To determine the influence of the timing of the removal of the ischaemia tourniquet on the transfusion needs in primary knee arthroplasty and its influence on post-operative complications.
Materials and methodsA retrospective analysis was performed on 201 primary knee arthroplasties. The patients were divided into two groups: group A (101 patients), where the tourniquet was removed before surgical closure, and group B (100 patients), where the tourniquet was removed after the surgical closure. A comparison was made of the blood transfusions (pools of red corpuscles) received by both groups, along with the surgical time, and the post-operative complications.
ResultsThe mean number of packed red cells transfused in group A was 0.62, while in the group B it was 0.61 (P=0.831). The mean time in the surgery in group A was 111min, and in group B it was 98min (P<0.05), with no significant difference between the surgical time and the number of packed cells transfused (P=0.055). The post-operative complications in the group A were 8, and in the B were 10 (P=0.79).
DiscussionThere were no differences between removing the tourniquet before or after surgical closure or in the transfusion needs or in the post-operative complications. Traditionally, the greater blood loss due to the removal of the tourniquet before surgical closure has been explained as due to the longer surgery time required. However, in the present study, this fact was not associated with greater transfusion needs, or with more post-operative complications.
Conocer la influencia del momento de la retirada del manguito de isquemia en la necesidad de transfusiones sanguíneas en las artroplastias primarias de rodilla y su influencia en las complicaciones postoperatorias.
Material y métodoFueron analizadas 201 artroplastias primarias de rodilla retrospectivamente. Se dividieron en 2 grupos: el grupo A (101 pacientes), en el que se retiró el manguito antes del cierre quirúrgico, y el grupo B (100 pacientes), en el que se retiró tras el cierre. Se compararon las transfusiones sanguíneas (medidas en concentrados de hematíes) llevadas a cabo por ambos grupos, contrastándolas con el tiempo quirúrgico, así como las complicaciones posquirúrgicas.
ResultadosLa media de concentrados de hematíes transfundidos en el grupo A fue de 0,62, mientras que en el grupo B fue de 0,61 (p=0,831). El tiempo medio quirúrgico en el grupo A fue de 111min, y en el grupo B, de 98min (p<0,05), no existiendo relación entre el tiempo quirúrgico y los concentrados de hematíes transfundidos (p=0,055). Las complicaciones posquirúrgicas en el grupo A fueron 8, y en el B, 10 (p=0,69).
DiscusiónNo hubo diferencias entre retirar el manguito de isquemia antes o después del cierre quirúrgico, ni en las necesidades transfusionales ni en las complicaciones posquirúrgicas. Tradicionalmente las mayores pérdidas sanguíneas al retirar el manguito antes del cierre han sido explicadas por el mayor tiempo quirúrgico que se requiere; sin embargo, este hecho no se relacionó con mayores necesidades transfusionales en nuestro estudio, ni con mayores complicaciones posquirúrgicas.
Blood loss derived from orthopaedic surgery should not be undervalued, as perioperative haemorrhage is estimated to be between 1000 and 1500ml in arthroplastic hip and knee surgery. It is estimated that a decrease of 1000ml represents the loss of 3gr/dl of haemoglobin (Hb).1 In our environment, a marked decrease in postoperative Hb is treated by transfusion of packed red blood cells (PRBC), however, this measurement is accompanied by an increase in morbidity and mortality,2 which take the form of infections, coagulopathies, immunosuppression, pulmonary lesions, disease transmission, etc. The criteria to carry out a transfusion are not always well defined, leaving the decision to the physician based on the symptoms and Hb levels in venous blood. However, these levels are not always completely reliable in the immediate postoperative period, which makes the clinical data essential in such situations (hypotension, tachycardia, pallor, decreased reactiveness, etc.).
Traditionally, a tourniquet applied through a pneumatic cuff placed in the thigh has been used to minimise perioperative blood loss. This causes ischaemia at the distal level of the cuff, which reduces intraoperative bleeding, and, in addition to entailing a decrease in patient morbidity and mortality, improves the view of the surgical field and favours the cementing technique. Nevertheless, this use is controversial, as it has been demonstrated that in 5min immediately after the release of the cuff there is an increase in reactive bleeding.3 In addition, the use of ischaemia seems to be a risk factor for deep vein thrombosis and greater postoperative pain due to neurovascular compression.4–7 On the other hand, some data show improved lateral tracking of the patella with the use of the cuff, due to the action of the vastus lateralis.8,9
The use of an ischaemia cuff was implemented in our environment in parallel to the knee prosthesis surgery itself and, at present, 100% of knee arthroplasties are carried out with a tourniquet. Nevertheless, the ideal way to remove the cuff is still under discussion, as there are two different possibilities: one advocates removing the ischaemia after cementing, prior to closing the surgical wound, carrying out haemostasis in the same action, whilst the other recommends removal after the closure, after applying a compressive bandage on the joint. Advocates of the first method argue the convenience of performing haemostasis through diathermic coagulation, as there is a periarticular arterial network (mainly the geniculated arteries deriving from the popliteal artery) which, if affected, would cause a haemorrhage. Those authors who opt for removing the ischaemia cuff after closing the surgical wound assure that an adequate approach avoids the geniculated arteries, and that the duration of surgery is reduced if haemostasis is not carried out, thus providing the added benefit of reducing haemorrhage and infection.
The majority of studies conducted show greater blood loss by removing the cuff before closure,10–15 when the lost blood is measured by an estimated calculation based on the Hb decrease in relation to the body mass index,16 whilst only one study has found greater blood loss by removing after closure.17 Nevertheless, an increased need for transfusions has been confirmed with cuff removal prior to closure, as shown by the metaanalysis of Rama et al.,18 28.6% versus 21.1%. In cases where the lost blood is measured directly (Hb, Hct, drainage, etc.) there are no differences in most studies10–12,18–25; only Yildiz et al.26 found differences in the mean direct blood loss (Hb). Thus, there is a notable error linked to imprecise measurements obtained postoperatively, as their variability is very high.18
On the other hand, there is a higher rate of local complications when the tourniquet is removed after closure,18 such as wound complications, deep vein thrombosis or the postoperative need for joint manipulation. There are also more reinterventions with release after closure, 3.1% versus 0.3%.
Furthermore, the use of a blood recovery system has not been linked with an increased need for transfusions based on whether it is removed before the cuff or after.27
Therefore, given this difference of results, and since there is a disparity regarding cuff removal in our hospital, we intend to review our series and verify the difference in terms of postoperative bleeding and clinical repercussions, manifested by normocytic and normochromic anaemia, with the subsequent increase in blood transfusions and possible complications derived thereof.
Materials and methodsPatientsWe retrospectively analysed 201 surgical interventions for primary total knee arthroplasty. We included patients aged between 55 and 85 years. We excluded patients who underwent anticoagulant or antiaggregant therapy (we did not take into consideration 100mg of acetyl salicylic acid and low molecular weight heparins) due to the variability in removal and reintroduction of these drugs based on the criteria of the surgeon, which could generate a bias among these patients. We did not include patients with coagulopathies, thrombopathies and other haematological diseases which affected haemostasis. Patients presented minimum preoperative Hb levels of 10gr/dl. Patients received one dose of low molecular weight heparin 12h before surgery, another 6h after the procedure, and then it was administered every 24h for 10 days.
DataPatients were operated on between June 30th 2010 and December 31st 2013. Patient data was obtained from their clinical histories, held by the archive of the hospital. We obtained approval from the regional committee for ethics and scientific research.
A total of eight surgeons specialising in Orthopaedic Surgery and Traumatology were responsible for the interventions.
SurgeryPatients were divided into two groups: one where the ischaemia cuff was removed prior to closing the surgical wound (group A) and another one where it was removed after closure (group B). The pneumatic cuff was placed in the proximal end of the thigh and inflated prior to the surgery at a pressure of between 270 and 350mmHg. A classical medial parapatellar approach was used in all cases. All the knee arthroplasties were stabilised posteriorly. In surgeries in which the cuff was removed before wound closure, the pressure was not loosened until cementing was completed. After applying compression on the surgical area with gauzes, haemostasis was carried out with a diathermic scalpel until it was confirmed that no vessels continued to bleed actively. Next, the surgical wound was closed, placing a drainage bulb or else a blood conservation system from the interior to the exterior of the joint. After this, a bandage was applied on the entire lower limb. The other alternative was to remove the cuff after closing the surgical wound (also leaving a drainage or recovery system) and applying a compressive bandage on the lower limb after the closure. The data on the method of tourniquet removal were extracted from the surgical protocol of the intervention, and contrasted with the protocols for nursing and anaesthesia. The choice of tourniquet removal method was the responsibility of each principal surgeon and was kept the same for all the interventions of each individual surgeon (except for one surgeon, who carried out two interventions with one method and the rest with another). Both the femoral and the tibial components were cemented in all the arthroplasties, as well as the patella, if this was replaced.
After the intervention, patients were taken to the reanimation area and from there to the Traumatology Service after 3–4h. The transfused blood was counted by collecting the data from the nursing history in the reanimation area and in the Traumatology Service. We took into account the number of transfused PRBC. We also considered autotransfusion with blood from the recovery system, counting every 250ml of recovered blood as 1 PRBC. The transfusion criteria were analytical, with an Hb level under 8gr/dl without comorbidities, or 10gr/dl with associated comorbidities or clear symptoms of anaemia. The symptoms were the reason for the decision to start transfusion during the stay in the reanimation room (based on systolic hypotension <80mmHg, tachycardia >120lpm or unreactive condition, with skin and mucosal pallor). We took into account the PRBC transfused during hospital admission in the Traumatology Service in the 72h after the intervention. The choice of blood recovery or drainage depended on the decision adopted by each surgeon during the intervention.
The monitoring of complications was carried out following the clinical history of each patient, from the intervention until the present moment. We took into account acute complications (during the first month), as well as the subacute (during the first 6 months) and the chronic (after 6 months). We distinguished between infectious and mechanical complications which required readmission. In addition, infections were classified as superficial and deep.
Statistical analysisThe descriptive analysis of Gaussian variables was expressed as mean±standard deviation and that of the non-Gaussian as median and first-third quartile. The Kolmogorov–Smirnov test was conducted to assess the normality of the variables. Qualitative variables were expressed as frequencies and percentages.
We conducted nonparametric tests (Chi-squared and Mann–Whitney U) to determine the association between removal of the ischaemia cuff (before, after) and the study variables.
A Mann–Whitney U test was carried out to verify if there were differences in surgery time between transfused and non-transfused patients.
Lastly, the Kruskal–Wallis test was applied to analyse the differences in surgery time between the different numbers of total PRBC. We also carried out multiple 2 on two comparisons using the Mann–Whitney U test with Bonferroni correction.
Differences of P<0.05 were considered as statistically significant in all cases. The analyses were carried out with the free software package R (http://www.r-project.org).
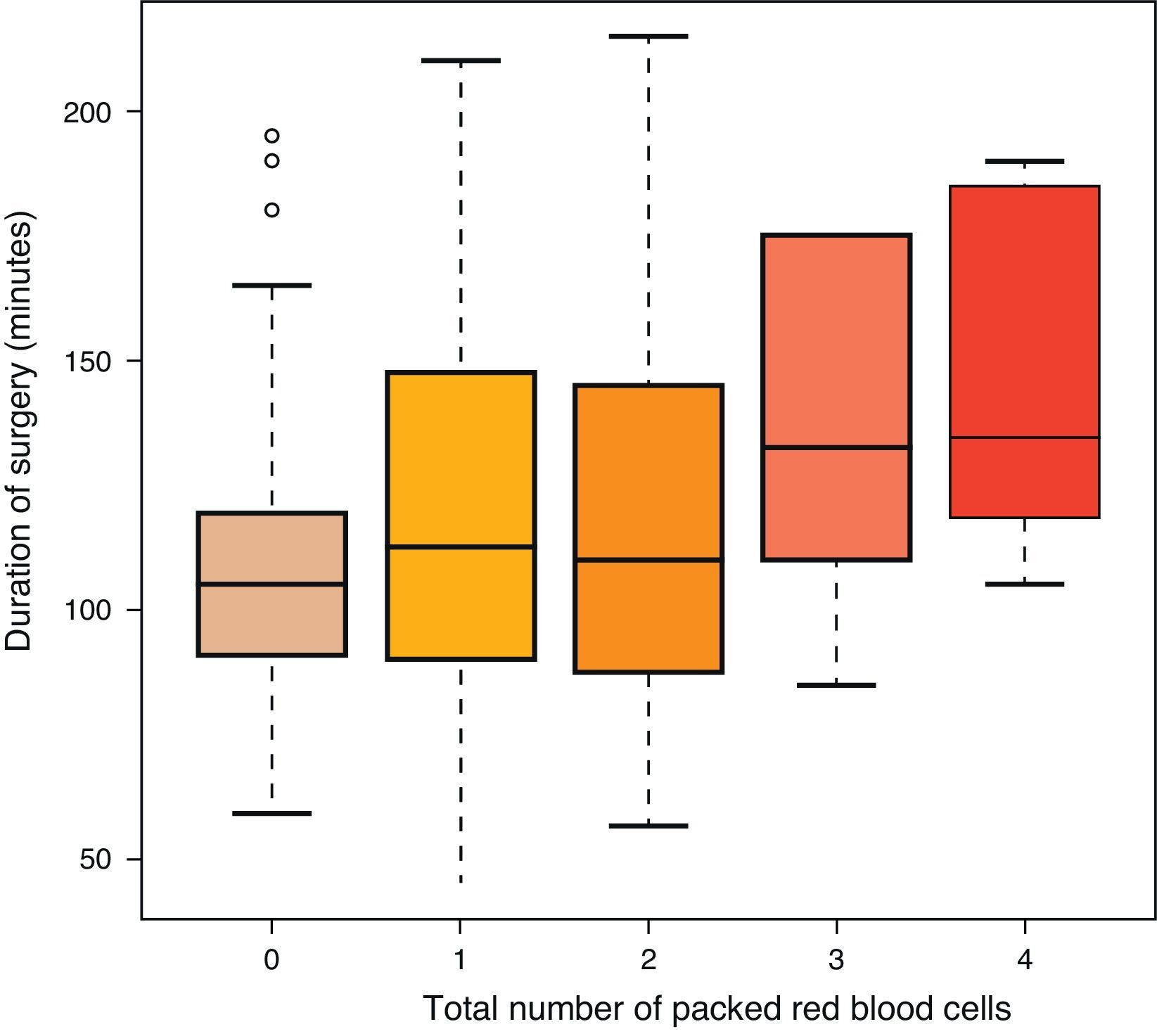
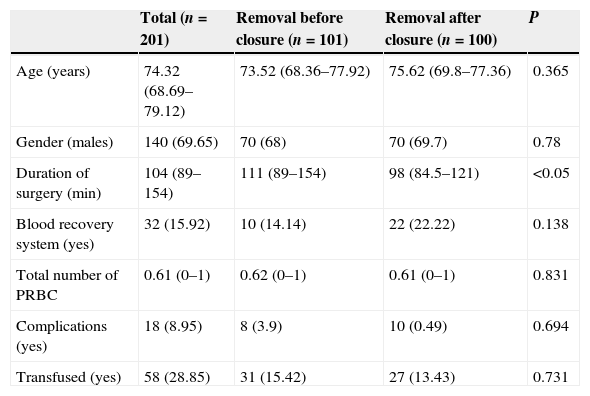
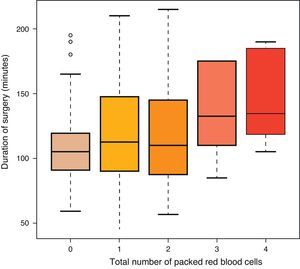
ResultsA total of 140 females (mean age of 72 years) and 61 males (mean age of 71 years) were intervened. A total of 101 patients underwent removal method A (prior to closure) and 100 underwent method B (after closure). There were no differences between groups in terms of age and gender. Each surgeon carried out a mean 25 arthroplasties (range: 18–31). The recovery system was applied to 32 patients and simple drainage was applied to the rest (169), with no differences between both groups regarding the use of the recovery system (Table 1). The mean duration of the intervention was 111min in group A and 98min in group B, with this difference being statistically significant (P<0.05) (Table 1). The mean transfused PRBC was 0.62 in group A and 0.61 in group B, with no statistically significant differences between both groups. No correlation was established between the surgery time and the transfused PRBC (P=0.055), although there was tendency to have more transfusions among cases with a longer surgery time (Table 2 and Fig. 1). There were 18 complications in total; 8 in group A and 10 in group B, with no differences between them. There were 12 infections (8 superficial and 4 deep). Among the 8 superficial, 7 were acute or subacute and 1 was chronic, whilst all the 4 deep cases were chronic and required replacement of the prosthesis in two stages. The results according to groups were the following: 5 in group A, 3 superficial and 2 deep, and 7 in group B, 5 superficial and 2 deep. There were no significant differences between both groups (P=0.69) (Table 1).
Patient data according to moment of pneumatic cuff removal.
| Total (n=201) | Removal before closure (n=101) | Removal after closure (n=100) | P | |
|---|---|---|---|---|
| Age (years) | 74.32 (68.69–79.12) | 73.52 (68.36–77.92) | 75.62 (69.8–77.36) | 0.365 |
| Gender (males) | 140 (69.65) | 70 (68) | 70 (69.7) | 0.78 |
| Duration of surgery (min) | 104 (89–154) | 111 (89–154) | 98 (84.5–121) | <0.05 |
| Blood recovery system (yes) | 32 (15.92) | 10 (14.14) | 22 (22.22) | 0.138 |
| Total number of PRBC | 0.61 (0–1) | 0.62 (0–1) | 0.61 (0–1) | 0.831 |
| Complications (yes) | 18 (8.95) | 8 (3.9) | 10 (0.49) | 0.694 |
| Transfused (yes) | 58 (28.85) | 31 (15.42) | 27 (13.43) | 0.731 |
PRBC: packed red blood cells.
Quantitative variables are represented by median (first-third quartile) and qualitative variables by absolute frequencies (percentages).
Our study did not find differences between removing the ischaemia cuff before or after closing the surgical wound, or regarding the need for transfusions, or in relation to postoperative complications. In the first of two metaanalyses on this topic, Rama et al.18 concluded that removing the tourniquet before closure increased the estimated postoperative bleeding. Zan et al.28 corroborated this finding in the last study conducted, whilst adding to this increase in blood loss the blood lost directly and the intraoperative loss. This loss could be explained by two reasons: on the one hand, the duration of surgery was increased, thus causing more bleeding, and on the other, there would be reactive haemorrhage after removing the cuff, which would increase the physiological bleeding.3 This result has been supported by an increased need for transfusions among these patients. In our series, the mean number of transfusions was far lower than in other studies, around 0.6 PRBC (Table 1) per patient in both groups, which is in contrast with the values of 1.3 in removal prior to closure and 1.4 in removal after closure reported in the study by Harvey et al.4 Similarly, Page et al.17 registered a mean value of 2.6 PRBC in both groups. This could be explained by various factors; firstly, the selection of patients in our study was very strict, as the number of subjects ruled out due to therapies and disease was almost 90, nearly 30% of the total arthroplasties performed, thus selecting our population and giving it less chance for intraoperative and postoperative complications, unlike most of the published studies, where prior treatments and protocols for removal of anticoagulation or antiaggregation are not specified. In addition, various studies do not specify the duration of surgery or the existence of differences between groups, a reason which seems to entail a greater postoperative blood loss.18 However, we did not find a direct relationship between the duration of surgery and the need for blood transfusions, although we did observe a tendency that was not statistically significant (P=0.055) (Fig. 1). Urbano Manero and Miguelena Bobadilla24 did not find differences in the number of transfusions (with mean values of 0.3 and 0.4 PRBC, similar to our results) between both groups, but they did find them between the blood lost intraoperatively and the total blood lost, which would not be consistent with an identical transfusion requirement with similar prior Hb levels. On the other hand, Abbas et al.25 obtained a decrease in the number of PRBC transfused in the group with intraoperative removal, compared to the group with postoperative removal, although, interestingly, they did not find any difference in the blood loss measured directly. We did find significant differences in duration of surgery between both groups (111min in group A and 98min in group B) (Table 2), as did Hersekli et al.,20 who obtained a mean duration of surgery shorter than ours, but also superior in the case of removal prior to closure (87min for prior removal and 80min for subsequent removal), as well as Yavarikia et al. (104 versus 96min).23 Furthermore, Steffin et al.,29 Barwell et al.,30 Burkhart et al.,19 and Newman et al.13 did not report any differences between both groups.
Thus, the reduction in transfusions could have influenced the possibility of finding differences between both groups. In any case, in our study we were only able to measure the blood lost through the need for transfusions, which confers a degree of subjectivity to the decision of transfusing patients. Likewise, it seems that the surgical technique plays a key role in postoperative bleeding, since a careful approach whilst recognising the vascular anatomy would avoid damaging the geniculated arteries, the main vessels responsible for bleeding in this intervention. The published studies comparing the surgical technique with both types of cuff removal do not report any cases of section of the popliteal artery. Likewise, in our series we did not register any cases, thus diminishing the importance of removal prior to closure in order to verify that the artery had not been affected.
The metaanalyses of Rama et al.18 and Zan et al.28 also compared the complications based on the time of tourniquet removal, with a different result to ours, since, in this case, removal prior to closure reduced the complications and the need for reintervention. This has been explained by the greater incidence of subcutaneous haematomas, which are produced after surgical closure when haemostasis is not carried out, which would cause an increase in ischaemia at a local level during the tissue recovery phase, with a subsequent predisposition towards infection and wound dehiscence. In addition, unlike Rama et al.,18 Zan et al.28 concluded that complications also increased in the group with removal after closure, in terms of rehabilitation and mobility. In our series we did not observe more complications with removal after closure. The number of severe complications was also low, and once again this could be explained by the characteristics of our patients, with less comorbidities and, therefore, less predisposed to complications, although the number of local complications, particularly superficial infections, was high. This reduction of complications also reduced the possibility of observing differences between both groups.
In light of our results, the point during the intervention at which ischaemia should be removed does not seem determinant in reducing blood transfusions. In any case, an in-depth knowledge of the vascular anatomy of the knee is essential to conduct the approach. Likewise, the postoperative complications seem to be more related to the characteristics of each patient, in terms of their comorbidities, than to the surgical technique applied. Further prospective studies would be necessary in order to determine the influence of ischaemia in blood loss, as well as the repercussion on complications deriving from ischaemia and blood transfusions.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that this investigation did not require experiments on humans or animals.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data.
Right to privacy and informed consentThe authors declare that this work does not reflect any patient data.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Paredes-Carnero X, Rosero-Ruíz GL, Centeno-García JJ, Pombo-Taboada FJ. Efecto de la retirada del manguito de isquemia sobre las necesidades transfusionales en el paciente intervenido de artroplastia primaria de rodilla. Rev Esp Cir Ortop Traumatol. 2015;59:394–399.