanium surface. These findings are consistent with those of other studies which show that farnesol causes cytoskeletal disorganisation and apoptosis in other types of cells, such as tumoural ones.21,22 There are currently no in vivo studies that analyse the possible long-term local or general toxicity of this substance. It would be necessary to undertake in vivo studies for histological and radiological evaluation over the long-term of the possible negative effect of farnesol on titanium osteointegration.
The chief limitation of this work is its sample size, which means we do not have the minimum population in the different subgroups that would be required to evaluate other variables, such as the influence of the size of the bacterial inoculate on the bactericide effect of the farnesol on Ti6Al4V needles.
Another major restriction is that this study uses an attenuated strain of S. aureus (ATCC 29213). The results obtained with this strain may not correspond with those resulting from the use of clinical strains with intact pathogenecity factors. More studies in different clinical strains will therefore be necessary to confirm that the results may be of clinical importance and could be extrapolated to medical care.
In this work we found a wide variation in bacterial counts obtained from femurs and needles, regardless of whether or not they were impregnated with farnesol. On the one hand this variability in the number of bacteria reflects the intrinsic peculiarities of an in vivo model (anatomical variations in the femur, in bleeding within the canal, in the immune system of the rat, etc.). On the other hand, possible errors that may have been committed in the study techniques may increase variation, such as differences in bone reaming, in the positioning of the implant, in the preparation of the inoculates or in sample processing, etc. We therefore believe that it would be very difficult to reproduce the results of an in vitro study in vivo, given that several variables influence the results, and we are completely unable to control them.
It would also be advisable to conduct an in vitro study that would make it possible to establish the kinetics of farnesol release from the titanium needles. If this is not uniform it may explain some of the variability in the results.
Based on the results of this study, and in spite of the intrinsic variability of in vivo models, we are able to conclude that treating Ti6Al4V with a 30mM concentration of farnesol seems to reduce the rate of colonisation by S. aureus ATCC 29213 when the said needles are implanted in rat femurs.
Level of evidenceLevel of evidence I.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed conform to the ethical norms of the responsible human experimentation committee and the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that no patient data are shown in this work.
Right to privacy and informed consentThe authors declare that no patient data are shown in this work.
Conflict of interestThe authors have no conflict of interests to declare.
This work was undertaken thanks to all of the members of the Microbial Adhesion Research Group of Extremadura University (AM-UEX) in the Networked Bioengineering, Biomaterials and Nanomedicine Biomedical Research Centre (CIBERBBN).
Please cite this article as: Constantino JA, Delgado-Rastrollo M, Pacha-Olivenza MA, Pérez-Giraldo C, Quiles M, González-Martín ML, et al. Eficacia bactericida in vivo del farnesol sobre implantes de Ti6Al4V. Rev Esp Cir Ortop Traumatol. 2016;60:260–266.