To assess the efficacy of implanting concentrated bone marrow rich in mesenchymal stem cells (MSC) for the treatment of femoral head avascular necrosis (AVN) to prevent or delay total hip replacement (THR).
Material and methodsA single-centre, prospective, non-controlled clinical study was conducted on patients with a diagnosis of AVN. The parameters assessed were, patient demographics, Harris Hip Score (HHS), imaging test (X-ray and NMR), and staging using the Arlet–Ficat scale. The patients were followed up for 1, 6, 12 and 24 months. The bone marrow was aspirated from the iliac crest, concentrated with the Harvest SmartPReP 2 system, and infused into the necrotic area by means of core decompression.
ResultsA total of 22 hips in 17 patients were recruited between the years 2006 and 2012, with a minimum follow-up of 2 years. A mean of 119.5ml of aspirate was extracted, with 15.25ml of MSC being implanted. During the first 2 years of the infusion, 5 hips (24.7%) required THR, with no differences in the baseline Arlet–Ficat stage, and 4 of these (80%) had femoral head involvement equal to or higher than 30%. A significant increase of 14.27 (P=.026) in the HHS and a 0.98 (P=.089) decrease in the VAS was observed two years after the infusion in the remaining 17 hips. The results suggest that the infusion of concentrated bone marrow rich in MSC, combined with surgical decompression of the nucleus, improves hip function (HHS), and avoids THR in 75.3% of patients with AVN treated during the first 2 years.
Evaluar la utilidad del aporte de concentrado de médula ósea rico en células troncales mesenquimales (MSC) como tratamiento de la necrosis avascular de cabeza femoral (NAV) para evitar o retrasar la artroplastia total de cadera (ATC).
Material y métodosEstudio clínico unicéntrico, prospectivo, no controlado, de pacientes con diagnóstico de NAV. Evaluación de parámetros demográficos, Harris Hip Score (HHS), pruebas de imagen (radiografía y RMN); estadificación mediante escala de Arlet-Ficat. Seguimiento a 1, 6, 12 y 24 meses. Aspirado de médula ósea de cresta ilíaca, concentrado con sistema Harvest SmartPReP 2, e infusión en zona de necrosis mediante forages.
ResultadosVeintidós caderas en 17 pacientes fueron reclutados entre los años 2006-2012, seguimiento mínimo 2 años. Extracción media de 119,5ml de aspirado, implantándose 15,25ml de concentrado de MSC. Durante los primeros 2 años de la infusión 5 caderas (24,7%) precisaron ATC, sin diferencias en estadio de Ficat basal, y 4 (80%) de ellas presentaban afectación de cabeza femoral igual o superior al 30%. En las 17 caderas restantes, se observó a los 2 años de la infusión un aumento significativo del HHS (14,27) (p=0,026) y disminución del dolor (0,98) en la EVA (p=0,089). Nuestros resultados sugieren que la infusión de concentrado de médula ósea rico en MSC asociado a descompresión quirúrgica del núcleo mejora significativamente la funcionalidad de la cadera (HHS) y evita la ATC en el 75,3% de los pacientes con NAV tratados durante los primeros 2 años.
Avascular necrosis of the femoral head (AVN) is a disease caused by a deficiency in the blood supply of a bone. Its peak incidence appears between the third and fourth decades of life. The high functional demand presented by these patients and the natural evolution of the disease towards a collapse of the femoral head pose a challenge when deciding the optimal treatment for these patients. There are numerous treatment options before the collapse, some of them with controversial efficacy. Conservative treatment through rest and unloading of the joint is effective in 22.7% of cases, whereas 66% of these patients will require a total hip arthroplasty (THA) (or total hip replacement [THR]) in the medium-term,1 with this indication accounting for between 5% and 12% of total hip replacements performed annually in the USA.2 Surgical decompression of the nucleus3 through forages remains the most commonly employed method,4 although it only appears effective in cases where less than 30% of the volume of the femoral head is affected.3 Other treatments, such as vascularised bone grafts or femoral osteotomies, present highly irregular results and involve complex techniques which may hinder any possible, subsequent interventions.5–7 Recently, some authors have chosen to implement treatments through concentrates of mesenchymal stem cells (MSC),8–13 infusing concentrate obtained from bone marrow (BM) aspirates into the necrosis area through forages. This technique is very mildly aggressive and has been used in the last decade with encouraging results, not only in the treatment of avascular necrosis of the femur, but also in cases of pseudoarthrosis or nonunion of the diaphysis of long bones.9
The aim of our study was to evaluate the clinical outcome and the need for THA in patients suffering AVN in the pre-collapse phase during the first 2 years after infusion of autogenous BM.
Material and methodsStudy designThis was a prospective, non-controlled, open clinical study of 22 hips in 17 consecutive adult patients with a diagnosis of AVN who were attended at the Orthopaedic Surgery and Traumatology consultation of our hospital between November 2006 and January 2012. The study was approved by the Ethics and Research Committee of our hospital and all patients gave their informed consent to participate in it.
We excluded patients with AVN and age over 70 years, radiological collapse of the femoral head, prior treatment with cytostatic agents, active alcoholism, skin lesions in the lower extremities, prior radiation affecting the pelvis, active infection, anaemia (Hb <10g/dl), leukopoenia (WBC <4000/mm3) and active tumoural processes.
Parameters analysed and evaluation scheduleWe conducted a preoperative evaluation through the clinical histories: demographic and general data, information on the disease (unilateral or bilateral, causes and risk factors), pain measured through a visual analogue scale (VAS), Harris Hip Score (HSS), and imaging tests: anteroposterior and axial hip radiographs, magnetic resonance imaging (MRI) scans (quantifying lesion volume in percentage and staging through the Arlet–Ficat scale for AVN assessment2).
Upon hospital discharge, patients walked without load using canes for 2 weeks, with a partial load being authorising thereafter, according to tolerance. We conducted outpatient follow-up at 1, 6, 12 and 24 months after the procedure. Each visit included radiographic and clinical evaluation (VAS and HSS) for the entire period of 24 months or until an arthroplasty was performed.
We defined treatment failure as the evolution towards collapse, requiring a THA. Indication for the latter was the absence of clinical improvement and the existence of radiological collapse equal to or greater than 30% on plain radiographs during follow-up visits.
Obtention and implant of autogenous bone marrow concentrateIn the operating room, under aseptic conditions and usually under epidural anaesthesia, we carried out BM aspiration by puncture of the ipsilateral iliac crest immediately posterior to the anterosuperior iliac spine, using a cannulated trocar of 1.5mm internal diameter and lateral orifices.9,13 This trocar was inserted through the cortical until the cancellous bone was reached and aspiration was started. In order to prevent haemodilution, the direction and depth of the needle was varied after 2ml of aspiration.14 Between 2 and 3 perforations were performed 2cm apart on each patient, and the trocar was turned in each aspiration.
The aspirate was injected into a sterile container. In addition, 3ml of a dilution containing 0.9% physiological saline and heparin at a concentration of 455 units/ml were added for each 60ml of BM aspirate. The resulting final concentration was 22.7 heparin units per ml of BM aspirate.
The stem cell concentrate was obtained using a Harvest SmartPReP 2® system (Harvest Technologies Corp.). This system centrifuges the aspirate bags for 15min and, through an automated process, obtains a separation of plasma and cellular layers, thus facilitating the recovery of a mononuclear cell suspension. The resulting extract of mononuclear cells was then reinfused into the area of bone necrosis.
During the surgical procedure we extracted a mean volume of 119.5ml (±31.87; range: 60–180ml) of BM. For every 60ml of BM aspirate we obtained about 10ml of concentrate, which contained a mean total of 376×106 mononuclear cells, 16×106 CD34+ cells (haematopoietic stem cells) and 3000 colony-forming units (CFU) per millilitre.7
Subsequently, we conducted forages with a 5mm drill by percutaneous technique under fluoroscopic control, reaching the centre of the bone necrosis area in the femoral head. Using the perforated trocar, we infused a mean volume of 15.25ml (±4.86) of BM concentrate at a slow and constant pace, following the technique standardised by other authors.11
There was a minor surgical complication, defined as a haematoma at the point of extraction in the iliac crest.
Statistical analysisStatistical analysis was performed using the software package SPSS 18.0. Data were expressed as: arithmetic mean (±standard deviation [SD]; range). Hypothesis testing was performed using nonparametric tests, the Wilcoxon signed ranks test and the Student t test for independent data.
ResultsDuring the study period we recruited 22 hips from 17 patients, of which 14 were male and 3 were female. The mean age of patients was 37.5 years (±9.5; range: 25–59 years). In total, 12 patients suffered unilateral AVN, whilst the remaining 5 suffered bilateral AVN (4 males and 1 female). AVN affected the right side in 11 cases and the left in the other 11 cases. The cause of AVN was traumatic in 6 hips, corticoid in 4 hips and idiopathic in the remaining 12 hips.
Pain assessment by VAS before infusion of the BM concentrate was 5.61 (±1.65; range: 1–9), whereas the mean HSS score was 60.23 (±18.8; range: 28–100). Two hips were in stage I of the Arlet–Ficat scale, 14 hips in stage II and 6 in stage III, with a mean value of 2.18 (±0.6). The mean percentage of affected femoral head, as measured by magnetic resonance imaging, was 26.07% (±14.57%; range: 10–60%).
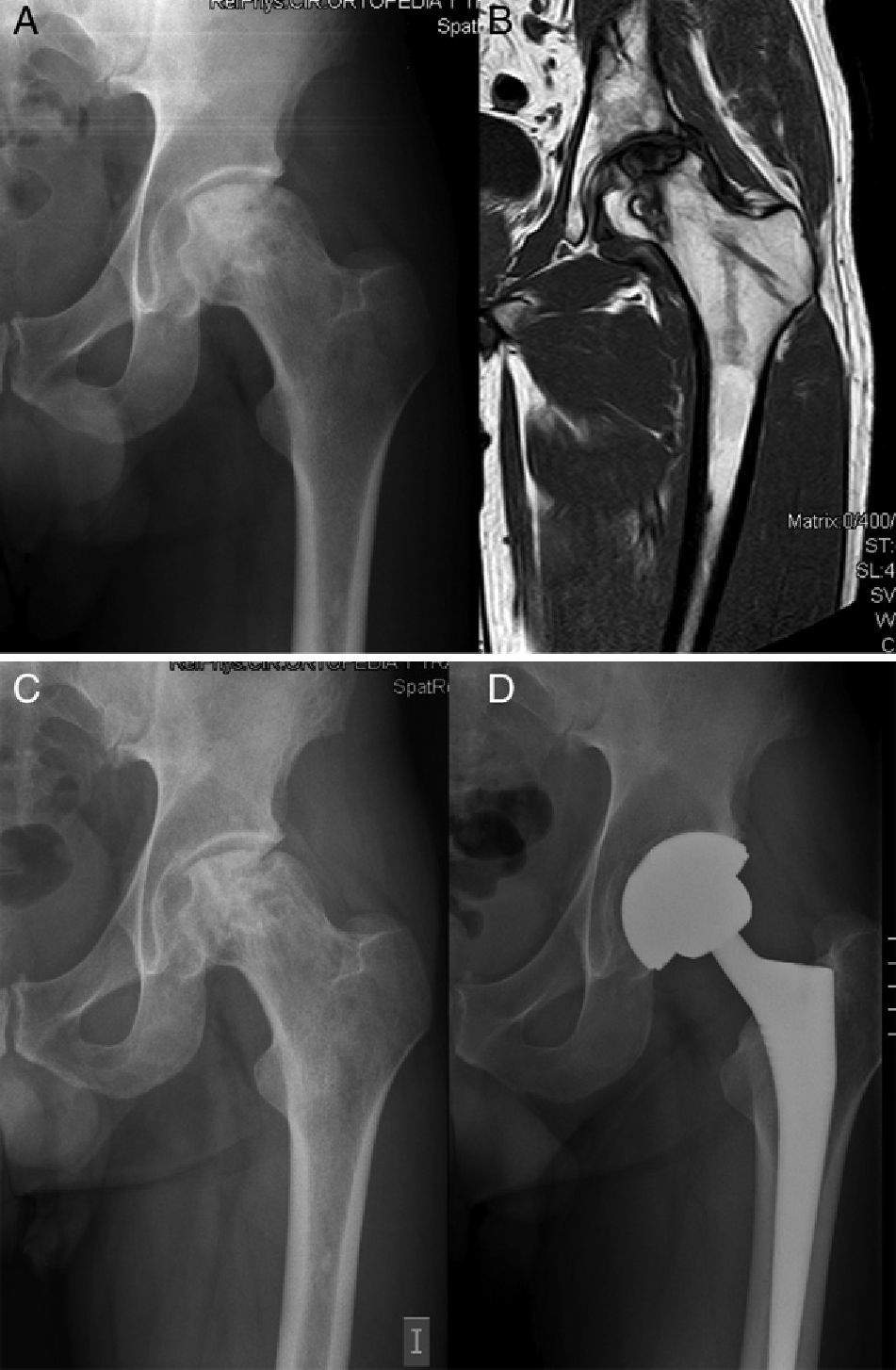
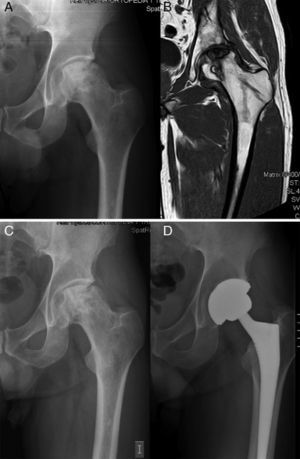
During the first 2 years after the intervention, hip arthroplasty was indicated in 5 (24.7%) of the 22 hips, after a mean period of 17.2 months following the infusion of BM concentrate. The baseline Arlet–Ficat stage in these cases was stage I in 1 case, stage II in 2 cases and stage III in the remaining 2 cases. A total of 3 arthroplasties were conducted on the 16 hips in stages I and II (18.75%) (Fig. 1) and 2 on the 6 hips in stage III (33.3%) (P=.9256). Out of the 17 hips which did not require arthroplasty, the VAS evaluation improved from a baseline value of 5.61 (±1.65) to 4.09 (±2.16); 4.66 (±2.26); 4.55 (±2.81) and 4.64 (±2.81) at 1, 6, 12 and 24 months, respectively. The mean decrease in VAS at 24 months compared to the baseline value was 0.98 points (P=.089).
In these 17 hips, the HHS assessment also improved significantly from a baseline value of 60.23 (±18.8) to 70.81 (±13.98); 70.04 (±18.31); 75.61 (±19.73) and 74.5 (±20.57) at 1, 6, 12 and 24 months, respectively. The mean increase in the HHS at 24 months compared to the baseline value was 14.27 points (P=.026).
DiscussionThe success of different treatments for AVN is directly related to the degree of the disease,5,9 and several studies have indicated which is the best technique to use depending on the osteonecrosis stage.5,10,13 Decompression by perforation is the most common technique in the pre-collapse phase, and the contribution of bone morphogenetic protein (BMP) or mesenchymal stem cells (MSC) improves outcomes by reducing the need for joint replacement.4 Adult MSC have the potential to differentiate into tendon, muscle, fat, bone, scar tissue, etc. The capacity of these cells to generate osteoblasts has been well defined in vitro and in vivo. This capacity is favoured by the presence of osteogenic factors, such as BMP, fibroblast growth factor, dexamethasone and insulin-like growth factor.13,15,16 Therefore, considering that the number of osteoprogenitors in the femoral head9 is reduced after the age of 25, and that this decline is more pronounced in cases of necrosis,8 AVN is an ideal indication for this treatment. However, successful treatment using this technique appears to be directly related to the number of implanted MSC, although there are no articles which establish an ideal figure.5,9 In a study on the consolidation of pseudoarthrosis or nonunion, Hernigou et al. established that success took place in those cases that had been implanted with over 30×103 osteoprogenitors in total.9
The number of cells present in the BM of each patient is variable and becomes decreased in certain situations (alcohol abuse, smoking and steroid treatment).5,9,10,17,18 The osteogenic potential of a BM sample is directly correlated with its cell concentration,13,15,16 and since the number of MSC present in the aspirate is small (1×105 mononuclear cells), various systems have been developed to increase this cell concentration. These systems use centrifugal separation to generate a concentration of nucleated cells which is 5 times higher than that in the original aspirate. There is currently no consensus in the literature regarding the number of stem cells required to treat avascular necrosis. However, recent works have established a mean amount of 25×103 cells per hip as the lower limit for clinical efficacy.8,9 In our patients, we infused a mean volume of 15ml per patient, which, according to the published data,7 represents a mean total of 45×103 cells.
The extent of disease is another determining factor in deciding the therapeutic approach. This mainly benefits those patients who have not yet suffered a collapse of the femoral head (grades I and II in the Ficat scale). However, the Ficat classification is descriptive of the lesion and does not consider its quantitative aspects, that is, the affected percentage of the femoral head.
Nevertheless, the rate of progression towards collapse in this disease left to its natural evolution would be 67% at 5 years on average in stages I and II.19 In the only prospective and controlled study published so far, Gangji et al. injected MSC and found that, after a mean 2 years follow-up, only 10% of the AVN cases treated with infusion of autogenous BM concentrate reached stage III, versus 62.5% in the control group. If we focus on the need for THA, in a recent study on 59 patients treated with this method, Wang estimated a value of 12.9% after a follow-up period of 27.6 months.20 Unfortunately, in our study we had to conduct twice as many prostheses within a slightly longer mean period (24.7% at 31 months follow-up). Nevertheless, these were less than half of the total expected if the disease were left to its natural course. This may be because, unlike the 2 articles mentioned previously, we used a classification which was scarcely adapted to the severity of necrosis. According to Saito et al., the evolution of the disease depends fundamentally on the extension of the lesion, thus observing that when it was less than 50% it only progressed 9%, whereas when it was greater than 50% it increased 73% within 5 years.7 In total, 4 of the 5 hips (80%) which required an arthroplasty presented femoral head involvement equal to or greater than 30% (2 in stage II and 2 in stage III). The use in clinical practice of other classifications which assess not only the degree of collapse but also the affected surface of the femoral head, such as the mentioned International Classification of Osteonecrosis (ARCO) and modified Pennsylvania,6 which offer descriptive and quantitative information on the lesion, may help to lay the foundations for the therapeutic indication of mesenchymal stem cells of the BM and reduce failures.
Our results suggest that the infusion of MSC from BM associated with surgical decompression of the nucleus in patients with AVN significantly improves the functionality of the hip, as measured by the HHS, and prevents THA in 75.3% of patients treated with this technique during the first 2 years. The treatment has minimal complications, is reproducible and easy to implement in everyday clinical practice and helps to decrease the morbidity of this disease.
In our experience, the Arlet–Ficat preoperative classification through magnetic resonance was not useful to discriminate the extent of the lesion and provide a correct indication and prognosis which improved the results of treatment with MSC.
The main limitation of this study was the lack of a randomised control group, so that no conclusions can be drawn with a high level of evidence on the usefulness of infusion of a BM concentrate, compared to its non-use and treatment of patients by unloading. Another partial limitation of the study was the short duration of the follow-up period. The results presented in this work represent preliminary data at 24 months from an ongoing study in which we continue to monitor these patients over a longer time period.
Level of evidenceLevel of evidence II.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation adhered to the ethical guidelines of the Committee on Responsible Human Experimentation, as well as the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that this work does not reflect any patient data.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Cuervas-Mons M, et al. Implante de concentrado de médula ósea autógeno en el tratamiento de la necrosis isquémica de cabeza femoral: evolución clínica al segundo año de seguimiento de un estudio prospectivo no controlado. Rev Esp Cir Ortop Traumatol. 2013;57:106-10.