Recent publications have questioned the efficacy of using therapeutic or intermediate doses of low molecular weight heparin (LMWH) in COVID-19 patients, especially in the most severe patients. In order to update these recommendations, a non-systematic review has been carried out in the main medical databases. A total of 14 randomized clinical trials, 14 meta-analyses and the recommendations of 12 scientific societies were selected, stratified according to the type of patient (outpatient, hospitalized, admitted to critical care or post-discharge). The efficacy of LMWH and other therapeutic approaches (rivaroxaban, apixaban, sulodexide, acetylsalicylic acid and P2Y12 inhibitors) has been analyzed. The findings recommend using standard doses of LMWH as thromboprophylaxis in critically hospitalized COVID-19 patients and therapeutic doses in non-critically hospitalized patients if the risk of bleeding is low. In outpatients and those discharged from the hospital, LMWH could be used at a prophylactic dose if there are thrombotic risk factors, and the bleeding risk is low. It is not recommended to associate antiplatelet agents with LMWH unless previously indicated.
Recientes publicaciones han puesto en duda la eficacia de la utilización de dosis terapéuticas o intermedias de heparina de bajo peso molecular (LMWH) en pacientes COVID-19, especialmente en los pacientes más graves. Con el objetivo de actualizar estas recomendaciones se ha realizado una revisión no sistemática en las principales bases de datos médicas. Se seleccionaron un total de 14 ensayos clínicos aleatorizados, 14 metaanálisis y las recomendaciones de 12 sociedades científicas, estratificadas según el tipo de paciente (ambulatorio. hospitalizado, ingresado en cuidados críticos o post-alta). Se ha analizado la eficacia de LMWH y también de otras aproximaciones terapéuticas (rivaroxabán, apixabán, sulodexida, ácido acetilsalicílico e inhibidores P2Y12). Los hallazgos recomiendan utilizar dosis estándar de LMWH como tromboprofilaxis en los pacientes COVID-19 hospitalizados críticos y dosis terapéutica en hospitalizados no críticos si el riesgo de sangrado es bajo. En los pacientes ambulatorios y dados de alta del hospital podría utilizarse LMWH a dosis profiláctica si existen factores de riesgo trombótico y el riesgo hemorrágico es bajo. No se recomienda asociar antiagregantes plaquetarios a la LMWH salvo indicación previa.
SARS-CoV-2 (COVID-19) infection is characterized by the presence of a severe inflammatory response associated with a high mortality rate and a high prevalence of thrombotic complications. A recent meta-analysis1 reported an overall prevalence of venous thromboembolism of 14.1%, a figure that increase to 45.6% in patients admitted to intensive care units (ICU).
Histopathology studies of patients who died from COVID-19 confirm a high incidence of pulmonary macroembolism, severe endothelial injury, increased angiogenesis, macroembolism, and capillary congestion.2 This hypercoagulability is shown by increased viscoelasticity, platelet hyperactivation and, above all, increased D-dimer values, with evidence of a direct relationship between the increase in D-dimer values and poor prognosis.3
Several strategies have been developed to prevent thrombotic complications in these patients, including parenteral anticoagulants, and, more recently, oral anticoagulants and antiplatelet agents.
The SEDAR-SEMICYUC4 consensus statement on the management of haemostasis disorders in severe COVID-19 patients published in May 2020 put special emphasis on anticoagulant prophylaxis, and recommended 3 different doses of low molecular weight heparin (LMWH): standard (equivalent to 40 mg of enoxaparin for a body weight less than or equal to 80 kg), intermediate (1 mg/kg/24 h) and therapeutic (1 mg/kg/12 h), depending on the patient's thrombotic risk. The authors of some articles that have appeared in Spain and elsewhere since the publication of the consensus have questioned the usefulness of thromboprophylaxis with higher than standard doses, particularly in critically ill patients.5–7
In light of the new evidence, the previous recommendations need to be updated to bring them in line with the current knowledge. For this reason, we performed an non-systematic review of the scientific literature published in the last two years related to thromboprophylaxis in COVID-19 patients.
Material and methodWe searched PubMed, Google Academic, Embase and Cochrane for articles published between 2020 and 2022 using the keywords COVID-19, thromboprophylaxis, heparin, low molecular weight heparin, anticoagulant, aspirin, clinical trial, meta-analysis, clinical practice guideline. We limited our selection to randomized clinical trials, meta-analyses, and clinical practice guidelines published by scientific societies or specific task forces.
ResultsWe selected a total of 14 randomized clinical trials; LMWH was used in 7, rivaroxaban in 2, apixaban in 1, sulodexide in another, aspirin in 3, and P2Y12 inhibitors in 2. Table 1 shows the characteristics and references of these studies. We also analysed 14 meta-analyses or systematic reviews that compared different thromboprophylactic doses of heparin (standard, intermediate or therapeutic). Finally, we collected recommendations made by 12 scientific societies and task forces and stratified them according to patient type (ambulatory, hospitalized, admitted to critical care or post-discharge). Table 2 shows the characteristics and references.
Prospective randomized clinical trials in thromboprophylaxis in COVID-19.
| Patients clinical scenario | Study titleMain finding | Intervention | Patients | Primary outcome |
|---|---|---|---|---|
| Outpatients | OVID22NO DIFFERENCE | Enoxaparin (40 mg/day) (n = 234) vs. no anticoagulation (n = 238) for 14 days | Outpatients with COVID-19 aged >50 years |
|
| ACTIV-4B25NO DIFFERENCE | Antiplatelet (acetylsalicylic acid 81 mg/day, n = 164) or anticoagulant therapy with prophylactic doses of apixaban (2.5 mg/12 h, n = 165) or therapeutic doses of apixaban (5 mg/12 h, n = 164) vs. placebo (n = 164) | Outpatients studied for 45 days. |
| |
| González-Ochoa AJ24LOWER HOSPITAL ADMISSION | Sulodexide (500 U twice daily n = 124) versus placebo (n = 119) for 21 days | Outpatients at high risk of complications |
| |
| Non-critical hospitalized patients | ATTACC, ACTIV-4a, and REMAP-CAP17INCREASED LIKELIHOOD OF SURVIVAL | Therapeutic anticoagulation with heparin (n = 699) vs. standard prophylaxis (n = 699) | Moderate-to-severe hospitalized patients |
|
| HEP-COVID18INCREASED LIKELIHOOD OF SURVIVAL IN NON-CRITICAL PATIENTS | Therapeutic-dose anticoagulation with enoxaparin (n = 129) vs. standard prophylaxis with LMWH or UFH (n = 124) | High-risk hospitalized patients (32.8% in Critical Care) |
| |
| RAPID19INCREASED LIKELIHOOD OF SURVIVAL | Therapeutic-dose anticoagulation (n = 228) vs. prophylactic dose (n = 237) of heparin (LMWH or UFH) | Hospitalized patients with elevated D-dimer |
| |
| ACTION23NO DIFFERENCEGREATER RISK OF BLEEDING | Therapeutic-dose anticoagulation (oral rivaroxaban 15/20 mg/day in stable patients or subcutaneous enoxaparin 1 mg/kg/12 h or i.v. UFH followed by rivaroxaban at previous doses until day 30) (n = 311) vs. standard prophylactic LMWH or UFH (n = 304) | Hospitalized patients with elevated D-dimer |
| |
| RECOVERY27NO DIFFERENCE | Aspirin (150 mg/day) combined (n = 7351) with usual treatment or not (n = 7541) | Hospitalized patients |
| |
| ACTIV-4a29NO DIFFERENCE | P2Y12 inhibitor (clopidogrel or ticagrelor) (293 patients) + therapeutic heparin vs. therapeutic heparin alone (269 patients) | Non-critical hospitalized patients |
| |
| Critical hospitalized patients | REMAP-CAP, ATTACC and ACTIV-416NO DIFFERENCE | Therapeutic-dose anticoagulation with heparin (n = 529) vs. standard prophylaxis (n = 545) | Patients admitted to intensive care |
|
| INSPIRATION20NO DIFFERENCE | Intermediate-dose heparin (n = 276) vs. standard prophylactic dose (n = 286) | Patients admitted to intensive care (43% with low-flow oxygen therapy) |
| |
| Perepu US et al J 202121NO DIFFERENCE | Intermediate-dose enoxaparin (n = 84) vs. standard prophylactic dose (n = 85) | Patients with severe disease (62% admitted to intensive care) |
| |
| REMAP-CAP28SLIGHTLY LOWER LIKELIHOOD OF ORGAN SUPPORT UP TO 21 DAYS | Aspirin (80−100 mg/day) (565 patients) or clopidogrel (75 mg/day), ticagrelor (60 mg/12 h), prasugrel (60 mg loading dose + 5−10 mg/day) (455 patients) combined with prophylactic-, intermediate- or therapeutic-dose anticoagulant | Critical hospitalized patients | Aspirin + therapeutic heparin vs. therapeutic heparin alone
| |
| Post-discharge | MICHELLE26LESS RISK OF THROMBOSIS/DEATH | Rivaroxaban (10 mg/day for 35 days) vs. no anticoagulation at discharge | Post discharge COVID-19 patients | Composite of symptomatic VTE, VTE-related death, VTE detected by bilateral lower limbs CT scan, and symptomatic arterial thromboembolism or cardiovascular death up to day 35 (3% vs. 15%) (RR 0.33 [95% CI 0.12–0, 90; p = 0 0293])
|
CI, confidence interval; Crl, credible interval; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; LMWH, low molecular weight heparin; UFH, unfractionated heparin.
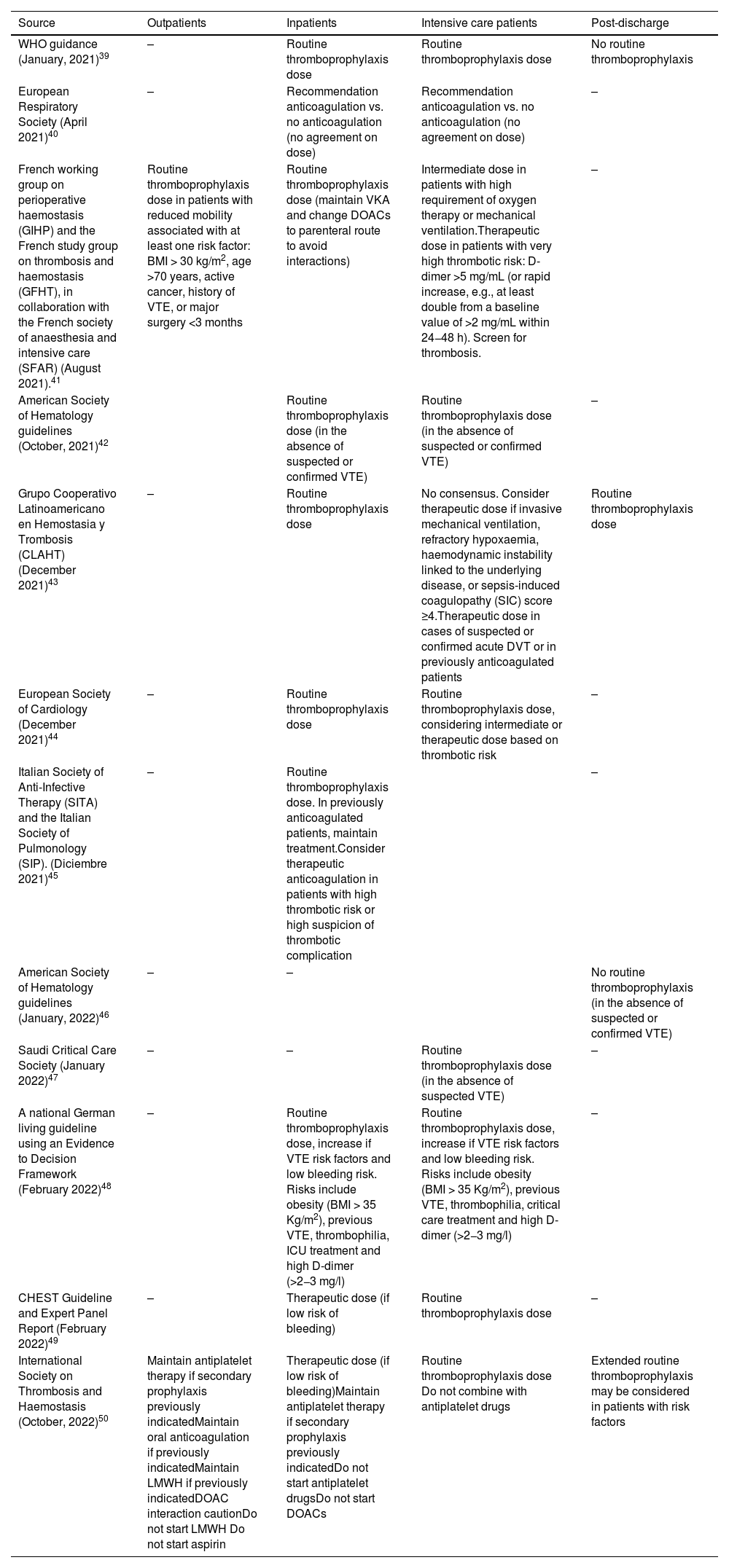
Clinical practice guideline recommendations on thromboprophylaxis in COVID-19.
| Source | Outpatients | Inpatients | Intensive care patients | Post-discharge |
|---|---|---|---|---|
| WHO guidance (January, 2021)39 | – | Routine thromboprophylaxis dose | Routine thromboprophylaxis dose | No routine thromboprophylaxis |
| European Respiratory Society (April 2021)40 | – | Recommendation anticoagulation vs. no anticoagulation (no agreement on dose) | Recommendation anticoagulation vs. no anticoagulation (no agreement on dose) | – |
| French working group on perioperative haemostasis (GIHP) and the French study group on thrombosis and haemostasis (GFHT), in collaboration with the French society of anaesthesia and intensive care (SFAR) (August 2021).41 | Routine thromboprophylaxis dose in patients with reduced mobility associated with at least one risk factor: BMI > 30 kg/m2, age >70 years, active cancer, history of VTE, or major surgery <3 months | Routine thromboprophylaxis dose (maintain VKA and change DOACs to parenteral route to avoid interactions) | Intermediate dose in patients with high requirement of oxygen therapy or mechanical ventilation.Therapeutic dose in patients with very high thrombotic risk: D-dimer >5 mg/mL (or rapid increase, e.g., at least double from a baseline value of >2 mg/mL within 24−48 h). Screen for thrombosis. | – |
| American Society of Hematology guidelines (October, 2021)42 | Routine thromboprophylaxis dose (in the absence of suspected or confirmed VTE) | Routine thromboprophylaxis dose (in the absence of suspected or confirmed VTE) | – | |
| Grupo Cooperativo Latinoamericano en Hemostasia y Trombosis (CLAHT) (December 2021)43 | – | Routine thromboprophylaxis dose | No consensus. Consider therapeutic dose if invasive mechanical ventilation, refractory hypoxaemia, haemodynamic instability linked to the underlying disease, or sepsis-induced coagulopathy (SIC) score ≥4.Therapeutic dose in cases of suspected or confirmed acute DVT or in previously anticoagulated patients | Routine thromboprophylaxis dose |
| European Society of Cardiology (December 2021)44 | – | Routine thromboprophylaxis dose | Routine thromboprophylaxis dose, considering intermediate or therapeutic dose based on thrombotic risk | – |
| Italian Society of Anti-Infective Therapy (SITA) and the Italian Society of Pulmonology (SIP). (Diciembre 2021)45 | – | Routine thromboprophylaxis dose. In previously anticoagulated patients, maintain treatment.Consider therapeutic anticoagulation in patients with high thrombotic risk or high suspicion of thrombotic complication | – | |
| American Society of Hematology guidelines (January, 2022)46 | – | – | No routine thromboprophylaxis (in the absence of suspected or confirmed VTE) | |
| Saudi Critical Care Society (January 2022)47 | – | – | Routine thromboprophylaxis dose (in the absence of suspected VTE) | – |
| A national German living guideline using an Evidence to Decision Framework (February 2022)48 | – | Routine thromboprophylaxis dose, increase if VTE risk factors and low bleeding risk. Risks include obesity (BMI > 35 Kg/m2), previous VTE, thrombophilia, ICU treatment and high D-dimer (>2−3 mg/l) | Routine thromboprophylaxis dose, increase if VTE risk factors and low bleeding risk. Risks include obesity (BMI > 35 Kg/m2), previous VTE, thrombophilia, critical care treatment and high D-dimer (>2−3 mg/l) | – |
| CHEST Guideline and Expert Panel Report (February 2022)49 | – | Therapeutic dose (if low risk of bleeding) | Routine thromboprophylaxis dose | – |
| International Society on Thrombosis and Haemostasis (October, 2022)50 | Maintain antiplatelet therapy if secondary prophylaxis previously indicatedMaintain oral anticoagulation if previously indicatedMaintain LMWH if previously indicatedDOAC interaction cautionDo not start LMWH Do not start aspirin | Therapeutic dose (if low risk of bleeding)Maintain antiplatelet therapy if secondary prophylaxis previously indicatedDo not start antiplatelet drugsDo not start DOACs | Routine thromboprophylaxis dose Do not combine with antiplatelet drugs | Extended routine thromboprophylaxis may be considered in patients with risk factors |
BMI, body mass index; DVT, deep vein thrombosis; VTE, venous thromboembolism; AVK, anticoagulantes anti-vitamina K; HBPM, heparina de bajo peso molecular; ACODS, anticoagulantes orales directos; AAS, ácido acetilsalicílico.
The following sections describe the main findings obtained with the drugs used.
Thromboprophylaxis with heparinMultiple studies, most of them retrospective, observational, and meta-analyses, have provided evidence of the potential benefit of parenteral anticoagulant treatment (especially with LMWH). However, it has not always been possible to compare the results due to methodological differences in study design - primarily differences in LMWH dosage. Because of these differences, several well-designed, large randomized controlled trials were started to assess the efficacy and safety of anticoagulant treatment in patients with COVID-19. Many of these have recently ended, and others are ongoing.
At least 14 meta-analyses and a recent Cochrane systematic review analysed the effect of different doses of prophylactic heparin on the clinical course. In most studies8–14 (almost all retrospective), the variables analysed were survival at 30 days, need for ICU admission, need for and days on ventilatory support (invasive and non-invasive), appearance of thrombotic complications, and bleeding events, among others. Most of these studies conclude that increasing the heparin dose provides little or no net benefit over administration of a standard thromboprophylactic dose. Although some of these studies report that high doses of heparin are associated with a slight reduction in pulmonary embolism and deep vein thrombosis (DVT), this beneficial effect is offset by an increased risk of bleeding, which is probably why the mortality rate is similar in high and standard doses.
At the start of the pandemic, several prospective, randomized clinical studies were designed to investigate the thromboprophylactic action of unfractionated heparin (UFH) and LMWH in the evolution of COVID-19 patients15 (Table 1). Four studies compared the effect of therapeutic anticoagulation with heparin vs. prophylactic anticoagulation on the rate of thrombotic events and mortality, one focussed on critically ill patients16 and the other 3 evaluated moderately severe hospitalized patients or those considered to be at high thrombotic risk.17–19
The study performed exclusively in critically ill patients16 found fewer thrombotic events in patients assigned to therapeutic anticoagulation (6% vs. 10%), but in-hospital mortality was similar between groups (37.3% vs. 35.5%).
The first of the other 3 studies17 included non-critical hospitalized patients and used the same study variables. Unlike the previous study, therapeutic anticoagulation was associated with a slight increase in 28-day survival (79.3% vs. 75.4%) in patients who did not require organ support. The other 2 studies also investigated whether early therapeutic anticoagulation had any positive effect on disease progression. The HEP-COVID study18 included 253 patients (therapeutic doses of LMWH vs. standard prophylactic doses of LMWH or UFH). In this study, 32.8% of the patients were admitted to the ICU. The primary endpoint was venous or arterial thrombosis or death from any cause, and the main safety endpoint was major bleeding within the first 30 days. The results showed that the primary endpoint was reduced in non-ICU patients (36.1% vs. 16.7%; (relative risk) RR 0.46; [95% CI 0.27−0.81]; p = 0.004), but not in ICU patients (55.3% vs. 51.1%; RR 0.92 [95% CI 0.62−1.39]; p = 0.71). Similar results were obtained in another study with the same characteristics (RAPID),19 confirming a lower death rate in the therapeutic (1.8%) vs. prophylactic (7.6%) anticoagulation group (OR 0.22 [95% CI 0.07−0.65] p = 0.006) in non-critical patients.
The seemingly paradoxical finding of a significant decrease in mortality in non-critical patients receiving therapeutic vs. prophylactic doses of heparin reported in these 3 previous studies suggests that therapeutic anticoagulation may be effective in early stages of the disease, in which a lower incidence of major bleeding is found in non-critical vs, critically ill patients could contribute to a lower rate of mortality.
Two other prospective studies compared the efficacy and safety of intermediate prophylactic doses of LMWH vs. the standard prophylactic dose (Table 1). In the first study (INSPIRATION20) occurrence of the primary endpoint (a composite of adjudicated venous or arterial thrombosis, treatment with extracorporeal membrane oxygenation, or mortality within 30 days) was similar in both groups, with a slight increase in major bleeding in the intermediate-dose group (2.5% vs. 1.4%). In the second study, which had a similar design but a smaller number of patients, no differences were found between the 2 dosage regimens.21 In both, around half of the patients included were admitted to the ICU.
Finally, other studies have analysed the effect of prophylactic doses of enoxaparin (40 mg/day) vs. no anticoagulation in ambulatory (non-hospitalized) COVID-19 patients (OVID study).22 Occurrence of the primary study endpoint (a composite of any untoward hospitalisation and all-cause death within 30 days of randomisation) was similar in patients receiving heparin vs. controls.
Thromboprophylaxis with other anticoagulantsTable 1 shows the main characteristics of the 4 publications analysing the effect of: (i) rivaroxaban in moderately ill hospitalized patients, (ii) sulodexide in outpatients, (iii) apixaban in outpatients, and (iv) rivaroxaban in post-discharge patients.
The decrease in mortality observed after the administration of therapeutic doses of heparin in moderately ill COVID-19 patients was not observed in patient receiving another anticoagulant compound. For example, in the ACTION study,23 no differences were observed in study variables (death, length of stay, time on suplemental oxygen, or thrombotic events) between groups anticoagulated with oral rivaroxaban (15/20 mg/day for 31 days) vs. prophylactic doses of LMWH or subcutaneous unfractionated heparin (UFH), but a greater increase in bleeding was observed the group anticoagulated with rivaroxaban.
Another randomized controlled study analysed the use of sulodexide (Table 1) in 243 non-hospitalized patients with COVID-19 who were at high risk of severe clinical progression due to chronic comorbidities.24 Sulodexide, a natural glycosaminoglycan with antithrombotic and profibrinolytic action, affects haemostasis to a lesser extent than heparin and is associated with a very low risk of bleeding. In the study in question, 22 of 124 patients required hospital admission at 21-day follow-up in the sulodexide group compared with 35 of 119 in the placebo group (RR 0.6 [95% CI 0.37−0.96], p = 0.03). The number of venous thrombotic events was low, and similar in both groups, and there was no statistically significant difference in mortality or length of hospital stay.
Another study (ACTIV-4B)25 (Table 1), also performed in outpatient COVID-19 patients, analysed the preventive efficacy of oral apixaban (2.5 or 5 mg/12 h) and found no differences with respect to placebo in the primary outcome measure (a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause).
Finally, the MICHELLE study26 (Table 1) compared administration of rivaroxaban (10 mg/day) vs. oral placebo for 35 days in patients discharged from hospital (52% admitted to the ICU). Rivaroxaban reduced (3% vs. 9%, p = 0.0293) the risk of occurrence of a composite variable of thromboembolic complications or death of cardiovascular origin.
The latest evidence does not seem to support the use of anticoagulants other than heparin, except in post-discharge patients, where rivaroxaban could be beneficial.
Thromboprophylaxis with antiplatelet agentsTo date, only 4 randomised prospective studies have analysed the effect of antiplatelet drugs on thromboprophylaxis in COVID-19 patients (Table 1). The effect of aspirin has been evaluated in outpatients, non-critical hospitalized patients, and critical hospitalized patients. In outpatient clinics (ACTIV-4B25), the administration of aspirin (81 mg/day) vs. apixaban or placebo did not change the main study endpoint, which was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause. In non-critically ill hospitalized patients (RECOVERY27), the administration of aspirin (150 mg/day) combined or not with prophylactic doses of heparin did not reduce mortality at 28 days or the risk of mechanical ventilation. The REMAP-CAP28 trial studied the the effect of aspirin (75−100 mg/day) combined or not with heparin at different doses (routine, intermediate or therapeutic prophylactic). The addition of aspirin to heparin did not provide significant advantages in any of the variables studied (organ support–free days, survival, or number of thrombotic events), but it did increase the risk of major bleeding.
The effect of P2Y12 inhibitor drugs (iP2y12) in antithrombotic prophylaxis in COVID-19 has also been reported. (ACTIV-4A29 and the aforementioned REMAP-CAP28) (Table 1). The first study was performed in hospitalised non-critical patients and analysed the effect of standard doses of clopidogrel, ticagrelor or prasugrel combined with heparin at therapeutic doses compared to heparin alone. The addition of iP2Y12 did not change the primary endpoint (a composite of survival or days free of organ support at 21 days), although it did increase the probability of major bleeding (2% vs. 0.7%). The second study compared the effect of various doses of heparin (routine, intermediate, or therapeutic prophylactic) alone or in combination with iP2Y12 treatment in critically ill patients. As with aspirin, the addition of iP2Y12 did not provide any advantage (it did not reduce days free of organ support, survival, or number of thrombotic events) and also increased the risk of major bleeding.
The finding that the addition of antiplatelet agents to heparin has no advantages in COVID-19 patients is in contrast with the results of previous observational studies, such as 2 extensive meta-analyses30,31 (with 34,415 and 14,000 patients, respectively from studies that were basically retrospective) that found that mortality was reduced if aspirin was maintained or started at the time of admission. A prospective cohort study, however, found no major advantage. A large study by Santoro et al.32 reported that the use of antiplatelets (acetylsalicylic and others, in single treatment or dual therapy) had no impact on in-hospital mortality (18% vs. 19%, p = 0.64), need for invasive ventilation (8.7% vs. 8.5%; p = 0.88), embolism (2.9% vs. 2.5%; p = 0.34) and bleeding (2.1% vs. 2.4%; p = 0.43), although it did shorten the duration of mechanical ventilation (8 ± 5 days vs. 11 ± 7 days; p = 0.01). In addition, mortality was lower in patients treated exclusively with antiplatelet agents (without concurrent anticoagulation) (RR 0.39; [95% CI 0.48−0.32], p < 0.001), suggesting that the beneficial effect of antiplatelet therapy could be masked by anticoagulant treatment.
Based on recently published randomized studies, we can conclude that the combination of antiplatelet drugs and heparin prophylaxis has no significant advantages.
Previous anticoagulation and COVIDAs mentioned above, the high risk of thrombosis associated with COVID-19 justifies the administration of antithrombotic prophylaxis in the most severely ill patients, many of whom will have already been receiving anticoagulant drugs for various indications (venous thromboembolism, atrial fibrillation or prosthetic heart valve). This led some experts to hypothesise that prior oral anticoagulant treatment with vitamin K antagonists (acenocoumarol or warfarin) or direct oral anticoagulants (DOAC) might have a beneficial effect on the course of the disease. Several studies, mostly retrospective cohorts together with 2 meta-analysis, have been performed to test this hypothesis.
The largest study was performed by Rivera-Caravaca et al.,33 who selected 26,006 patients (13,003 under treatment with DOACs at the time of diagnosis) from a cohort of 738,423 COVID-19 patients. After a 30-day follow-up, the authors concluded that DOAC-treated patients had a higher relative risk of all-cause mortality (RR 1.27 [955 CI 1.12–1.44]), hospitalization and re -hospitalization at the time of COVID-19 diagnosis (RR 1.72 [95% CI, 1.64–1.82]) and venous thromboembolism (RR 4.51 [95% CI 3.91–5.82]). Three other studies,34–36 however, found that prior anticoagulant treatment had a beneficial effect on various composite variables that included 30 to 60-day mortality and/or risk of hospitalization. The largest of these studies34 (23,159 patients treated with warfarin or DOACs at diagnosis), found that the 60-day risk of death or hospitalization was 29.2% (95% CI 27.4%–31.2%) for anticoagulated patients and 32.1% (95% CI 30.7%–33.5%) for non-anticoagulated patients.
Meta-analyses have been unable to clarify the situation either. One such study,37 reported that chronic oral anticoagulants in patients with COVID-19 is associated with reduced mortality, while another38 found no significant difference compared with controls.
There is therefore no clear evidence on whether prior oral anticoagulants (vitamin K antagonists or DOACs) has a protective effect on the course of the disease and reduces mortality or thrombotic complications.
Recommendations from clinical practice guidelinesTable 239–50 gives an overview of the recommendations of various clinical practice guidelines on thromboprophylaxis in COVID-19, based on clinical staging (admitted to ICU, non-critical hospitalised patients, outpatients, or post-discharge patients). Due to the absence of high-quality randomised trials, some of these recommendations lack the preferred strength, although the results of recent clinical trials have shown that the latest recommendations differ markedly from those published just one year ago. The basic recommendations of these guidelines are as follows:
- 1
COVID-19 is a highly thrombogenic disease, and all guidelines agree that heparin thromboprophylaxis should be administered to patients admitted to hospital with COVID-19.
- 2
At the start of the epidemic, some guidelines recommended thromboprophylaxis with intermediate-dose heparin in patients admitted to intensive care units or non-critical patients with thrombotic risk factors. However, this recommendation was not supported by two subsequent clinical trials20.21 and is discouraged in the latest guidelines.49,50
- 3
Administration of therapeutic-dose heparin in patients admitted to intensive care units is not currently recommended either; instead, the standard prophylactic dose should be used because a therapeutic dose of LMWH or UFH does not improve clinical outcome and is associated with a greater risk of bleeding complications.
- 4
Therapeutic-dose heparin, however, is recommened in hospitalized non-critical patients with a low risk of bleeding, since this strategy has been reported to reduce mortality.17–19
- 5
Antiplatelet therapy or anticoagulant therapy with DOACs should not be started in patients hospitalised with COVID-19, but antiplatelet therapy should be maintained if the patient is receiving it as secondary prophylaxis.
- 6
The latest guidelines50 do not recommend routine administration of heparin thromboprophylaxis22 or aspirin in COVID-19 outpatients due to lack of efficacy.25 However, they recommend maintaining antithrombotic treatment (with heparin, oral anticoagulants, or antiplatelet agents) if previously indicated. Some less recent guidelines advise administering prophylactic-dose heparin in patients with a high risk of thromboembolism and no risk factors for bleeding.
- 7
In patients who have recovered from COVID-19 and have been discharged from hospital, prolonged thromboprophylaxis after discharge is not routinely recommended. However, in patients with long-term immobility or severe inflammation, or both, anticoagulant thromboprophylaxis could be considered if there is no increased risk of bleeding. None of the guidelines published so far have included recommendations based on the positive results obtained with rivaroxaban in this population.26
Although experts agree that thromboprophylaxis with heparin should be started hospitalized patients with COVID-19, there is no unanimity regarding the dosage. In patients admitted to ICUs, all most recent recommendations, based on the results of randomized prospective studies, advise against high (therapeutic) doses of low molecular weight heparin, and recommend instead standard thromboprophylaxis doses. In non-critically hospitalized patients, most older guidelines recommend using standard doses, but several recent randomized studies have shown that therapeutic doses should be used if the risk of bleeding is low. Randomized prospective studies have found no advantage in using intermediate-dose low molecular weight heparin, in adding antiplatelet agents to heparin, or in replacing heparin with rivaroxaban.
In discharged patients, most guidelines advise against the routine use of thromboprophylaxis, and reserving this therapy for patients with a high thrombotic and low bleeding risk. However, a large group of clinical practice guidelines remain silent on this issue, showing the lack of a generally accepted criterion. In the absence of a definitive recommendation, it may be reasonable to maintain thromboprophylaxis at standard doses for 7–10 days or until the patient is fully ambulatory.
Many guidelines are also silent on the issue of thromboprophylaxis in outpatient COVID-19 cases. Some recommend using standard-dose thromboprophylaxis if there are certain risk factors (D-dimer >1500 ng/mL, and/or reduced mobility associated with at least one risk factor: obesity, age >70 years, active cancer, history of thromboembolism, or major surgery <3 months), particularly in the presence of clinical signs of pneumonia. Nevertheless, a recent clinical practice guideline that includes the latest results from randomized studies advises against the general use of heparin or aspirin. Antithrombotic treatment with antiplatelet agents or anticoagulants (oral or parenteral) should be maintained only if previously indicated.
FundingThis study has not received specific funding from agencies in the private, public and nonprofit sectors.
Conflict of interestsNone of the authors have a conflict of interest